Abstract
Background and Objectives
Arterial stiffness is well known as an important risk factor for cardiovascular disease. At our institution, we assessed the association between arterial stiffness, as determined by brachial ankle pulse wave velocity (baPWV), and the extent of coronary artery disease (CAD), as detected by conventional coronary angiography (CAG) in patients who visited the outpatient clinic for angina without any previous history of heart disease. In addition, we evaluated if the level of baPWV could predict the revascularization as a clinical outcome.
Subjects and Methods
On a retrospective basis, we analyzed the data of 651 consecutive patients who had undergone baPWV and elective CAG for suspected CAD between June 2010 and July 2011, at a single cardiovascular center.
Results
The baPWV was one of the statistically meaningful predictors of significant CAD (diameter of stenosis >50%) in addition to male gender, age, the level of high density lipoprotein-cholesterol, and hemoglobin A1c in multivariate analysis. However, baPWV was not the significant predictor of revascularization. When the extent of CAD was classified into following 4 groups; no significant CAD, 1-, 2- and 3-vessel disease, there was significant difference of baPWV between the significant and non-significant CAD group, but there was no difference of baPWV among the 3 significant CAD groups, although there was a trend toward the positive correlation.
Conclusion
Although baPWV was an independent predictor of significant CAD, it was neither associated significantly with the extent of CAD nor with the risk of revascularization. Therefore, baPWV has a limited value for portending the severity of CAD in patients with chest pain.
Keywords: Arterial stiffness, Coronary artery disease, Atherosclerosis
Introduction
Arterial stiffness is emerging as a marker of cardiovascular disease.1-3) Over the past decade, many studies have focused on arterial stiffness resulting in clarification of the associated factors. Elevated arterial stiffness is associated with a number of cardiovascular risk factors that include age, hypertension, diabetes mellitus, and end stage renal disease4),5); furthermore, arterial stiffness is increased in patients with atherosclerotic disease.2) Although arterial stiffness can be evaluated by various parameters, there is no consistent method.
Among these methods, pulse wave velocity (PWV) has been becoming a widely adopted as an index of arterial stiffness because of its simplicity, reproducibility, and inexpensiveness. A large number of studies have suggested that a relationship exists between increased PWV and coronary artery disease (CAD)6-8); one of these studies was the Framingham study, which has included over 2000 participants and has demonstrated an association between high aortic PWV and first CVD event in white middle aged and in elderly subjects.3)
Among the several ways evaluating arterial stiffness with PWV, carotid-femoral PWV (cfPWV), which is a measurement of stiffness of the thoracic and abdominal aorta has mostly been used in previous studies. However, the brachial-ankle (ba) PWV has been used increasingly in clinical research and in practice currently, because ease of use for clinical staff and its comfort to subjects who take the examination. Thus, this method allows a greater convenience of screening the general population than other methods of measuring PWV.9)
Although baPWV contains both the components of central and peripheral arterial stiffness when compared to cfPWV, which reflects stiffness of large arteries such as the aorta, it can be thought that there are associations between arterial stiffness measured through baPWV and significant CAD.6),9-13)
On these grounds, we assessed, retrospectively, the association between arterial stiffness, as determined by baPWV, and the presence and extent of CAD, as detected by conventional coronary angiography (CAG) in patients who visited outpatient clinic for chest pain without any previous history of ischemic heart disease. Furthermore, we evaluated how the baPWV is related to the revascularization as a clinical outcome.
Subjects and Methods
Study population
From June 2010 to July 2011, 1276 patients visited the outpatient clinic in the Department of Cardiology at Sejong General Hospital for the evaluation of effort chest pain and underwent both CAG and baPWV. Of the 1276 patients, we enrolled adults of 30 years to 80 years old, but excluded patients with a past history of ischemic heart disease, myocardial infarction, percutaneous coronary intervention (PCI), coronary artery bypass surgery (CABG), chronic kidney disease (the estimated glomerular filtration rate <60 mL/min/1.73 m2 or serum creatinine >2.0 mg/dL), or heart failure (left ventricular ejection fraction <45%).
Among the patients, those who underwent PCI or CABG urgently or who were diagnosed with unstable angina or myocardial infarction by electrocardiogram (ECG) or elevation of cardiac enzyme (creatine kinase-MB, TnT) were not included. Also, the patients who had a previous history of peripheral artery disease (PAD), symptoms of intermittent claudication, limb ischemic signs, or ankle brachial index (ABI) <0.9 were excluded from this study. Because previous studies have suggested that a decreased ABI is related to an increased PWV; severe PAD is related with increased arterial wave reflection, which may confound our result.13) Our final study population included 651 subjects and was approved by the institutional review board of Sejong General Hospital.
Other variables of interest including cardiovascular risk factors
We obtained basic demographic data from participant's medical records. Each subject was interviewed closely regarding information on any medical history including hypertension, diabetes mellitus, stroke or myocardial infarction, tobacco use, and current medication. Height and weight were measured for all subjects. Body mass index (BMI) {weight (kg)/height2 (m2)} was calculated. Mean arterial pressure was determined as the diastolic pressure plus one third pulse pressure. All patients had a thorough physical examination and routine biochemical analysis of the blood and urine. After patients had fasted for at least 12 hours, the following parameters were measured: plasma triglycerides, total cholesterol, low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), glycated hemoglobin A1c (HbA1c), fasting blood glucose, uric acid, blood urea nitrogen, and serum creatinine.
Measurements of baPWV
The baPWV measurement was performed using a volume-plethysmographic device (Omron Healthcare, Kyoto, Japan), which uses a method based on blood pressure cuffs wrapped on the arm near the brachial artery and tibial artery of ankle14); thus, blood pressure, ABI, ECG, bilateral baPWV, and heart sounds are measured simultaneously. Subjects were examined in the supine position after at least 5 minutes rest, with ECG electrodes placed on both wrists, and a microphone for detecting heart sounds placed on the left edge of the sternum. Brachial and ankle pulse arterial pressure waveforms were stored for 10 seconds interval sampling times with automatic gain analysis and quality adjustment. The mean of bilateral baPWV value was used for the analysis. The validity and reproducibility of such baPWV measurements have been described elsewhere.9) The baPWV measurement had been performed within at least 1 week before undergoing CAG as a part of the clinical pathway.
Coronary angiography
Coronary angiography was performed in all patients using a standard Judkins technique via the right femoral artery or right radial artery at the physician's discretion. The coronary angiograms were assessed by 3 experienced physicians who were blinded to the information about an individual patient's baPWV. We defined significant CAD as a more than 50% narrowing of the lumen by visual estimation. Subjects were assigned to the non-significant, 1-, 2-, or 3-vessel disease group based on the presence of a 50% or greater diameter narrowing for each of the 3 main coronary arteries (the patients with left main disease were included in the 2-vessel disease group).
Clinical outcomes
We categorized the subjects into three groups in the light of clinical outcomes; non-significant CAD, intermediate-CAD, and revascularization groups. The non-significant CAD group was defined as follows; normal CAG, <50% of luminal narrowing of main coronary artery, or small vessel disease. Patients who had >50% luminal narrowing of the main coronary artery, but had a negative stress test result, were included in the intermediate CAD group. Both of these groups received medical treatment alone. Subjects who had undergone PCI or CABG were categorized into the revascularization group.
Statistical analysis
All data are expressed by mean±SD. Categorical and continuous variables were compared between groups using chi-squared, unpaired Student's t-test, and the analysis of covariance. A multivariate logistic regression analysis was carried out to assess the odds ratios of factors related to significant CAD and of those patients who underwent revascularization. A receiver operating characteristic (ROC) curve was used to show a positive correlation between the baPWV and clinical outcomes in patients with angina. Cut-off values were determined as the sum of sensitivity and specificity. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) 12.0 statistical package (SPSS Inc., Chicago, IL, USA) and the values of p<0.05 were considered to indicate statistical significance.
Results
Baseline characteristics of the overall study population and of each group of patients classified as significant versus non-significant CAD are summarized in Table 1. The sample was composed of 378 male and 273 female patients. The mean age of participants was 61.5±9.9 years and the mean BMI was 24.7±3.1 kg/m2. Three-hundred-seventy-six patients (57.8%) were hypertensive, 182 (28%) were diabetic, and 112 (17.2%) were current smokers. The Significant CAD group was comprised of 401 (61.6%) subjects, in which baPWV was significantly increased compared to the non-significant CAD group (1729±387.2 cm/s vs. 1514±287.6 cm/s, p<0.001).
Table 1.
Baseline patient characteristics
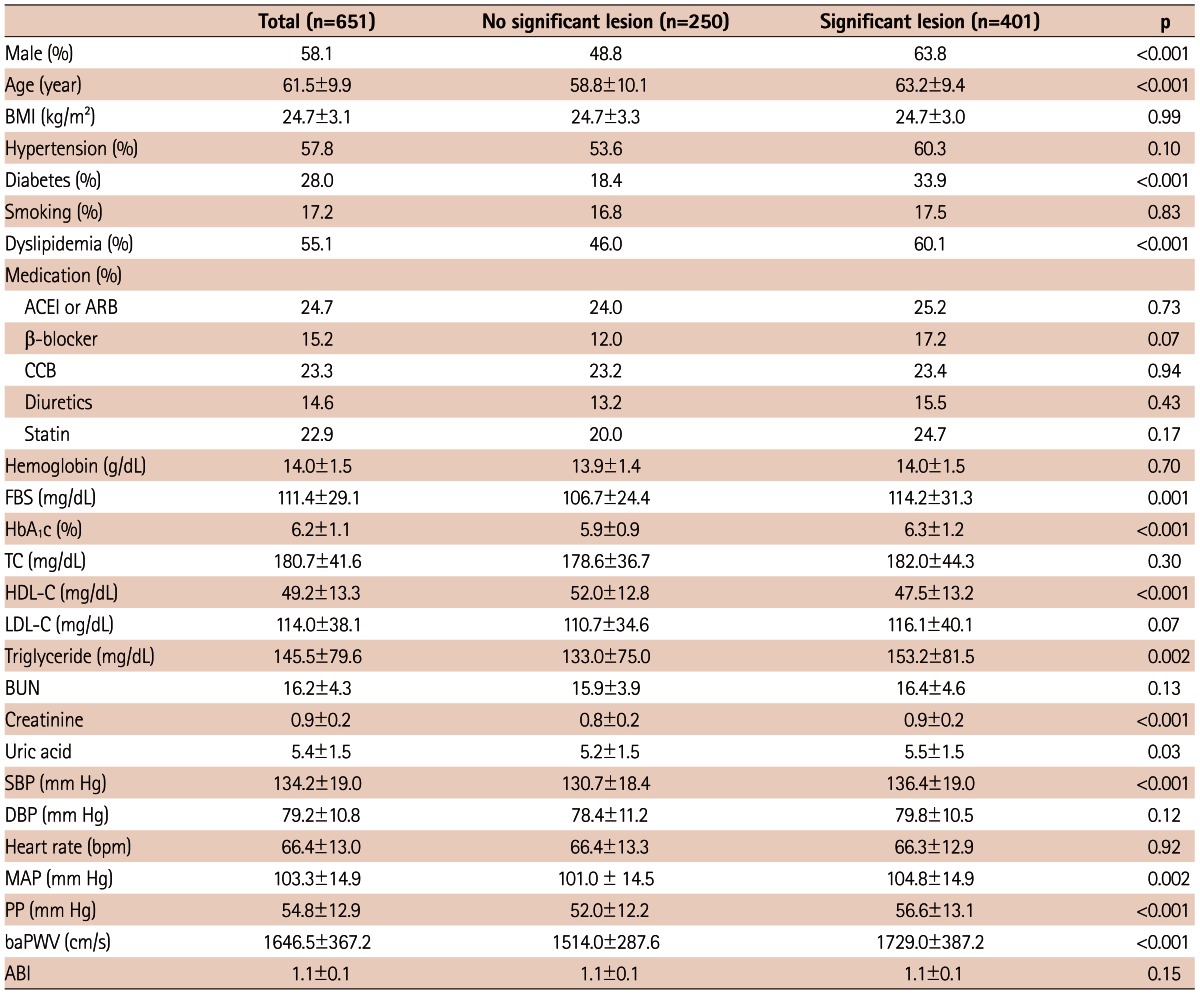
Data were presented as % or mean±standard deviation. Significant stenosis was defined as >50% luminal narrowing of coronary artery on conventional coronary angiogram. BMI: body mass index, ACEI: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker, CCB: calcium channel blocker, FBS: fasting blood sugar, HbA1c: hemoglobin A1c, TC: total cholesterol, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, BUN: blood urea nitrogen, SBP: systolic blood pressure, DBP: diastolic blood pressure, MAP: mean arterial pressure, PP: pulse pressure, baPWV: brachial-ankle pulse wave velocity, ABI: ankle/brachial pressure index
Among the significant CAD group, revascularizations were performed in 256 patients (63.8%), which included 226 PCI and 30 CABG. In terms of extent of CAD, 207 patients had single vessel disease, 133 patients had 2-vessel disease and 61 patients had 3-vessel disease. Table 2 showed that the more the number of vessel diseased, the more invasive treatment is needed (p<0.001).
Table 2.
The clinical outcomes in patients with significant coronary disease
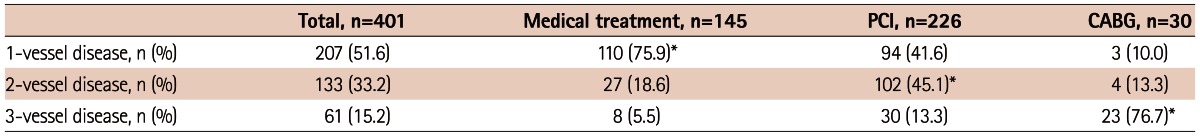
*p<0.001, by chi-square test. PCI: percutaneous coronary intervention, CABG: coronary artery bypass graft
We carried out multivariate logistic regression analysis to detect the factors related to significant CAD and revascularization. As shown in Table 3, male gender, age, HDL-C, HbA1C and baPWV were meaningfully related to the existence of significant CAD. When it came to the clinical outcome of revascularization, male gender, age, HDL-C, LDL-C and HbA1C were significantly associated with the patient who received revascularization (Table 4). However, the level of baPWV was not independently associated with increased risk of revascularization (odds ratio=1.001, 95% confidence interval: 1.000-1.001, p=0.191)
Table 3.
Multivariate logistic regression analysis of factors related to significant coronary artery disease
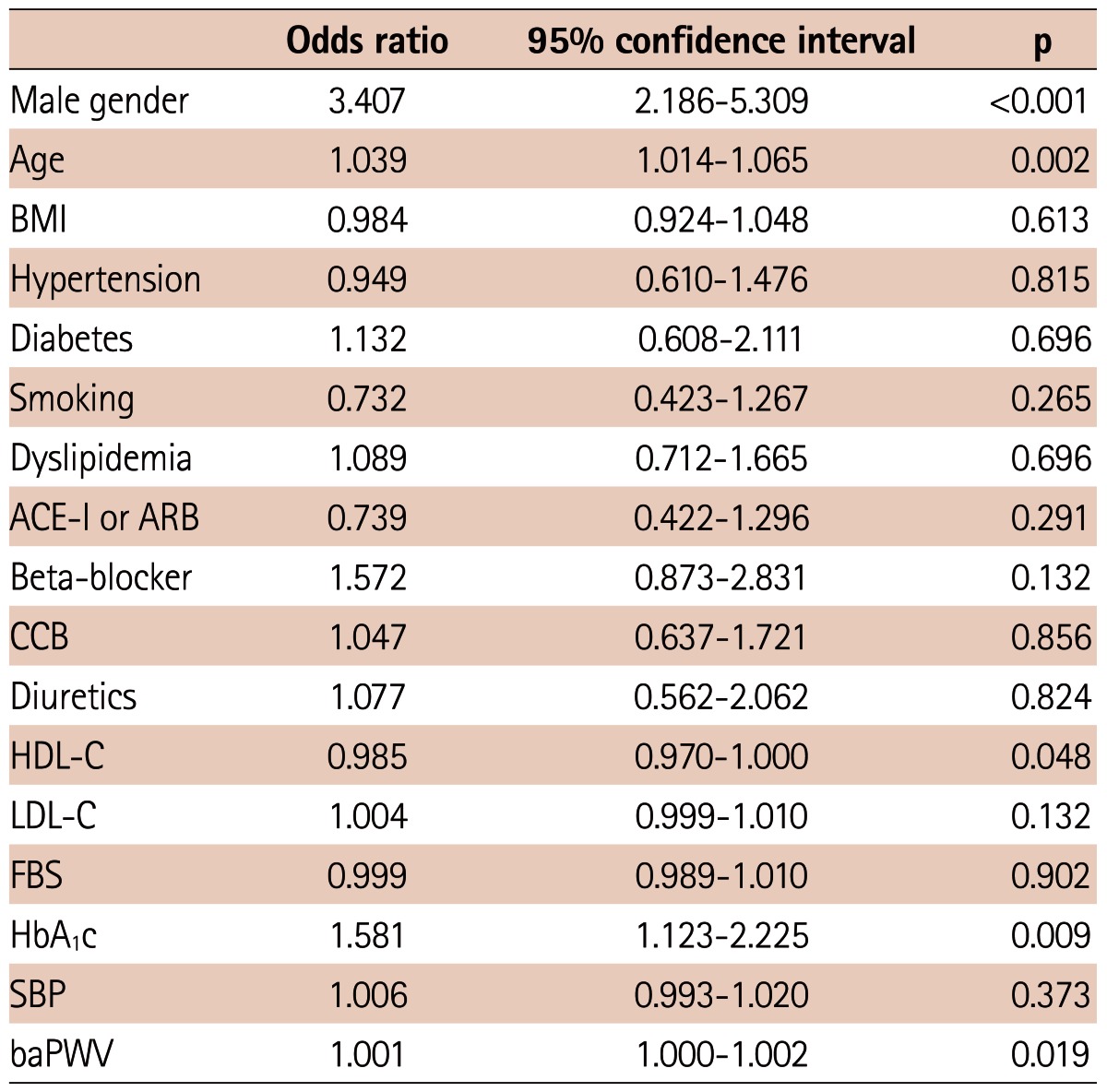
BMI: body mass index, ACE-I: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, CCB: calcium channel blocker, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, FBS: fasting blood glucose, HbA1c: hemoglobin A1c, SBP: systolic blood pressure, baPWV: brachial-ankle pulse wave velocity
Table 4.
Multivariate logistic regression analysis of factors related to the revascularization
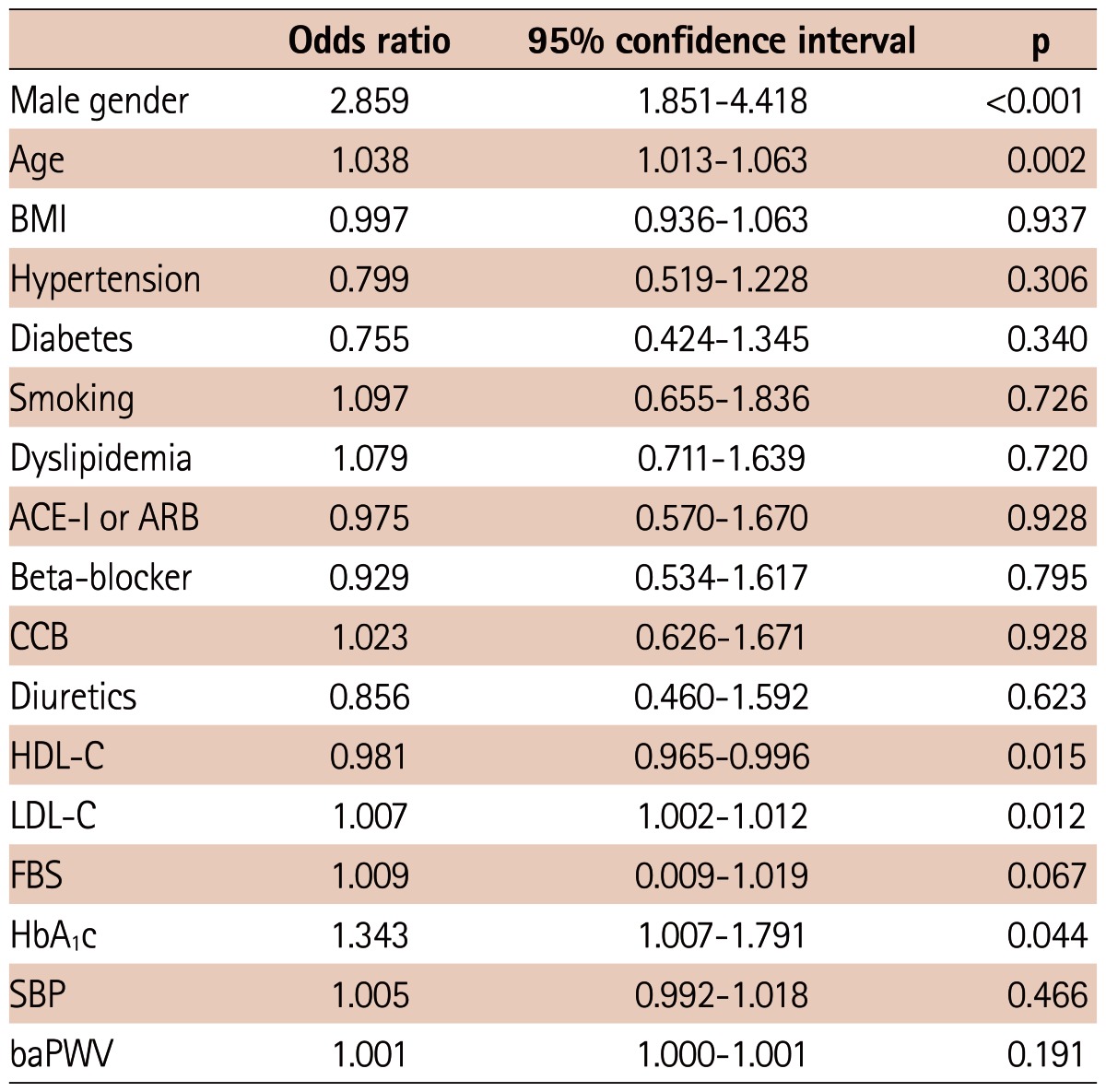
BMI: body mass index, ACE-I: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, CCB: calcium channel blocker, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, FBS: fasting blood glucose, HbA1c: hemoglobin A1c, SBP: systolic blood pressure, baPWV: brachial-ankle pulse wave velocity
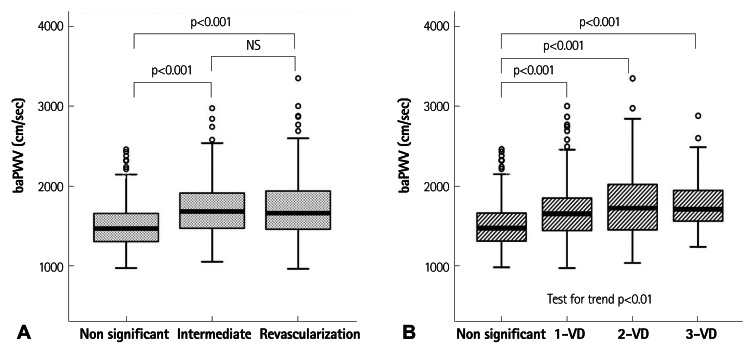
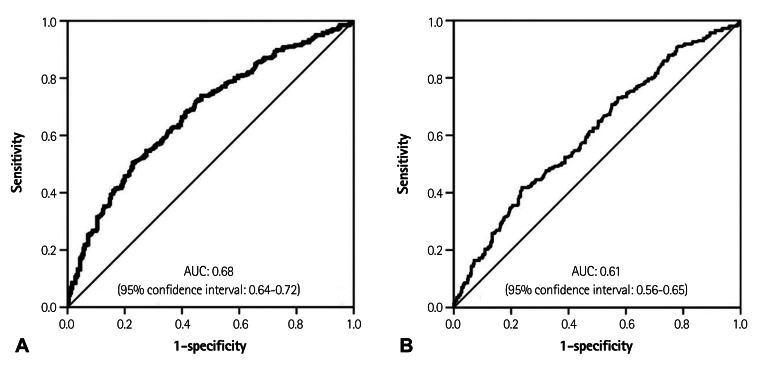
Fig. 1 shows the value of baPWV according to the history of revascularization and the presence of CAD. In the analysis of clinical outcomes, the non-significant CAD group's baPWV was significantly lower than other groups, but the baPWV values were not significantly different between intermediate CAD group and the revascularization group (Fig. 1A). In the view of extent of CAD, we assorted the subjects into one of 4 groups: non-significant stenosis, 1, 2 and 3-vessel disease. A significant difference of baPWV was not observed between each group except for the subjects without significant stenosis. However, a trend toward positive association was found between the extent of CAD and baPWV (p<0.01) (Fig. 1B). The ROC curve analysis of the association between baPWV and CAD is shown in Fig. 2. In a ROC curve of baPWV for significant CAD (Fig. 2A), the area under the curve was 0.68 (95% confidence interval=0.64-0.72). For revascularization (Fig. 2B), the area under the curve was 0.61 (95% confidence interval=0.56-0.65). The cut-off point of 1540 cm/s showed 65% sensitivity and 61% specificity in predicting significant CAD and the cut-off point of 1570 cm/s showed 60% sensitivity and 54% specificity in predicting revascularization.
Fig. 1.
The value of baPWV according to the history of revascularization and the presence of CAD. A: comparison of baPWV between the subjects divided into 3 groups based on clinical outcomes. The baPWV of the patients with normal or minimal CAD was significantly lower than that of the patients who had intermediate CAD or received revascularization, but there was no significant difference in baPWV between the intermediate CAD and revascularization group. B: comparison of baPWV according to the extent of coronary artery disease. There was no significant difference in baPWV between each group except the subjects without significant stenosis, but there was a linear trend between the number of stenosed vessels and increased baPWV. baPWV: brachial-ankle pulse wave velocity, CAD: coronary artery disease, VD: vessel disease, NS: no significant difference.
Fig. 2.
Receiver operating characteristic (ROC) curve analysis. A: ROC curve of baPWV for significant coronary artery disease, baPWV cut-off value at 1540 cm/s showed sensitivity 65%, specificity 61% (area under the ROC curve=0.68; 95% confidence interval=0.64-0.72). B: ROC curve of baPWV for revascularization, 1570 cm/s showed sensitivity 60%, specificity 54% (area under the ROC curve=0.61; 95% confidence interval=0.56-0.65). baPWV: brachial-ankle pulse wave velocity, AUC: area under curve.
Discussion
In this study, the baPWV was independently associated with significant CAD in patients who had no previous history of ischemic heart disease, heart failure, peripheral arterial occlusive disease or chronic renal failure. However, it did not show the meaningful difference among the classified groups by the extent of CAD or by the clinical outcomes of medical treatment versus revascularization, though there was a trend toward positive association between the extent of CAD and baPWV.
Increased arterial stiffness hinders the hemodynamic buffering effect of the cardiovascular system, contributing to elevated systolic blood pressure and pulse pressure, coronary arterial disease, and left ventricular hypertrophy.1),15) Therefore, measuring aortic PWV is suggested as the standard method for the evaluation of aortic stiffness in assessing subclinical target organ damage.2),16) Previous studies have suggested that the measurement of PWV could be useful to screen for vascular damage in a large population.17),18)
The cfPWV measurement has been used as a conventional method for assessing arterial stiffness. The cfPWV in patients with atherosclerotic diseases has been well established19) and the Rotterdam study, which included over 3000 elderly subjects, showed that cfPWV is a strong predictor of CAD and stroke.20) Although cfPWV measurement by tonometric method is accurate and widely used, it may not be suitable for routine use in clinical practice, because this method is dependent upon the transducers that must be placed exactly over the arteries and this is a time consuming job. Of the problems related to data acquisition, the application of baPWV in assessing arterial stiffness has been used on increasing basis in recent clinical research studies.6),9),10),21-23) The baPWV reflects the stiffness of both central and peripheral muscular arteries and serves as a simple index of the arterial stiffness severity and of atherosclerosis. Moreover, the validity and the reproducibility are high, regardless of clinical staff technique; thus, it is appropriate for routine examinations and large clinical trials. The baPWV has a close association with aortic PWV, which is an index of central arterial stiffness9),22) and is a positive correlation with the intima-media thicknes of the carotid artery that predicts the severity of atherosclerosis.24)
Many studies showed that an increased baPWV reflects the presence of CAD. Yamashina et al.11) found that an increased baPWV (baPWV ≥1400 cm/s) serves to discriminate the patients with coronary heart disease, especially in middle-aged subjects and suggested that baPWV has the potential as a marker of cardiovascular risk stratification. Nam et al.6) have recently reported that asymptomatic patients with baPWV ≥1426 cm/s showed a significantly higher rate of stenosis on coronary CT angiography. Tomiyama et al.21) revealed that baPWV predicts a prognosis of patients with acute coronary syndrome. In their results, a baPWV of 1700 cm/s or higher indicated a risk of post-hospitalization cardiovascular events and 1800 cm/s or higher indicated a risk of a major cardiovascular event. There are several mechanisms that could explain the association between increased PWV and CAD. Arterial stiffness causes premature return of the reflected pulse wave in late systole, leading to an increase in central pulse pressure and, consequently, to increased load on the left ventricle. The results are decreased ejection fraction and increased myocardial oxygen demand.25) In addition, the decreased absorption capacity of the arterial wall leads to wall injury, which accelerates the progression of atherosclerosis.26) The results of the present study resemble the results of earlier studies, which reported that the patients with significant CAD have a higher level of baPWV than normal subjects. However, our data showed that the cut-off value of baPWV for significant CAD was 1540 cm/s, while the cut-off value for the revascularization was 1570 cm/s. It was too small to put meaning to the difference between both groups. In addition, the level of baPWV was not associated independently with an increased risk of revascularization. Thus, we suggest that baPWV has a limited value for predicting the severity of the disease in patients with significant CAD.
With regard to the usefulness of baPWV measurement in the diagnosis of CAD, there have been several studies that have been in conflict. Seo et al.27) reported that baPWV may be of limited value in identifying patients at risk for CAD on the study of 174 patients with high-risk of cardiovascular disease. The results demonstrated that baPWV did not directly correlate with coronary artery calcification and severity of CAD in high-risk patients. In their study, the Gensini score was calculated to evaluate the extent and severity of coronary atherosclerosis, though this is not commonly used in clinical practice. In addition, their study population group was comprised of the patients who were at a relatively high-risk for CAD compared to our patients. So, the differences in study populations may cause disparity from our results. Igarashi et al.28) used myocardial perfusion imaging to evaluate the diagnostic value of ABI and baPWV for the extent and severity of CAD and showed that ABI, but not baPWV, correlated with the percent myocardial ischemia. However, we excluded patients who had an abnormal value of ABI less than 0.9 or had a previous history of peripheral arterial disease from the beginning in this study; peripheral arterial stenosis can affect baPWV. Therefore, we did not include ABI for the analysis of independent variables associated with CAD.
Thus far, a number of reports have studied the association between baPWV and CAD. However, this study is of relatively large scale research compared to others. In additional, the present study was based on general patients with chest pain visiting the outpatient clinic, whose pain was documented as angina. These points may enable our results to be clinically applicable in daily practice. All the subjects underwent conventional CAG, which confirmed the CAD and made it available to assess not only the correlation between baPWV and the presence of CAD, but the correlation between extent of CAD and clinical outcome.
However, the present study is subject to several limitations. First, our research is a retrospective study, so the association between baPWV and other clinical figures cannot have an intrinsic causal relationship. In addition, we could not figure out the patients who refused revascularization, but we think that those cases were so small that it made no difference in the results.
Second, since the current study was performed based on same ethnic group and in a single medical center, the generalizability of our data remains uncertain. Third, many subjects were taking various medications including antihypertensive drugs that may exert significant influences on baPWV. It is difficult to evaluate how exactly those medications influenced the numbers for baPWV; nonetheless, there was no statistically significant difference in antihypertensives or lipid-lowering medications between the patients with significant CAD or with non-significant CAD. Thus, it can be assumed that the medications would not have had major effect on the results of this study.
In conclusion, this study demonstrated that the baPWV is associated independently with significant CAD in patients with angina who had visited the outpatient clinic. Although there was a trend toward a positive association between the extent of CAD and baPWV, it did not show the meaningful difference in baPWV, neither among the groups classified by the extent of CAD nor between the clinical outcomes of medical versus revascularization. Therefore, baPWV has limited value in predicting the severity of CAD, although baPWV may be utilized as an easily accessible screening tool for detecting significant CAD among those patients with newly developed angina in an outpatient clinic.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Amar J, Ruidavets JB, Chamontin B, Drouet L, Ferrières J. Arterial stiffness and cardiovascular risk factors in a population-based study. J Hypertens. 2001;19:381–387. doi: 10.1097/00004872-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehmann ED, Gosling RG, Sönksen PH. Arterial wall compliance in diabetes. Diabet Med. 1992;9:114–119. doi: 10.1111/j.1464-5491.1992.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 5.London GM, Marchais SJ, Safar ME, et al. Aortic and large artery compliance in end-stage renal failure. Kidney Int. 1990;37:137–142. doi: 10.1038/ki.1990.19. [DOI] [PubMed] [Google Scholar]
- 6.Nam HJ, Jung IH, Kim J, et al. Association between brachial-ankle pulse wave velocity and occult coronary artery disease detected by multi-detector computed tomography. Int J Cardiol. 2012;157:227–232. doi: 10.1016/j.ijcard.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 7.Kullo IJ, Bielak LF, Turner ST, Sheedy PF, 2nd, Peyser PA. Aortic pulse wave velocity is associated with the presence and quantity of coronary artery calcium: a community-based study. Hypertension. 2006;47:174–179. doi: 10.1161/01.HYP.0000199605.35173.14. [DOI] [PubMed] [Google Scholar]
- 8.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 9.Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–364. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Nam JS, Park JS, et al. Usefulness of brachial-ankle pulse wave velocity as a predictive marker of multiple coronary artery occlusive disease in Korean type 2 diabetes patients. Diabetes Res Clin Pract. 2009;85:30–34. doi: 10.1016/j.diabres.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Yamashina A, Tomiyama H, Arai T, et al. Brachial-ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res. 2003;26:615–622. doi: 10.1291/hypres.26.615. [DOI] [PubMed] [Google Scholar]
- 12.Liu CS, Li CI, Shih CM, et al. Arterial stiffness measured as pulse wave velocity is highly correlated with coronary atherosclerosis in asymptomatic patients. J Atheroscler Thromb. 2011;18:652–658. doi: 10.5551/jat.7021. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Wu Y, Li J, et al. The predictive value of brachial-ankle pulse wave velocity in coronary atherosclerosis and peripheral artery diseases in urban Chinese patients. Hypertens Res. 2008;31:1079–1085. doi: 10.1291/hypres.31.1079. [DOI] [PubMed] [Google Scholar]
- 14.Imanishi R, Seto S, Toda G, et al. High brachial-ankle pulse wave velocity is an independent predictor of the presence of coronary artery disease in men. Hypertens Res. 2004;27:71–78. doi: 10.1291/hypres.27.71. [DOI] [PubMed] [Google Scholar]
- 15.Kingwell BA, Waddell TK, Medley TL, Cameron JD, Dart AM. Large artery stiffness predicts ischemic threshold in patients with coronary artery disease. J Am Coll Cardiol. 2002;40:773–779. doi: 10.1016/s0735-1097(02)02009-0. [DOI] [PubMed] [Google Scholar]
- 16.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 17.Asmar R, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 18.Asmar R, Topouchian J, Pannier B, et al. Scientific, Quality Control, Coordination and Investigation Committees of the Complior Study. Pulse wave velocity as endpoint in large-scale intervention trial. The Complior study. J Hypertens. 2001;19:813–818. doi: 10.1097/00004872-200104000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Safar ME, Henry O, Meaume S. Aortic pulse wave velocity: an independent marker of cardiovascular risk. Am J Geriatr Cardiol. 2002;11:295–298. doi: 10.1111/j.1076-7460.2002.00695.x. [DOI] [PubMed] [Google Scholar]
- 20.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 21.Tomiyama H, Koji Y, Yambe M, et al. Brachial -- ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome. Circ J. 2005;69:815–822. doi: 10.1253/circj.69.815. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchikura S, Shoji T, Kimoto E, et al. Brachial-ankle pulse wave velocity as an index of central arterial stiffness. J Atheroscler Thromb. 2010;17:658–665. doi: 10.5551/jat.3616. [DOI] [PubMed] [Google Scholar]
- 23.Tomiyama H, Yamashina A, Arai T, et al. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement--a survey of 12517 subjects. Atherosclerosis. 2003;166:303–309. doi: 10.1016/s0021-9150(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 24.Yambe M, Tomiyama H, Hirayama Y, et al. Arterial stiffening as a possible risk factor for both atherosclerosis and diastolic heart failure. Hypertens Res. 2004;27:625–631. doi: 10.1291/hypres.27.625. [DOI] [PubMed] [Google Scholar]
- 25.Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 26.Demer LL. Effect of calcification on in vivo mechanical response of rabbit arteries to balloon dilation. Circulation. 1991;83:2083–2093. doi: 10.1161/01.cir.83.6.2083. [DOI] [PubMed] [Google Scholar]
- 27.Seo WW, Chang HJ, Cho I, et al. The value of brachial-ankle pulse wave velocity as a predictor of coronary artery disease in high-risk patients. Korean Circ J. 2010;40:224–229. doi: 10.4070/kcj.2010.40.5.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Igarashi Y, Chikamori T, Tomiyama H, et al. Diagnostic value of simultaneous brachial and ankle blood pressure measurements for the extent and severity of coronary artery disease as assessed by myocardial perfusion imaging. Circ J. 2005;69:237–242. doi: 10.1253/circj.69.237. [DOI] [PubMed] [Google Scholar]