Abstract
Background/Aims
The bedside index of severity in acute pancreatitis (BISAP) is a new, convenient, prognostic multifactorial scoring system. As more data are needed before clinical application, we compared BISAP, the serum procalcitonin (PCT), and other multifactorial scoring systems simultaneously.
Methods
Fifty consecutive acute pancreatitis patients were enrolled prospectively. Blood samples were obtained at admission and after 48 hours and imaging studies were performed within 48 hours of admission. The BISAP score was compared with the serum PCT, Ranson's score, and the acute physiology and chronic health examination (APACHE)-II, Glasgow, and Balthazar computed tomography severity index (BCTSI) scores. Acute pancreatitis was graded using the Atlanta criteria. The predictive accuracy of the scoring systems was measured using the area under the receiver-operating curve (AUC).
Results
The accuracy of BISAP (≥ 2) at predicting severe acute pancreatitis was 84% and was superior to the serum PCT (≥ 3.29 ng/mL, 76%) which was similar to the APACHE-II score. The best cutoff value of BISAP was 2 (AUC, 0.873; 95% confidence interval, 0.770 to 0.976; p < 0.001). In logistic regression analysis, BISAP had greater statistical significance than serum PCT.
Conclusions
BISAP is more accurate for predicting the severity of acute pancreatitis than the serum PCT, APACHE-II, Glasgow, and BCTSI scores.
Keywords: Prognosis, Severity, Serum procalcitonin, Acute pancreatitis
INTRODUCTION
Acute pancreatitis (AP) is highly variable in terms of its clinical presentation and severity [1,2]. Many scoring systems have been developed for the early detection of severe AP, but they are not convenient for predicting the severity of AP since they involve many parameters [3-5].
Ranson's score is relatively accurate at classifying the severity of AP, but it is difficult to calculate the score as it requires a 48-hour, missing the potential for early treatment [6]. The acute physiology and chronic health examination (APACHE)-II is more accurate than Ranson's score [7], and is more commonly used to predict the severity of AP [7-9], although it was designed originally to predict intensive care unit survival and required the collection of many parameters, some of which might not be relevant to AP prognosis [10]. The Glasgow score also requires many clinical parameters and needs 48 hours to complete. In 1990, Balthazar et al. [11] described the utility of contrast-enhanced computed tomography (CT) for evaluating the severity of AP and developed the Balthazar computed tomography severity index (BCTSI) based on contrast-enhanced CT, but this system was based on local complications and did not reflect the systemic inflammatory response. The biochemical marker serum procalcitonin (PCT) is a relatively accurate and convenient method for predicting the severity in AP and is easily measured [12,13]. Some studies revealed a strong relationship between an increased serum PCT and the severity of AP [12,14,15].
In our previous study, the serum PCT level in severe AP was significantly higher than in mild AP. The accuracy of serum PCT as biochemical marker was 77.3%, which was similar to the APACHE-II score, worse than Ranson's score (93.2%), and better than the BCTSI (65.9%) [16]. In 2008, Wu et al. [17] proposed new prognostic scoring system for the early determination of the severity of AP, which they named the bedside index of severity in acute pancreatitis (BISAP). BISAP incorporates f ive parameters: blood urea nitrogen > 25 mg/dL, impaired mental status, systemic inflammatory response syndrome (SIRS), age > 60 years, and detection of pleural effusion by imaging [17,18]. SIRS is defined by the presence of at least two of the following: pulse > 90 beats per minute, respirations > 20 per minute, PaCO2 < 32 mmHg, temperature > 38℃ or < 37℃, and white blood cell count > 12,000 or < 4,000 cells/mm3, or > 10% immature neutrophils (bands) [19,20]. A prospective validation of the BISAP scoring system as a method for the early detection of severe AP was published recently, and concluded that it is a reliable, accurate means for stratifying patients with AP [17] and that it was an accurate and convenient method of risk stratification compared with other multifactorial scoring systems in patients with AP [21]. However, the BISAP data are limited and insufficient for clinical application. The serum PCT is a simple, convenient method for predicting the severity of AP [12,14,15], with similar accuracy to the APACHE-II score [16]. As no study has compared BISAP with the serum PCT, we compared the accuracies of the BISAP, serum PCT, and other scoring systems in terms of predicting the severity of AP.
METHODS
Study population
This prospective study enrolled 50 consecutive patients with AP admitted to Dong-A University Hospital, Busan, Korea over a 14-month period. The diagnosis of AP was based on acute upper abdominal pain associated with a serum amylase level greater than three times the normal value or an elevated serum lipase level and radiological evidence of AP. The patients were treated using the accepted standard management of AP [22]. All patients were fasted soon after admission and given fluids, electrolytes, and analgesics parenterally. If necessary, systemic antibiotics were administered. Patients with a suspected biliary cause of AP underwent endoscopic retrograde cholangiopancreaticography within the first 24 hours.
Methods
Blood samples for PCT were collected on admission and biochemical tests for the other scoring systems were performed on admission (day 0) and within 48 hours (day 2). Appropriate physiological data were recorded and weighted for age and chronic health to permit calculation of the Ranson's, APACHE-II, and Glasgow scores. The BISAP and APACHE-II scores were calculated from data obtained within 24 hours of admission, while Ranson's score was calculated at and 48 hours after admission. Contrast-enhanced CT was performed within 48 hours of admission and repeated weekly if the symptoms worsened. For serum PCT, blood samples were centrifuged for 10 minutes at 3,000 rotations per minute at -4℃. The serum was removed and stored at -80℃ until biochemical analysis. The serum PCT concentration was measured using a chemiluminescent immunoassay (LUMItest PCT, Brahms Diagnostica, Berlin, Germany). The reference value range established for this method was < 0.05 ng/mL. All patients were classified as mild or severe AP according to the Atlanta criteria [23]. This study was approved by the Institutional Review Board of Dong-A University Hospital, Busan, South Korea.
Statistical analysis
We expressed the results as medians followed by range or 95% confidence interval. Groups were compared using the Mann-Whitney U test for noncategorical data; Fisher's exact test was used to examine differences in the sex ratio, etiology, and death ratio. The cutoff values of BISAP, serum PCT, and other parameters were determined using receiver operating characteristic (ROC) curves. Sensitivity, specificity, positive, and negative predictive values, accuracy, and likelihood ratios were also calculated. Logistic regression analysis was used to establish the influence of the chosen parameters on the prognosis of AP. Linear regression analysis was conducted to estimate the relationship between the BISAP scores and length of hospital stay. The relationship between the serum PCT and length of hospitalization was evaluated using the same statistical method. A p value < 0.05 was considered to indicate statistical significance. The PASW version 18.0 for Windows (IBM Co., Armonk, NY, USA) was used to perform all statistical analyses.
RESULTS
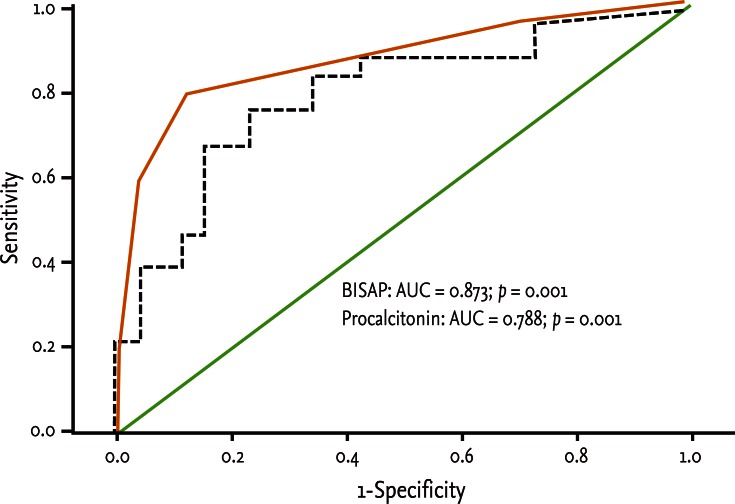
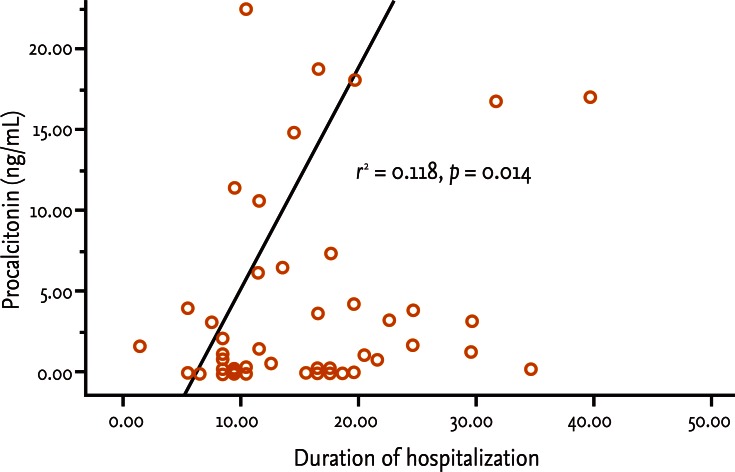
Fifty patients were enrolled in the study: 34 males and 16 females. The median patient age was 59.5 years. According to the Atlanta criteria, 26 patients were classified as mild AP and 24 as severe AP. There were no significant differences according to age (p = 0.228) and sex (p = 0.85). The causes of AP were alcoholic, biliary stone, and idiopathic or miscellaneous; differences were not significant (p = 0.465) (Table 1). Seven patients died: four of multiple organ failure and two of severe necrotizing pancreatitis; all six had severe AP, while one patient with mild AP and underlying thyroid cancer had a sudden cardiac arrest with no previous cardiac problems. The parameters according to the Atlanta criteria are described in Table 1. All six parameters were analyzed using the area under the receiver-operating curve (AUC) to predicting severe AP and the BISAP results were excellent (AUC = 0.873; p = 0.001) and the serum PCT was good (AUC = 0.788; p = 0.001) (Fig. 1). According to the analysis, Ranson's scores were the most accurate (AUC = 0.947) and the BISAP and APACHE-II scores had similar accuracy for predicting severe AP (AUC = 0.873 and AUC = 0.857, respectively). The serum PCT had relatively low accuracy (AUC = 0.788), but was better than the BCTSI score (AUC = 0.676). All six parameters were significant in terms of predicting the severity of AP (p < 0.05) (Table 2). To assess significance, BISAP scores were analyzed using two cutoff values and serum PCT levels were analyzed using four cutoff values. For the BISAP, scores of 2 were more accurate than scores of 3. For the serum PCT, 1.77 and 3.29 ng/mL had the same accuracy, but the sensitivity, positive predictive value, and likelihood ratio were higher for 3.29 ng/mL, making it the best cutoff (Table 3). The sensitivity, specificity, positive and negative predictive values, accuracy, and likelihood ratio for the other parameters with the best cutoff value were also analyzed (Table 4). Logistic regression analysis of risk factors for severe AP revealed that the odds ratios of the BISAP and serum PCT were 29.13 (≥ 2, p < 0.001) and 11.00 (≥ 3.29 ng/mL, p < 0.001), respectively (Table 5). In a simple linear regression analysis of admission duration, the BISAP had no significant relationship with hospital stay (p = 0.073), while the serum PCT was correlated with the length of hospital stay (p = 0.014), although it had a low r2 value (r2 = 0.118) (Fig. 2).
Table 1.
Characteristics of the patients with acute pancreatitis
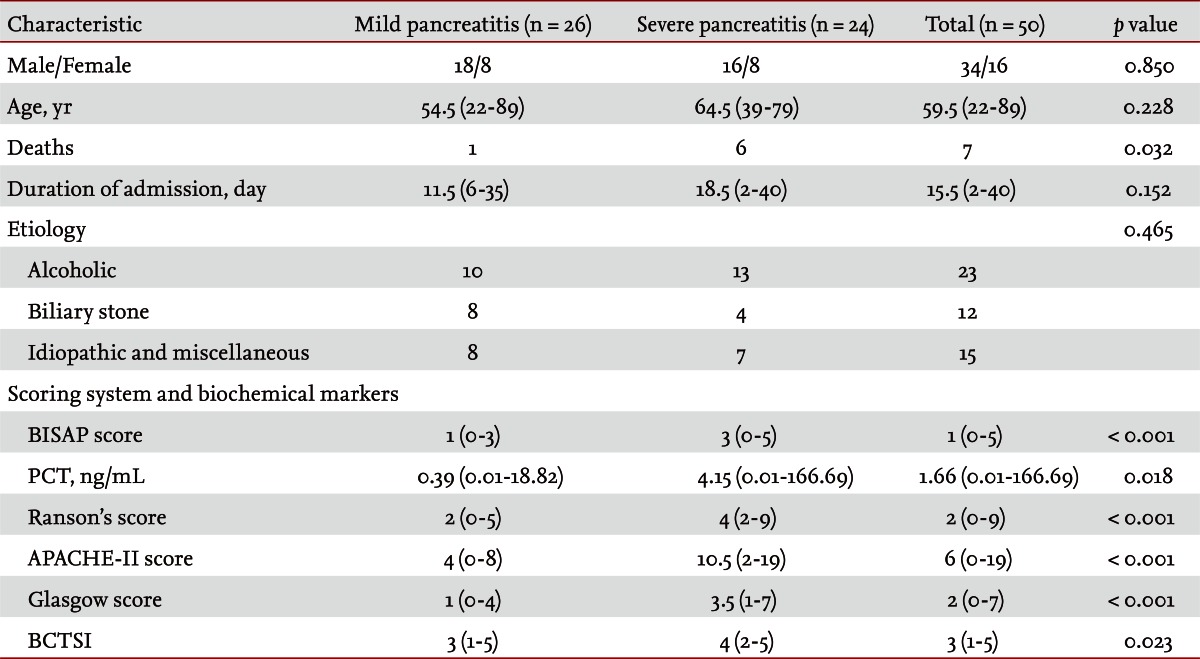
Values are presented as median (range).
BISAP, bedside index of severity in acute pancreatitis; PCT, procalcitonin; APACHE-II, acute physiology and chronic health examination-II; BCTSI, Balthazar computed tomography severity index.
Figure 1.
Receiver operation characteristic curve of serum levels of bedside index for severity in acute pancreatitis (BISAP) and procalcitonin in prediction of severity of acute pancreatitis. BISAP had excellent value (AUC = 0.873; p = 0.001) and serum procalcitonin had good value (AUC = 0.788; p = 0.001). AUC, area under the receiver-operating curve.
Table 2.
Area under the receiver-operating curve of scoring systems for predicting the severity of acute pancreatitis

BISAP, bedside index of severity in acute pancreatitis; PCT, procalcitonin; APACHE-II, acute physiology and chronic health examination-II; BCTSI, Balthazar computed tomography severity index; AUC, area under the receiver-operating curve; CI, confidence interval.
Table 3.
Analysis of BISAP scores and serum procalcitonin
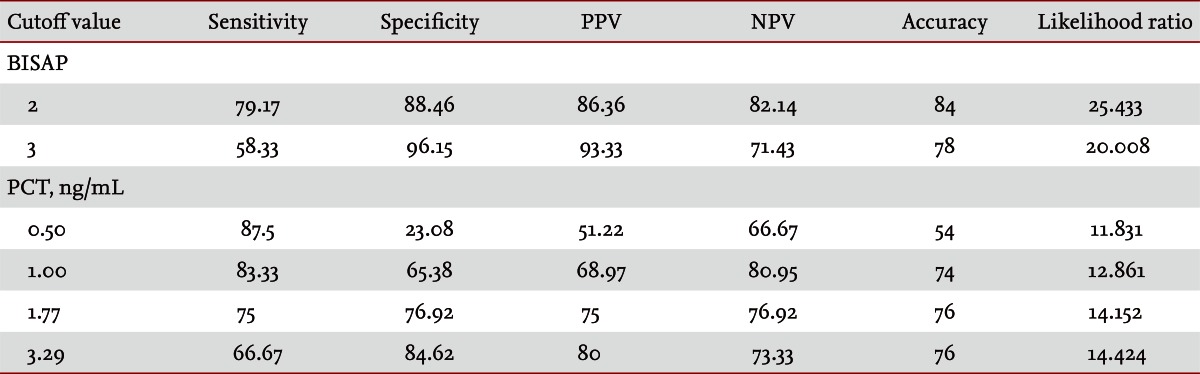
Values are presented as percentage.
BISAP, bedside index of severity in acute pancreatitis; PPV, positive predictive value; NPV, negative predictive value; PCT, procalcitonin.
Table 4.
Analysis of various parameters
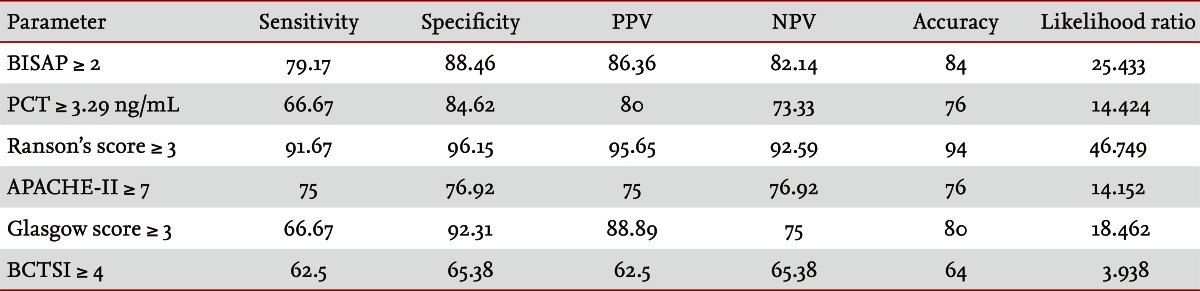
Values are presented as percentage.
PPV, positive predictive value; NPV, negative predictive value; BISAP, bedside index of severity in acute pancreatitis; PCT, procalcitonin; APACHE-II, acute physiology and chronic health examination-II; BCTSI, Balthazar computed tomography severity index.
Table 5.
Logistic regression analysis of risk factors for severe acute pancreatitis
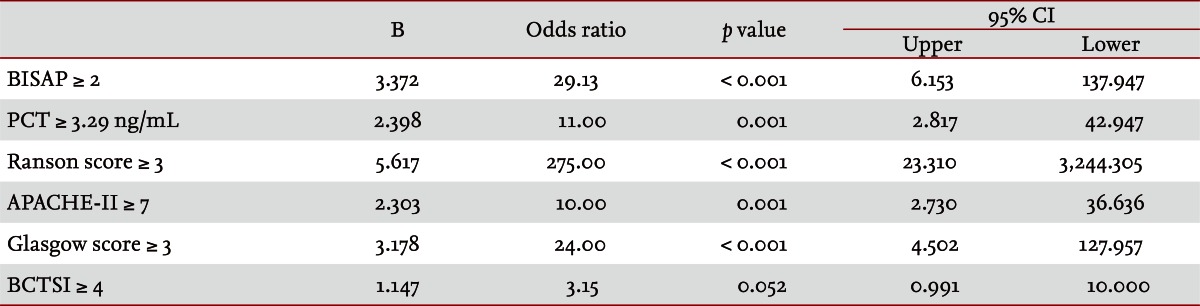
CI, confidence interval; BISAP, bedside index of severity in acute pancreatitis; PCT, procalcitonin; APACHE-II, acute physiology and chronic health examination-II; BCTSI, Balthazar computed tomography severity index.
Figure 2.
Correlation between serum procalcitonin (PCT) levels and duration of admission in patients with acute pancreatitis by simple linear regression and expressed as a graph. Serum PCT were correlated with length of hospital stay (p = 0.014) but with ratively low R square (0.118).
DISCUSSION
AP is a common disorder that places a substantial burden on the healthcare system [24]. The clinical course of AP is usually mild and it often resolves without sequelae. Nonetheless, 10% to 20% of patients experience a severe AP attack, resulting in an intense inflammatory response, a variety of local and systemic complications, a prolonged hospital course, and significant morbidity and mortality [25]. The scoring of patients with AP is important for several reasons. First, the clinician can be alerted to the presence of potentially severe disease. Second, severity can be compared both within and between patient series. Third, a rational selection of patients can be made for inclusion in trials of potential new treatments or interventions. Unfortunately, the scoring systems used at present are often inadequate in patients with severe AP, which is characterized by rapidly progressive multiple system organ dysfunction [26-28]. Therefore, a simple scoring system or single biomarker that predicts the severity of AP is needed. Serum C-reactive protein (CRP) was reported (CRP ≥ 15 mg/dL on admission) to predict severe AP with an overall accuracy of 69% [29]. Consequently, many studies have described its clinical limitation; i.e., a potential failure to detect severe cases of AP in the early stage [30-33]. Serum PCT was introduced as prognostic marker of AP, focusing on systemic inflammation and organ failure in the early stage of AP [34]. In 2008, Gurda-Duda et al. [12] stated that the early prediction of severe AP was achievable by measuring PCT, and many subsequent studies reported the efficacy of PCT as a predictor of AP, although there is some debate [14,15,30,35]. Our study revealed that the serum PCT was a significant predictor of severe AP (p = 0.018) and was consistent with our previous report [16]. In 2008, Wu et al. [17] introduced the BISAP and their results were relatively satisfactory [18,21], although more data were needed to determine its clinical applicability. In this study, we compared the BISAP with the serum PCT as a predictor of AP, and included other multifactorial scoring systems that predict the severity of AP as control parameters. In our series, Ranson's scores had the greatest accuracy for predicting the severity of AP (94%), followed by the BISAP (84%); the AUC of the BISAP was 0.873, which was superior to the results in Wu et al. [17] (AUC = 0.82) and Papachristou et al. [21] (AUC = 0.81). We found that the best cutoff score of BISAP was 2, while most other studies have proposed 3 to be the best cutoff score [17,18,21]. In our analysis, a BISAP score of 2 had greater accuracy, with a better likelihood ratio than a score of 3 (84% vs. 78% and 25.433 vs. 20.008, respectively). With a BISAP score of 2 in severe AP had 80% of SIRS, which was not observed with the same score of 2 in mild AP, which suggests that SIRS is an independent risk factor in the BISAP scoring system. In our study, the accuracy of serum PCT was 76% and the AUC was 0.788, which is similar to other reports [14,15,30,34-36]. In the analysis of four cutoff values of the serum PCT, 1.77 and 3.29 ng/mL had the same accuracy (76%), but 3.29 ng/mL had a slightly higher likelihood ratio (14.152 vs. 14.424), making 3.29 ng/mL the best cutoff level. Comparing BISAP with the serum PCT, the former had better sensitivity, specificity, accuracy, likelihood ratio, odds ratio (29.13 vs. 11.00) and p value (< 0.001 vs. 0.001) in the logistic regression analysis. Regarding the duration of hospitalization, the BISAP was not significantly related (p = 0.073), while the serum PCT had a significant p value (0.014), although r2 was low (0.118). Nevertheless, the BISAP was superior to serum PCT in terms of predicting the severity of AP. We did not measure serial PCT levels because this study investigated the potential role of the PCT level in the early prediction (on the day of admission) of disease severity in patients with AP and compared it with the BISAP within 24 hours. However, Kylanpaa-Back et al. [14] demonstrated the influence of a delayed peak in PCT on predicting the severity of attacks, reporting greater sensitivity (92%) with the PCT strip test 24 hours after admission to hospital. Our study enrolled 50 consecutive patients with AP over a 14-month period: using the Atlanta classification, 52% (26/50) had mild pancreatitis and the other 48% (24/50) had severe AP. Most recent studies included only a few cases, which were usually classified as severe necrotizing pancreatitis. In contrast, our series contained only a few patients with severe necrotizing pancreatitis and the diagnosis of necrotizing pancreatitis was made using imaging tools and laboratory findings, not histologically. Therefore, we think that larger populations are needed to obtain more precise results.
In conclusion, the serum PCT is useful for predicting the severity of AP and has an accuracy similar to that of the APACHE-II score. The BISAP is relatively simple and had greater accuracy than other multifactorial scoring systems, except Ranson's score, making it a promising method of predicting the clinical severity of AP.
KEY MESSAGE
1. Bedside index of severity in acute pancreatitis (BISAP) scoring is simple and convenient method for predicting the severity of acute pancreatitis.
2. The accuracy of BISAP (≥ 2) at predicting severe acute pancreatitis was superior to the serum procalcitonin (≥ 3.29 ng/mL, 76%) which was similar to the acute physiology and chronic health examination (APACHE)-II score.
Footnotes
No potential conflict of interest relevant to this article is reported.
References
- 1.Triester SL, Kowdley KV. Prognostic factors in acute pancreatitis. J Clin Gastroenterol. 2002;34:167–176. doi: 10.1097/00004836-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Lee SK. Medical treatments of necrotizing pancreatitis. Korean J Med. 2007;73:237–242. [Google Scholar]
- 3.Kusske AM, Rongione AJ, Reber HA. Cytokines and acute pancreatitis. Gastroenterology. 1996;110:639–642. doi: 10.1053/gast.1996.v110.agast960639. [DOI] [PubMed] [Google Scholar]
- 4.Larvin M. Circulating mediators in acute pancreatitis as predictors of severity. Scand J Gastroenterol Suppl. 1996;219:16–19. doi: 10.3109/00365529609104993. [DOI] [PubMed] [Google Scholar]
- 5.Windsor AC, Kanwar S, Li AG, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42:431–435. doi: 10.1136/gut.42.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranson JH, Pasternack BS. Statistical methods for quantifying the severity of clinical acute pancreatitis. J Surg Res. 1977;22:79–91. doi: 10.1016/0022-4804(77)90045-2. [DOI] [PubMed] [Google Scholar]
- 7.Yeung YP, Lam BY, Yip AW. APACHE system is better than Ranson system in the prediction of severity of acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2006;5:294–299. [PubMed] [Google Scholar]
- 8.Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet. 1989;2:201–205. doi: 10.1016/s0140-6736(89)90381-4. [DOI] [PubMed] [Google Scholar]
- 9.Wilson C, Heath DI, Imrie CW. Prediction of outcome in acute pancreatitis: a comparative study of APACHE II, clinical assessment and multiple factor scoring systems. Br J Surg. 1990;77:1260–1264. doi: 10.1002/bjs.1800771120. [DOI] [PubMed] [Google Scholar]
- 10.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 11.Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331–336. doi: 10.1148/radiology.174.2.2296641. [DOI] [PubMed] [Google Scholar]
- 12.Gurda-Duda A, Kusnierz-Cabala B, Nowak W, Naskalski JW, Kulig J. Assessment of the prognostic value of certain acute-phase proteins and procalcitonin in the prognosis of acute pancreatitis. Pancreas. 2008;37:449–453. doi: 10.1097/MPA.0b013e3181706d67. [DOI] [PubMed] [Google Scholar]
- 13.Modrau IS, Floyd AK, Thorlacius-Ussing O. The clinical value of procalcitonin in early assessment of acute pancreatitis. Am J Gastroenterol. 2005;100:1593–1597. doi: 10.1111/j.1572-0241.2005.41456.x. [DOI] [PubMed] [Google Scholar]
- 14.Kylanpaa-Back ML, Takala A, Kemppainen E, Puolakkainen P, Haapiainen R, Repo H. Procalcitonin strip test in the early detection of severe acute pancreatitis. Br J Surg. 2001;88:222–227. doi: 10.1046/j.1365-2168.2001.01673.x. [DOI] [PubMed] [Google Scholar]
- 15.Ammori BJ, Becker KL, Kite P, et al. Calcitonin precursors in the prediction of severity of acute pancreatitis on the day of admission. Br J Surg. 2003;90:197–204. doi: 10.1002/bjs.4036. [DOI] [PubMed] [Google Scholar]
- 16.Woo SM, Noh MH, Kim BG, et al. Comparison of serum procalcitonin with Ranson, APACHE-II, Glasgow and Balthazar CT severity index scores in predicting severity of acute pancreatitis. Korean J Gastroenterol. 2011;58:31–37. doi: 10.4166/kjg.2011.58.1.31. [DOI] [PubMed] [Google Scholar]
- 17.Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57:1698–1703. doi: 10.1136/gut.2008.152702. [DOI] [PubMed] [Google Scholar]
- 18.Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol. 2009;104:966–971. doi: 10.1038/ajg.2009.28. [DOI] [PubMed] [Google Scholar]
- 19.Buter A, Imrie CW, Carter CR, Evans S, McKay CJ. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg. 2002;89:298–302. doi: 10.1046/j.0007-1323.2001.02025.x. [DOI] [PubMed] [Google Scholar]
- 20.Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738–744. doi: 10.1002/bjs.5290. [DOI] [PubMed] [Google Scholar]
- 21.Papachristou GI, Muddana V, Yadav D, et al. Comparison of BISAP, Ranson's, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105:435–441. doi: 10.1038/ajg.2009.622. [DOI] [PubMed] [Google Scholar]
- 22.Toouli J, Brooke-Smith M, Bassi C, et al. Guidelines for the management of acute pancreatitis. J Gastroenterol Hepatol. 2002;17(Suppl):S15–S39. doi: 10.1046/j.1440-1746.17.s1.2.x. [DOI] [PubMed] [Google Scholar]
- 23.Bradley EL., 3rd A clinically based classification system for acute pancreatitis: summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 24.Villatoro E, Mulla M, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2010;(5):CD002941. doi: 10.1002/14651858.CD002941.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Servin-Torres E, Velazquez-Garcia JA, Delgadillo-Teyer G, Galindo-Mendoza L, Bevia-Perez F, Rivera-Bennet F. Severe acute pancreatitis: surgical management in a third-level hospital. Cir Cir. 2009;77:407–410. [PubMed] [Google Scholar]
- 26.Dervenis C, Bassi C. Evidence-based assessment of severity and management of acute pancreatitis. Br J Surg. 2000;87:257–258. doi: 10.1046/j.1365-2168.2000.01348.x. [DOI] [PubMed] [Google Scholar]
- 27.Lempinen M, Puolakkainen P, Kemppainen E. Clinical value of severity markers in acute pancreatitis. Scand J Surg. 2005;94:118–123. doi: 10.1177/145749690509400207. [DOI] [PubMed] [Google Scholar]
- 28.Wyncoll DL. The management of severe acute necrotising pancreatitis: an evidence-based review of the literature. Intensive Care Med. 1999;25:146–156. doi: 10.1007/s001340050808. [DOI] [PubMed] [Google Scholar]
- 29.Neoptolemos JP, Kemppainen EA, Mayer JM, et al. Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study. Lancet. 2000;355:1955–1960. doi: 10.1016/s0140-6736(00)02327-8. [DOI] [PubMed] [Google Scholar]
- 30.Muller CA, Uhl W, Printzen G, et al. Role of procalcitonin and granulocyte colony stimulating factor in the early prediction of infected necrosis in severe acute pancreatitis. Gut. 2000;46:233–238. doi: 10.1136/gut.46.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puolakkainen P, Valtonen V, Paananen A, Schroder T. C-reactive protein (CRP) and serum phospholipase A2 in the assessment of the severity of acute pancreatitis. Gut. 1987;28:764–771. doi: 10.1136/gut.28.6.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson C, Heads A, Shenkin A, Imrie CW. C-reactive protein, antiproteases and complement factors as objective markers of severity in acute pancreatitis. Br J Surg. 1989;76:177–181. doi: 10.1002/bjs.1800760224. [DOI] [PubMed] [Google Scholar]
- 33.Chen CC, Wang SS, Lee FY, Chang FY, Lee SD. Proinflammatory cytokines in early assessment of the prognosis of acute pancreatitis. Am J Gastroenterol. 1999;94:213–218. doi: 10.1111/j.1572-0241.1999.00709.x. [DOI] [PubMed] [Google Scholar]
- 34.Kylanpaa-Back ML, Takala A, Kemppainen EA, et al. Procalcitonin, soluble interleukin-2 receptor, and soluble E-selectin in predicting the severity of acute pancreatitis. Crit Care Med. 2001;29:63–69. doi: 10.1097/00003246-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Ammori BJ, Becker KL, Kite P, et al. Calcitonin precursors: early markers of gut barrier dysfunction in patients with acute pancreatitis. Pancreas. 2003;27:239–243. doi: 10.1097/00006676-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Mofidi R, Suttie SA, Patil PV, Ogston S, Parks RW. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review. Surgery. 2009;146:72–81. doi: 10.1016/j.surg.2009.02.013. [DOI] [PubMed] [Google Scholar]