Abstract
Cellular senescence, a stress induced growth arrest of somatic cells, was first documented in cell cultures over forty years ago, however its physiological significance has only recently been demonstrated. Using novel biomarkers of cellular senescence we examined whether senescent cells accumulate in tissues from baboons of ages encompassing the entire lifespan of this species. We show that dermal fibroblasts, displaying markers of senescence such as telomere damage, active checkpoint kinase ATM, high levels of heterochromatin proteins and elevated levels of p16, accumulate in skin biopsies from baboons with advancing age. The number of dermal fibroblasts containing damaged telomeres reaches a value of over 15% of total fibroblasts, whereas 80% of cells contain high levels of the heterochromatin protein HIRA. In skeletal muscle, a postmitotic tissue, only a small percentage of myonuclei containing damaged telomeres were detected regardless of animal age. The presence of senescent cells in mitotic tissues might therefore be a contributing factor to aging and age related pathology and provides further evidence that cellular senescence is a physiological event.
Keywords: Senescence, telomeres, DNA damage, aging, p16, p21
Introduction
Organismal aging is accompanied by a progressive loss of tissue structure and function, however the cause of tissue degeneration remains poorly understood (Kirkwood and Austad, 2000). Consequently the function of many organ systems progressively declines with increasing age giving rise to the many pathological changes observed as we grow older. For example, skin aging is characterized by a flattening of the dermo-epidermal junction, reduced thickness of the epidermis, wrinkling due to changes in the collagen and elastin matrix, and impaired wound healing, a process that requires functional dermal fibroblasts (Boukamp, 2005). In skeletal muscle, sarcopenia, an age associated loss of muscle mass and strength causes impairment and frailty. Contributing factors of sarcopenia include decreased synthesis rate of muscle proteins, reduction of muscle fibers and an age-associated decline of satellite cells essential for muscle regeneration (Carmeli et al., 2002; Morley et al., 2001; Renault et al., 2002). Why many of these changes occur, is currently unknown.
One model that might partly explain the functional decline of various organ systems with increasing age is that cells essential for tissue function and regeneration increasingly become senescent and accumulate in the organism. Not only would this deplete the pool of mitotically competent and functional cells within these tissues, but it would also change the surrounding microenvironment and compromise tissue repair and renewal since senescent cells secrete a number of matrix metalloproteinases and inflammatory cytokines that can alter the surrounding tissue structure and cause local inflammation (Campisi, 2005). However, due to the lack of suitable markers that can detect senescent cells in tissues, this model remains largely speculative. In order to better understand whether, and to what extent cellular senescence contributes to organismal aging, it is essential to develop novel markers to detect senescent cells in tissues and use these markers to analyze the various organ systems affected by age.
At least two converging mechanisms regulate growth arrest in senescent human cell cultures (Herbig and Sedivy, 2006). A cell intrinsic mechanism, referred to as replicative senescence, is triggered by short, dysfunctional telomeres which are sensed as double strand DNA breaks. Recruitment of DNA damage response and repair factors to dysfunctional telomeres triggers the G1 DNA damage checkpoint which leads to activation of p53, upregulation of the cyclin dependent kinase (Cdk) inhibitor p21, and consequently cell cycle arrest (d'Adda di Fagagna et al., 2003; Gire et al., 2004; Herbig et al., 2004). In cultured human somatic cells, dysfunctional telomeres induce a permanent growth arrest (Herbig et al., 2004), likely due to the inability of the cell to efficiently repair damaged chromosome ends. Although telomere shortening with age has been detected in a variety of human tissues including skin, liver, kidney and lymphocytes (Allsopp et al., 1992; Hastie et al., 1990) it is currently unclear whether telomere dysfunction and the associated senescence growth arrest occurs in tissues of living organisms.
The second growth arrest, termed “stress or aberrant signaling induced senescence” (STASIS) (Drayton and Peters, 2002) is induced independently of telomere damage in cultured cells (Herbig et al., 2004), although high levels of DNA damage have also been shown to trigger this response (Robles and Adami, 1998; Smogorzewska and de Lange, 2002). Other factors that induce STASIS include modifiers of chromatin structure and hypermitogenic signaling elicited by dysregulated oncogenes to name a few (Herbig and Sedivy, 2006). Growth arrest in STASIS is mediated primarily by p16INK4a, an inhibitor of cyclin D/Cdk4,6 complexes. A critical role for STASIS in limiting the progression of malignant lesions in tissues of laboratory animals and humans has recently been demonstrated by a number of laboratories (Braig et al., 2005; Chen et al., 2005; Collado et al., 2005; Michaloglou et al., 2005).
In culture, senescent cells have a characteristic enlarged and flattened morphology, which is accompanied by changes in gene expression, protein processing, and altered metabolic processes. In addition, chromatin structure is reorganized leading to transcriptional silencing of growth-promoting genes in heterochromatin clusters called senescence associated heterochromatin foci (SAHF) (Narita et al., 2003). SAHF formation is driven by the chromatin regulators HIRA and ASF1a (Zhang et al., 2005) which promote the accumulation of heterochromatin proteins such as lysine 9 methylated histone H3 (Me-K9-H3), the histone H2A variant macroH2A (Zhang et al., 2005), and HP1α, β and γ (Narita et al., 2003). Although it is likely that accumulation of these heterochromatin proteins in SAHF contributes to the stable growth arrest in senescent cell, it is currently unknown whether SAHF formation and heterochromatinization of nuclear DNA in naturally senescing cells is a result of replicative senescence, STASIS, or independent mechanisms.
Unlike other model organisms such as mice, baboons share many genetic, anatomical, and physiological similarities with humans including age-associated telomere shortening in leukocytes (Baerlocher et al., 2003; Martin et al., 2002). The non human primate baboon is a relatively long-lived organism with an average life expectancy of 21 years in captivity, although many animals live longer (Bronikowski et al., 2002). Since these similarities make this organism an attractive model system to study the significance of telomere-dysfunction induced senescence in organismal aging, we examined whether senescent cells accumulate in tissues of baboons with increasing age of the animals. Recently, we have shown that senescent cells displaying dysfunctional telomeres accumulate with increasing age in dermal fibroblasts of skin biopsies of aging baboons (Herbig et al., 2006). We now extend these studies and provide further evidence to substantiate the presence of senescent cells in baboon skin biopsies. In addition, we demonstrate that in postmitotic skeletal muscle only a small fraction of myonuclei display telomere damage in both young and old animals.
2. Experimental Procedures
2.1 Tissue Culture
Baboon skin fibroblasts were cultured in Ham’s F10 nutrient mixture (Invitrogen, Carlsbad, CA) supplemented with 15% fetal bovine serum (Hyclone, Logan, UT), 20 mM L-glutamine (Sigma-Aldrich, St. Louis, MO), and penicillin/streptomycin (Sigma- Aldrich, St. Louis, MO). Cultures were maintained using a 1:4 subculture regimen and incubated at 37°C in an atmosphere of 93% N2, 5% CO2, and 2% O2. Cells were split into fresh medium after reaching 70–80% confluence.
2.2 Tissues
30 healthy baboons from the Southwest Foundation for Biomedical Research (SFBR, San Antonio, TX) were included in this study. Baboon sampling and processing of skin tissues were previously described (Herbig et al., 2006). Muscle biopsies were performed as aseptic procedures. Animals were immobilized with ketamine (10 mg/kg, intra-muscularly) and supplemented with diazepam (0.25 mg/kg, intra-venously). Incisions were made through the Vastus Lateralis muscle, biopsies were immediately flash frozen in O.C.T. (optimal cutting temperature) compound and stored at −80°C until used. Wounds were closed immediately with a simple continuous pattern. The yield was about 0.5 cm2 of skeletal muscle per animal. Postoperative analgesics were generally not administered after minor procedures. All procedures were approved by the IACUC (Institutional Animal Care and Use Committee). The SBRF animal program has been accredited by the AAALAC (Association for the Assessment and Accreditation of Laboratory Animal Care, International) since 1973. 8 µm tissue sections were cut using a cryomicrotome (Leica CM3050S) at −20°C.
2.3 Antibodies
The antibodies used and the dilution factors were as follows: anti-53BP1 (Novus; Littleton, CO; 1:500), anti-phospho p53 (S15) (Cell Signalling; Danvers, MA; 1:100), anti-p21 (C19) (Santa Cruz; Santa Cruz, CA; 1:100), anti-phospho ATM (S1981) (Abcam; Cambridge, MA; 1:50), anti-γH2AX (S139) (Upstate; Chicago, IL; 1:100), antip16 (JC8) (GeneTex; San Antonio, TX; 1:100), anti-p16 (H165) (Santa Cruz; Santa Cruz, CA; 1:100), anti-HIRA (generously provided by P.Adams, Fox Chase Cancer Center; 14 µg/ml). Incubation with all primary antibodies was for approximately 14 h at 4°C.
2.4 Immunofluorescence
For immunofluorescence analysis of baboon skin fibroblast cultures the cells were plated onto coverslips 48 h prior to harvesting. Cells were washed briefly with phosphate buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4 ·7H2O, 1.4 mM KH2PO4) and fixed for 20 min with freshly prepared 4% paraformaldehyde dissolved in PBS. Tissue sections were fixed immediately after sectioning under identical conditions. After permeabilization for 20 min with PBST (PBS with 0.2% Triton-X100) cells were incubated in blocking buffer (4% BSA in PBS) for 1 h. All fixation, permeabilization and blocking steps were performed at room temperature. Subsequent processing was performed as previously described (Herbig et al., 2004).
2.5 Immuno Fluorescence In Situ Hybridization (ImmunoFISH)
ImmunoFISH of cultured cells was performed as described previously (Herbig et al., 2004). Fixation, permeabilization and blocking of tissue sections were carried out as described above. After blocking, sections were incubated with a combination of anti- 53BP1 and anti-vimentin antibodies (omitted for muscle tissue sections) in 4% BSA-PBST for 2 h, washed three times with PBS and incubated with an Alexa-488-conjugated goat anti-rabbit antibody (Invitrogen Molecular Probes; Carlsbad, CA; 1:1000) for 1 h in 4% BSA-PBST. Subsequently, tissue sections were washed with PBS fixed again for 20 min with 4% paraformaldehyde in PBS, and incubated with 0.25 mM glycine in PBS for 20 min. Sections were dehydrated sequentially by placing them in 70%, 90% and 100% ethanol for 3 min each. Slides were air dried and incubated for 5 min at 80°C in hybridization buffer containing 0.5 µg/ml (C3TA2)3-Cy3 labelled peptide nucleic acid (PNA) telomeric probe (Applied Biosystems, Foster City, CA), 70% formamide (Sigma; St Louis, MO), 12 mM Tris-HCl pH 8,5 mM KCl, 1 mM MgCl2, 0.001% Triton X-100 and 2.5 mg/ml acetylated BSA (Sigma; St Louis, MO). After denaturation the tissue sections were further incubated in the same buffer at room temperature for 14 h in a humidified chamber. The slides were washed 3 times for 10 min with 70% formamide/2×SSC (0.3 M NaCl, 30 mM Na-citrate, pH 7), followed by a 10 min wash with 2×SSC and a 10 min wash with PBS. Sections were incubated for 1 h with Alexa-488-conjugated donkey anti-goat antibody (Invitrogen Molecular Probes; Carlsbad, CA; 1:1000) together with an Alexa-647-conjugated donkey anti mouse antibody (Invitrogen Molecular Probes; Carlsbad, CA; 1:1000) in PBST to detect vimentin (omitted for muscle tissue sections). After 3 5 min washes with PBS nuclei were counterstained with 0.1 µg/ml DAPI for 5 min. Microscopy was performed as previously described (Herbig et al., 2004). 200–600 nuclei were scored for each animal.
3. Results
3.1 Analysis of cellular senescence in Baboon fibroblast cultures
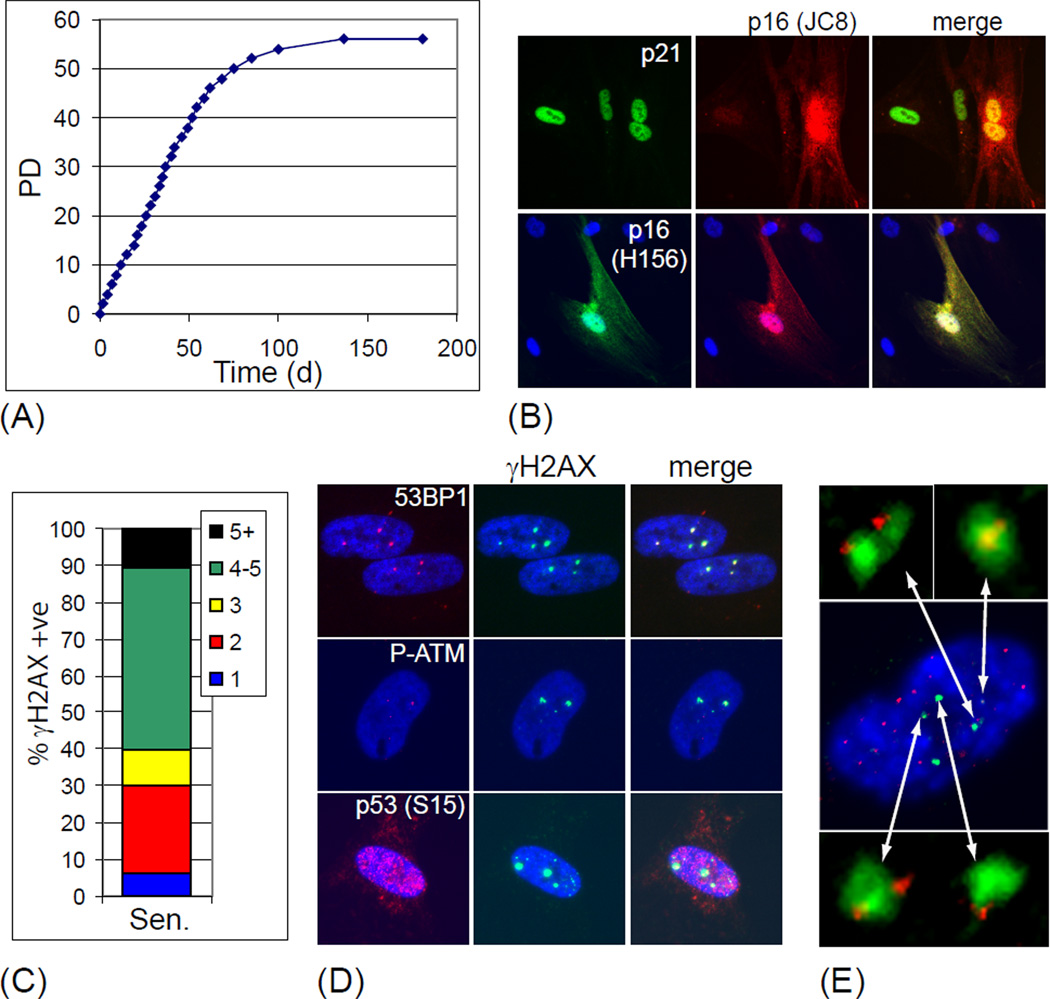
Continuous passage of cultured human fibroblasts results in cellular senescence, in part due to activation of the G1 DNA damage checkpoint by one or more dysfunctional telomeres (d'Adda di Fagagna et al., 2003; Herbig et al., 2004). Whereas human skin fibroblast cultures arrest in response to telomere dysfunction, lung fibroblasts will also spontaneously undergo STASIS in culture, a telomere independent growth arrest (Beausejour et al., 2003; Herbig et al., 2004). To determine whether skin fibroblasts from baboons also have limited replicative potential in culture, we serially passaged these cells into senescence, recording each population doubling (Figure 1A). Similar to humans, baboon skin fibroblasts arrested after 56 population doublings (Figure 1A) with a flattened and enlarged morphology (data not shown). Immunofluorescence analysis revealed that all cells in senescent cultures expressed high levels of the Cdk inhibitor p21 (Figure 1B; green nuclear staining). Only a minor fraction of senescent fibroblasts (less than 10%) displayed elevated levels of p16 (Figure 1B; red nuclear and cytoplasmic staining). In addition to the commonly used monoclonal antibody JC8 to detect endogenous p16 protein, we were also able to visualize p16 protein levels in both human and baboon cell cultures and in baboon tissues using a polyclonal antibody against p16 (Figure 1B, 3B; data not shown).
Fig. 1.
Telomere damage induced senescence in baboon fibroblast cultures. (A) Passage history of baboon skin fibroblasts. PD: population doublings (B) p21 and p16 levels in senescent baboon skin fibroblasts visualized by immunostaining with antibodies against p21 (green, top left panel), p16 using the monoclonal JC8 antibody (red, center column), and p16 using the polyclonal antibody H156 (green, bottom left panel). Merged images are shown in the right column. DNA was counterstained with DAPI (blue). (C) Accumulation of γH2AX foci in senescent baboon skin fibroblasts. Senescent cells were immunostained with a γH2AX antibody and the number of cells displaying one or more γH2AX foci were quantified. The bar is divided into the fraction of cells displaying 1 (blue), 2 (red), 3 (yellow), 4–5 (Green), or over 5 (black) γH2AX foci. A total number of 113 cell nuclei were scored. (D) Activated G1 DNA damage checkpoint proteins in senescent baboon fibroblasts. Cultures of senescent fibroblasts were immunostained with antibodies against γH2AX (green, center column, 53BP1 (red, top left), ATM (S1981) (PATM, red, center left), and p53 (S15) (red, bottom left). Merged images are shown in the right column. DNA was counterstained with DAPI (blue). (E) Senescent cells were processed by immunoFISH to visualize γH2AX foci (green) and telomeres (red). DNA was counterstained with DAPI (blue). Arrows point to enlarged images of TIF.
Fig. 3.
Fibroblasts in skin biopsies primarily contain a single 53BP1 focus and display markers of cellular senescence. (A) Accumulation of 53BP1 foci in fibroblasts of skin biopsies from baboons at indicated ages. Bars indicate the total number of 53BP1 positive fibroblasts in the tissue. The bars are divided into the fraction of fibroblasts displaying 1 (blue), 2 (red), 3 (yellow), 4–5 (Green), or over 5 (black) γH2AX foci. 200–600 fibroblasts were scored for each animal. (B) The number of 53BP1 positive fibroblasts displaying a single 53BP1 focus remains constant as animals age. 200–600 fibroblasts in baboon skin biopsies were scored for each animal. (C) Immunofluorescence analysis of fibroblasts in skin biopsies of a 25 year old baboon showing colocalization between 53BP1 and indicated proteins. P-ATM: ATM(Ser1981). Nuclear DNA was counterstained with DAPI (blue).
Since upregulation of p21 in senescent cultured human cells is a consequence of DNA damage signaling triggered by dysfunctional telomeres, we investigated whether the G1 DNA damage checkpoint was active in senescent baboon fibroblasts. Almost all senescent cells displayed double strand DNA breaks visualized using an antibody against γH2AX (Figure 1C). The vast majority of senescent cells (over 95%) contained multiple γH2AX foci (Figure 1C). γH2AX showed a high degree of colocalization with 53BP1, and phosphorylated ATM kinase (ATM-S1981), and levels of phosphorylated p53 (p53-S15) were elevated in cells displaying DNA damage foci (Figure 1D). Using a telomeric immunoFISH assay to detect telomere dysfunction induced foci (TIF) (Herbig et al., 2004) we observed that 80% or more of γH2AX foci in senescent baboon fibroblast colocalized with telomeric sequences (Figure 1E and data not shown). However, young cells rarely contained TIF (data not shown). Therefore, similar to cultured human fibroblasts, baboon skin fibroblasts undergo replicative senescence in culture, a growth arrest triggered by dysfunctional telomeres and mediated by G1 DNA damage checkpoint proteins.
3.2 Telomere dysfunction induced foci in Baboon skin and skeletal muscle
To test whether telomere dysfunction induced senescence is a physiological event we analyzed two tissues with different proliferating potentials for the appearance of double strand DNA breaks, one of several markers of senescent cells that recently have been described (d'Adda di Fagagna et al., 2003; Gire et al., 2004; Herbig et al., 2004). Dermal fibroblasts were examined as these cells are capable of dividing and telomeres have been observed to shorten in skin tissues with increasing age of the organism (Sugimoto et al., 2006). Skeletal muscle fibers on the other hand, consist of fully differentiated, postmitotic cells. Fewer than 3% of dermal fibroblasts and myonuclei of skeletal muscle contained visible 53BP1 foci in young baboons (animals 5 years of age) suggesting that DNA damage at early age is infrequent (Figure 2A and B). However, as the animals grew older the number of dermal fibroblasts displaying one or more 53BP1 foci increased with age reaching levels of over 30% in very old animals (Herbig et al., 2006) (Figures 2A and B). This was in stark contrast to skeletal muscle myonuclei in which, even in very old animals, the number of double strand DNA breaks did not increase with age (Figures 2A and B).
Fig. 2.
Accumulation of telomere dysfunction induced foci (TIF) in dermal fibroblasts of old baboons. (A) 8 µm skin (left column) or 8 µm skeletal muscle (right column) tissue sections from young (5 yr, top row) and old (30 yr, bottom row) baboons were immunostained with a 53BP1 antibody (red) to detect sites of double strand DNA breaks. 53BP1 foci are indicated by the arrows for better visualization. Nuclear DNA was counterstained with DAPI (blue). (B) Quantitation of 53BP1 positive cells in skeletal muscle and skin biopsies from young (5 animals between the ages of 5–6 years, grey bars) and old (5 animals between the ages of 26–30 years, black bars) baboons. 200–600 cell nuclei were scored for each animal (C) Fibroblasts in baboon skin biopsies (top panel, TIF positive) and myonuclei (bottom panel, TIF negative) were processed by immunoFISH to visualize 53BP1 foci (green) and telomeres (red). DNA was counterstained with DAPI (blue). Arrows point to enlarged images of the 53BP1/telomere signal. (D) Quantitation of TIF in skeletal muscle and skin biopsies from young (5 animals between the ages of 5–6 years, grey bars) and old (5 animals between the ages of 26–30 years, black bars) baboons. 200–600 cell nuclei were scored for each animal. A cell was considered TIF positive when 50% or more of its 53BP1 foci colocalized with telomeric sequences.
To determine whether the 53BP1 foci observed in skin biopsies from old animals localize to telomeric sequences, we performed the telomere-immunoFISH assay which detects dysfunctional telomeres. As shown in Figure 2C (top row), we could clearly detect DNA damage foci in dermal fibroblasts that colocalized with telomeres in old animals, however colocalization in skeletal muscle myonuclei in these animals were infrequent (Fig. 2C bottom row). To quantitatively assess the number of cells containing damaged telomeres we scored a cell TIF positive when 50% or more of its 53BP1 foci colocalized with telomere signals. Although colocalization was observed in animals of all ages, TIF’s were rarely detected in young baboons (5 year age group) due to the infrequent appearance of DNA damage foci. As animals aged to 25–31 years however, the number of TIF positive dermal fibroblasts increased and reached levels of over 15% of total fibroblasts. Often the telomere signal colocalizing with a DNA damage focus was significantly weaker compared to surrounding telomeric signals, suggesting that the dysfunctional telomere was comparatively short (Fig. 2C and data not shown). In contrast to dermal fibroblasts, skeletal muscle myonuclei did not accumulate double strand DNA breaks with age and therefore, myonuclei even in very old animals remained largely TIF negative (Fig. 2C and D; less than 2.5%).
3.3 Comparison of telomere dysfunction in vivo and in vitro
Contrary to cultured human and baboon fibroblasts that primarily display multiple DNA damage foci in old cells (Fig. 1C) (Gire et al., 2004; Herbig et al., 2004), fibroblasts in the dermis of baboons rarely contained more that one DNA damage focus, even in very old animals (Fig. 3A). Over 70% of 53BP1 positive cells in young animals displayed merely a single dysfunctional telomere and this number only slightly decreases to 65% in very old animals (Fig. 3B).
Dysfunctional telomeres in senescent cultured baboon fibroblasts (Fig. 1) and in senescent human fibroblasts (d'Adda di Fagagna et al., 2003; Gire et al., 2004; Herbig et al., 2004) are sensed as double strand DNA breaks which results in the recruitment of a number of DNA damage response factors to the telomeres. As a consequence, p53 becomes activated by post-translational modifications enabling expression of the Cdk inhibitor p21 (Herbig et al., 2004). Although we could clearly detect activation of the ATM kinase in fibroblasts of baboon skin biopsies, as evident by co-localization between ATM(S1981) and 53BP1 (Fig. 3C), we were unable to detect activation and/or upregulation of any downstream mediators and effectors of the G1 DNA damage checkpoint such as Chk1(S317), Chk2(T68), p53(S15), and p21 (data not shown) Currently however, we cannot rule out the possibility that the lack of these checkpoint proteins in 53BP1 positive fibroblasts is due to technical limitations of the antibodies used. Surprisingly, p16 protein levels were elevated in cells that also contained 53BP1 foci, an effect not observed in senescent fibroblast cultures (Herbig et al., 2004).
3.4 Levels of heterochromatin proteins in tissues
Cellular senescence in cultured human cells is associated with global changes in chromatin structure resulting in the accumulation of heterochromatin proteins 1 (HP1α, β, and γ), HIRA, and lysine 9-methylated histone H3 (Me-K9-H3) (Narita et al., 2003; Zhang et al., 2005) at a number of promoters that regulate cell growth genes. Some of these heterochromatin proteins are also elevated in premalignant lesions where they are thought to contribute to a stable cellular growth arrest to prevent the outgrowth of these lesions into malignant tumors (Collado et al., 2005; Michaloglou et al., 2005). We observed that nuclear HIRA levels were significantly elevated in dermal fibroblasts positive for 53BP1 (Fig. 3C), suggesting that these cells contained high levels of repressive chromatin and were senescent. Less than 20 % of fibroblasts in skin biopsies from young animals (5 year age group) contained elevated levels of HIRA but this number increased dramatically to over 70% in very old baboons (Fig. 4A and B). However, unlike telomere damage which increased in an exponential manner (Herbig et al., 2006), HIRA upregulation with increasing age of the animals followed a linear pattern (Fig. 4B). Although virtually all 53BP1 positive fibroblasts also stained positive for HIRA, the total number of HIRA positive cells far exceeded the number of 53BP1 positive fibroblasts suggesting that stresses other than telomere damage also contribute to accumulation of HIRA in dermal fibroblasts. In contrast to tissues from skin, no specific staining for HIRA could be detected in skeletal muscle biopsies (data not shown).
Fig. 4.
The number of fibroblasts in baboon skin biopsies displaying elevated levels of the heterochromatin protein HIRA increases with age of the animal. (A) Immunostaining of dermal fibroblasts of a young (5 year, top row) and an old (29 year, bottom row) animal with an antibody against HIRA (green). Nuclear DNA was counterstained with DAPI (blue). (B) Quantitation of HIRA positive fibroblasts in skin biopsies of baboons at indicated ages.
Although senescence of cultured cells has been correlated to increased levels and aggregation of heterochromatin proteins HP1β, Me2-K9-H3, and Me3-K9-H3 (Narita et al., 2003; Zhang et al., 2005) we could not detect either specific staining or a statistically significant and age dependent increase of these proteins in skin or skeletal muscle biopsies. In muscle myonuclei elevated levels of either HP1β or Me3-K9-H3 could not be detected, although the majority displayed high nuclear levels of nuclear Me2-K9-H3 in an age independent manner (data not shown). In contrast to myonuclei, dermal baboon fibroblasts displayed strong nuclear staining of all three heterochromatin markers. However the levels of these heterochromatin proteins did not increase significantly with age (data not shown) making them an unreliable marker for senescent cells in these tissues.
4. Discussion
Whether cellular senescence is a physiological mechanism or simply an artifact of tissue culture has been subject to debate for many years (Sherr and DePinho, 2000). Although markers for cellular senescence, such as staining for senescence-associated β galactosidase activity at pH=6.0 (Dimri et al., 1995), have been used widely in an attempt to detect senescent cells in culture and tissues from a variety of organisms, the use and specificity of this biomarker clearly has its limitations (Cristofalo, 2005; Severino et al., 2000; Yang and Hu, 2005). The availability of novel and specific biomarkers to detect senescent cells in vivo, has allowed us to reinvestigate the physiological significance of cellular senescence in living organisms. Together with our recently published data (Herbig et al., 2006) we demonstrate that senescent cells characterized by damaged-telomeres, activation of ATM kinase, elevated p16 levels, and heterochromatinization of the nuclear genome accumulate in the dermis of aging baboons. Although we were unable to quantitatively assess the accumulation of cells displaying some of these markers, due to antibody limitations, we were able to measure the age-associated increase in cells containing high levels of the heterochromatin marker HIRA. These data support our previous findings that senescent cells accumulate in mitotic tissue with advancing age (Herbig et al., 2006).
Pathogenesis of skin aging is caused by both intrinsic and extrinsic mechanisms (McCullough and Kelly, 2006). Whereas telomere-dysfunction induced senescence might be considered an intrinsic mechanism, environmental stresses, the major being exposure to ultraviolet (UV) radiation, are superimposed on intrinsic factors and accelerate age-associated changes in the skin. As shown in this report, over 15% of dermal fibroblast in very old animals contained damaged telomeres however, 80% displayed elevated levels of HIRA. Although virtually all fibroblasts that displayed 53BP1 foci also accumulated high levels of HIRA, not all HIRA positive cells contained DNA damage foci. Since many stresses, including telomere-dysfunction (Herbig et al., 2004) and exposure to UVA radiation (Naru et al., 2005), cause cellular senescence of cultured human fibroblasts, senescence in dermal fibroblasts of the skin might also be caused by multiple stresses. It is therefore possible that upregulation of HIRA is primarily caused by extrinsic, telomere-damage independent factors, which would explain the much higher frequency of cells displaying this heterochromatin marker. Alternatively, DNA damage signaling from dysfunctional telomeres for extended periods of time might result in a stable growth arrest characterized by heterochromatinization of nuclear DNA. It is thus possible that after an extensive period of chronic DNA damage, checkpoint signaling is no longer required to maintain a stable growth arrest due to upregulation of other growth suppressors, such as p16INK4a, and heterochromatin proteins, and the DNA damage signal eventually dissipates. Long term DNA damage checkpoint activation from dysfunctional telomeres might actually not be beneficial for an organism since growth arrest triggered by telomere-dysfunction can be bypassed in cultured cells simply by inactivating checkpoint proteins such as ATM (d'Adda di Fagagna et al., 2003; Herbig et al., 2004), Chk2 (Gire et al., 2004), p53 (Beausejour et al., 2003), or p21 (Herbig et al., 2004). Since little is known about the pathways involved in upregulation and aggregation of heterochromatin proteins in senescent cells, the other causes of elevated HIRA levels in dermal fibroblasts of baboons remain to be determined.
Given that the vast majority of senescent fibroblasts in culture display multiple DNA damage foci that colocalize with telomeric DNA (Herbig et al., 2004), the presence of only a single 53BP1 focus in over 65% of 53BP1 positive dermal fibroblasts in skin biopsies in both young and old baboons was a surprising observation. Although technical limitations might prevent us from detecting smaller and less bright DNA damage foci in tissues, these data suggest that a single dysfunctional telomere is sufficient to trigger and potentially maintain the senescence response in dermal fibroblasts. In agreement with this, we observed that 53BP1 positive dermal fibroblasts displaying a single DNA damage focus also contained elevated levels of p16, HIRA, and active ATM kinase at all ages of the animals (Fig. 3C, data not shown), conditions that have been demonstrated to prevent proliferation of cultured cells. Furthermore, we have previously shown that a single TIF is sufficient to induce a permanent growth arrest in cultured fibroblasts although the vast majority of senescent cells contain multiple TIF (Herbig et al., 2004). Our data might also point out important differences between in vitro and in vivo senescence such as an increase of stress in cells cultured under non-physiological conditions which might facilitate TIF formation. However, it is equally likely that continuous proliferation of fibroblasts in culture, which occurs infrequently in tissues, might shorten telomeres in a more uniform and synchronous manner compared to stochastic telomere shortening events that likely occur in tissues. This might lead to an accumulation of many critically short telomeres giving rise to the multiple TIF observed in nuclei of cultured cells.
The low abundance of visible telomere damage in skeletal muscle myonuclei of very old baboons suggests that telomere-dysfunction induced senescence does not universally occur in all tissues. Although we currently can not rule out the possibility that cells of other postmitotic tissues accumulate dysfunctional telomeres with age, it may suggest that cell proliferation may be required for telomeres to become dysfunctional. This is in agreement with the observation that cultured somatic cells progressively lose telomeric DNA with every cell division which, to a certain extent, is due to the inability of the replicative polymerase to completely duplicate linear chromosome ends. While it is likely that this end replication problem contributes to telomere-dysfunction in proliferating cells, it is clearly not the only mechanism that can lead to the deprotection of chromosome ends. Factors such as reactive oxygen species (ROS) (von Zglinicki, 2002), nucleases (Lydall, 2003), secondary structures in telomeric DNA (Ding et al., 2004) and sporadic loss of large telomeric stretches (Wang et al., 2004) have all been implicated to contribute to telomere erosion and telomere dysfunction in mammalian cells. Our observations that telomere signals in TIF’s of dermal fibroblast nuclei usually emit only a very faint fluorescence signal compared to other telomeric signals in the same cell nucleus suggests that the majority of intact, non-damaged telomeres are significantly longer than the dysfunctional one. Telomere erosion exclusively due to the end replication problem, which would affect all telomeres equally, is therefore unlikely. To what extent other potential telomere shortening mechanisms contribute to TIF formation in tissues remains to be determined.
Although telomere-dysfunction induced senescence of postmitotic myofibers is not prevalent and therefore would not be a contributing factor to the age-associated loss of muscle mass and strength, it is not inconceivable that this type of senescence occurs in progenitors of myofibers, called satellite cells. These progenitor cells are critical for the ability of skeletal muscle to grow and become repaired upon injury and a loss of satellite cells with age would impair the regenerative capacity of skeletal muscle (Jejurikar and Kuzon, 2003). In fact, satellite cells progressively lose telomeric DNA with every cell division (Decary et al., 1997) and undergo replicative senescence in culture (Cudre-Mauroux et al., 2003) suggesting that these cells are capable of undergoing a telomere-dysfunction induced arrest in tissues. Since the number of satellite cells declines with advancing age of the organism (Jejurikar and Kuzon, 2003) it is possible that these cells undergo replicative senescence in vivo. However, due to the low abundance of satellite cells in our skeletal muscle biopsies, we were unable to test this hypothesis directly.
Studies from a number of laboratories have recently demonstrated that oncogene-induced senescence is a physiological mechanism that limits the growth of premalignant neoplasms in both mouse and human tissues (Braig et al., 2005; Chen et al., 2005; Collado et al., 2005; Gray-Schopfer et al., 2006; Lazzerini Denchi et al., 2005;Michaloglou et al., 2005). Our studies demonstrate that telomere-dysfunction induced senescence may limit the replicative potential of fibroblasts in the dermis of baboons, which would lead to an accumulation of senescent cells in old animals. Telomere-dysfunction places a cell at risk for acquiring additional, potentially transforming mutations by promoting genomic instability in DNA damage checkpoint compromised cells (Greenberg, 2005). Therefore, both oncogene-and telomere dysfunction-induced senescence may be a mechanism to suppress the growth of cells that have acquired potentially hazardous mutations. However, at the cost of suppressing tumorigenesis, accumulation of senescent cells with advancing age might also contribute to organismal aging by depleting tissues of functional cells required to maintain organ homeostasis, making the senescence response antagonistically pleiotropic (Campisi, 2005).
Acknowledgements
This work was supported by NIH grant R01 AG016694 to J.M.S. Partial support to U.H. was provided by the NRSA postdoctoral fellowship F32 CA099388 from the NIH. The Brown Imaging facility was supported by the NIH COBRE grant P20 RR-15578. The anti-HIRA antibodies were a generous gift from P. Adams.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerlocher GM, Mak J, Roth A, Rice KS, Lansdorp PM. Telomere shortening in leukocyte subpopulations from baboons. Journal of Leukocyte Biology. 2003;73:289–296. doi: 10.1189/jlb.0702361. [DOI] [PubMed] [Google Scholar]
- Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. Embo J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P. Skin aging: a role for telomerase and telomere dynamics? Curr Mol Med. 2005;5:171–177. doi: 10.2174/1566524053586644. [DOI] [PubMed] [Google Scholar]
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- Bronikowski AM, Alberts SC, Altmann J, Packer C, Carey KD, Tatar M. The aging baboon: comparative demography in a non-human primate. Proc Natl Acad Sci. 2002;99:9591–9595. doi: 10.1073/pnas.142675599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Carmeli E, Coleman R, Reznick AZ. The biochemistry of aging muscle. Exp Gerontol. 2002;37:477–489. doi: 10.1016/s0531-5565(01)00220-0. [DOI] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon- Cardo C, Pandolfi PP. Crucial role of p53 dependent cellular senescence in suppression of Pten deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguria A, Zaballos A, Flores JM, Barbacid M, Beach D, Serrano M. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- Cristofalo VJ. SA beta Gal staining: biomarker or delusion. Exp Gerontol. 2005;40:836–838. doi: 10.1016/j.exger.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Cudre-Mauroux C, Occhiodoro T, Konig S, Salmon P, Bernheim L, Trono D. Lentivector-mediated transfer of Bmi-1 and telomerase in muscle satellite cells yields a duchenne myoblast cell line with long-term genotypic and phenotypic stability. Hum Gene Ther. 2003;14:1525–1533. doi: 10.1089/104303403322495034. [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- Decary S, Mouly V, Hamida CB, Sautet A, Barbet JP, Butler-Browne GS. Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum Gene Ther. 1997;8:1429–1438. doi: 10.1089/hum.1997.8.12-1429. [DOI] [PubMed] [Google Scholar]
- Dimri G, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Liskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Schertzer M, Wu X, Gertsenstein M, Selig S, Kammori M, Pourvali R, Poon S, Vulto I, Chavez E, Tam PP, Nagy A, Lansdorp PM. Regulation of murine telomere length by Rtel: an essential gene encoding a helicase-like protein. Cell. 2004;117:873–886. doi: 10.1016/j.cell.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Drayton S, Peters G. Immortalisation and transformation revisited. Curr Opin Genet Dev. 2002;12:98–104. doi: 10.1016/s0959-437x(01)00271-4. [DOI] [PubMed] [Google Scholar]
- Gire V, Roux P, Wynford-Thomas D, Brondello JM, Dulic V. DNA damage checkpoint kinase Chk2 triggers replicative senescence. Embo J. 2004 doi: 10.1038/sj.emboj.7600259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Schopfer VC, Cheong SC, Chong H, Chow J, Moss T, Abdel-Malek ZA, Marais R, Wynford-Thomas D, Bennett DC. Cellular senescence in naevi and immortalisation in melanoma: a role for p16. Br J Cancer. 2006;95:496–505. doi: 10.1038/sj.bjc.6603283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg RA. Telomeres, crisis and cancer. Curr Mol Med. 2005;5:213–218. doi: 10.2174/1566524053586590. [DOI] [PubMed] [Google Scholar]
- Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Herbig U, Ferreira M, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere Shortening Triggers Senescence of Human Cells through a Pathway Involving ATM, p53, and p21(CIP1), but Not p16(INK4a) Mol Cell. 2004;14:501–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- Herbig U, Sedivy JM. Regulation of growth arrest in senescence: telomere damage is not the end of the story. Mech Ageing Dev. 2006;127:16–24. doi: 10.1016/j.mad.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Jejurikar SS, Kuzon WM., Jr Satellite cell depletion in degenerative skeletal muscle. Apoptosis. 2003;8:573–578. doi: 10.1023/A:1026127307457. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Austad SN. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Lazzerini Denchi E, Attwooll C, Pasini D, Helin K. Deregulated E2F activity induces hyperplasia and senescence-like features in the mouse pituitary gland. Mol Cell Biol. 2005;25:2660–2672. doi: 10.1128/MCB.25.7.2660-2672.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydall D. Hiding at the ends of yeast chromosomes: telomeres, nucleases and checkpoint pathways. J Cell Sci. 2003;116:4057–4065. doi: 10.1242/jcs.00765. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Mahaney MC, Bronikowski AM, Dee Carey K, Dyke B, Comuzzie AG. Lifespan in captive baboons is heritable. Mech Ageing Dev. 2002;123:1461–1467. doi: 10.1016/s0047-6374(02)00083-0. [DOI] [PubMed] [Google Scholar]
- McCullough JL, Kelly KM. Prevention and treatment of skin aging. Ann N Y Acad Sci. 2006;1067:323–331. doi: 10.1196/annals.1354.044. [DOI] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LCW, Soengas MS, Denoyelle C, Kuilman T, van der Horst CMAM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. BRAFE600 associated senescence like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- Narita M, Nunez S, Heard E, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- Naru E, Suzuki T, Moriyama M, Inomata K, Hayashi A, Arakane K, Kaji K. Functional changes induced by chronic UVA irradiation to cultured human dermal fibroblasts. Br J Dermatol 153 Suppl. 2005;2:6–12. doi: 10.1111/j.1365-2133.2005.06964.x. [DOI] [PubMed] [Google Scholar]
- Renault V, Thornell LE, Eriksson PO, Butler-Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1:132–139. doi: 10.1046/j.1474-9728.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- Robles SJ, Adami GR. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998;16:1113–1123. doi: 10.1038/sj.onc.1201862. [DOI] [PubMed] [Google Scholar]
- Severino J, Allen RG, Balin S, Balin A, Cristofalo VJ. Is betagalactosidase staining a marker of senescence in vitro and in vivo? Exp Cell Res. 2000;257:162–171. doi: 10.1006/excr.2000.4875. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, DePinho RA. Cellular senescence: mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- Smogorzewska A, de Lange T. Different telomere damage signaling pathways in human and mouse cells. Embo J. 2002;21:4338–4348. doi: 10.1093/emboj/cdf433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto M, Yamashita R, Ueda M. Telomere length of the skin in association with chronological aging and photoaging. J Dermatol Sci. 2006;43:43–47. doi: 10.1016/j.jdermsci.2006.02.004. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Wang RC, Smogorzewska A, de Lange T. Homologous recombination generates T-loop-sized deletions at human telomeres. Cell. 2004;119:355–368. doi: 10.1016/j.cell.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Yang NC, Hu ML. The limitations and validities of senescence associatedbeta- galactosidase activity as an aging marker for human foreskin fibroblast Hs68 cells. Exp Gerontol. 2005;40:813–819. doi: 10.1016/j.exger.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Zhang R, Poustovoitov MV, Ye X, Santos HA, Chen W, Daganzo SM, Erzberger JP, Serebriiskii IG, Canutescu AA, Dunbrack RL, Pehrson JR, Berger JM, Kaufman PD, Adams PD. Formation of MacroH2Acontaining senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev Cell. 2005;8:19–30. doi: 10.1016/j.devcel.2004.10.019. [DOI] [PubMed] [Google Scholar]