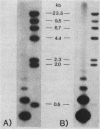
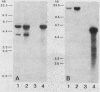
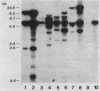
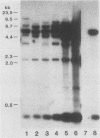
Abstract
Repetitive DNA sequences have been implicated in the mediation of DNA rearrangement in mammalian cells. We have tested this hypothesis by using a dihydrofolate reductase (DHFR) expression vector into which candidate sequences were inserted. DHFR- Chinese hamster ovary (CHO) cells were transfected with this vector, the amplification of which was then selected for by methotrexate (MTX) exposure. Cells transfected with the vector alone (and resistant to 0.02 or 1.0 microM MTX) or with a poly(dG-dT) insert (and resistant to 0.05 or 1.0 microM MTX) showed little change in chromosome aberrations or sister chromatid exchange frequencies. In contrast, transfection of DHFR- CHO cells with a vector containing either of two distinct 0.34-kilobase human alphoid DNA segments (and selection to 0.05 to 10.0 microM MTX) showed an approximately 50% increase in chromosome number and marked changes in chromosome structure, including one or two dicentric or ring forms per cell. The sister chromatid exchange frequency also increased, to more than double the frequency of that in cells transfected without insert or those containing poly(dG-dT). In situ hybridization of one 0.34-kilobase insert in some cells suggested clustering of homologous sequences in structurally abnormal recipient CHO cell chromosomes. The approach described provides an introduction to a unique means for a coordinate molecular and cytological study of dynamic changes in chromosome structure.
Full text
PDF

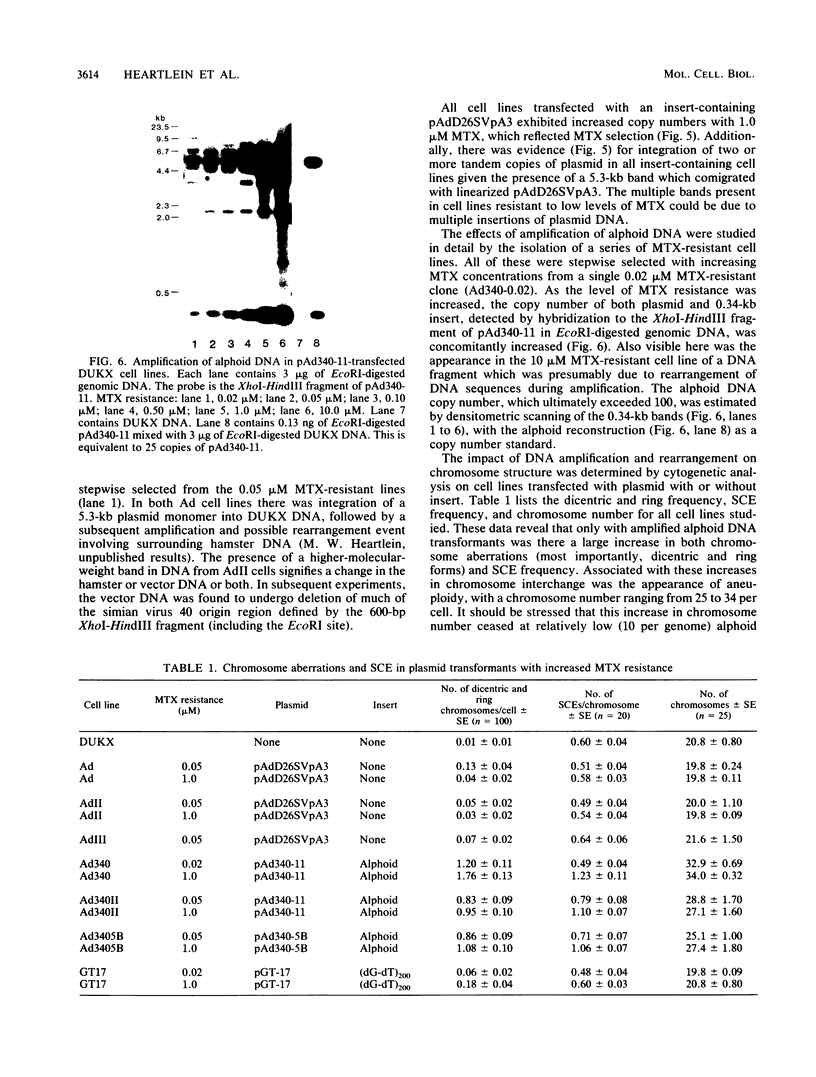
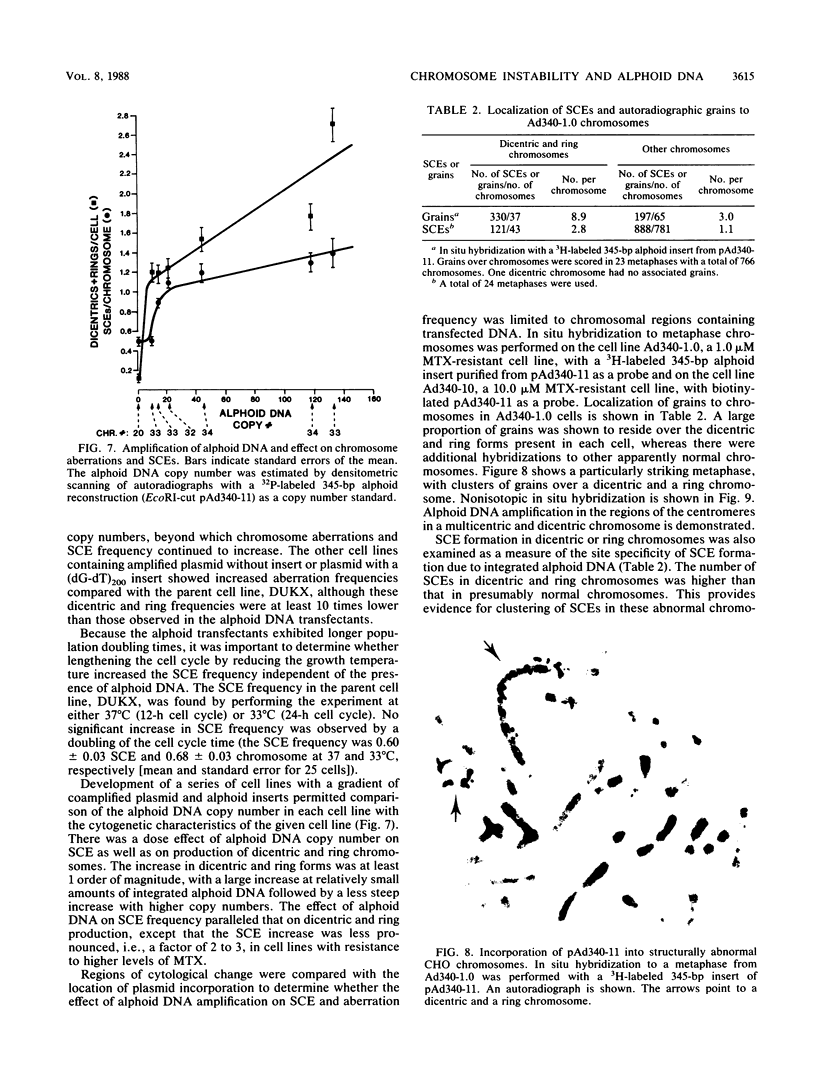



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge J., Kunkel L., Bruns G., Tantravahi U., Lalande M., Brewster T., Moreau E., Wilson M., Bromley W., Roderick T. A strategy to reveal high-frequency RFLPs along the human X chromosome. Am J Hum Genet. 1984 May;36(3):546–564. [PMC free article] [PubMed] [Google Scholar]
- Butner K. A., Lo C. W. High frequency DNA rearrangements associated with mouse centromeric satellite DNA. J Mol Biol. 1986 Feb 20;187(4):547–556. doi: 10.1016/0022-2836(86)90333-5. [DOI] [PubMed] [Google Scholar]
- Butner K., Lo C. W. Modulation of tk expression in mouse pericentromeric heterochromatin. Mol Cell Biol. 1986 Dec;6(12):4440–4449. doi: 10.1128/mcb.6.12.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling S. M., Crampton J. M., Williamson R. Organization of a family of highly repetitive sequences within the human genome. J Mol Biol. 1982 Jan 5;154(1):51–63. doi: 10.1016/0022-2836(82)90416-8. [DOI] [PubMed] [Google Scholar]
- Disteche C. M., Gandy S. L., Adler D. A. Translocation and amplification of an X-chromosome DNA repeat in inbred strains of mice. Nucleic Acids Res. 1987 Jun 11;15(11):4393–4401. doi: 10.1093/nar/15.11.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlon T. A., Litt M., Newcom S. R., Magenis R. E. Localization of the restriction fragment length polymorphism D14S1 (pAW-101) to chromosome 14q32.1 leads to 32.2 by in situ hybridization. Am J Hum Genet. 1983 Nov;35(6):1097–1106. [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hamada H., Kakunaga T. Potential Z-DNA forming sequences are highly dispersed in the human genome. Nature. 1982 Jul 22;298(5872):396–398. doi: 10.1038/298396a0. [DOI] [PubMed] [Google Scholar]
- Hamada H., Seidman M., Howard B. H., Gorman C. M. Enhanced gene expression by the poly(dT-dG).poly(dC-dA) sequence. Mol Cell Biol. 1984 Dec;4(12):2622–2630. doi: 10.1128/mcb.4.12.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Ullrich A., Saunders G. F. Localization of the human insulin gene to the distal end of the short arm of chromosome 11. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4458–4460. doi: 10.1073/pnas.78.7.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heartlein M. W., Tsuji H., Latt S. A. 5-Bromodeoxyuridine-dependent increase in sister chromatid exchange formation in Bloom's syndrome is associated with reduction in topoisomerase II activity. Exp Cell Res. 1987 Mar;169(1):245–254. doi: 10.1016/0014-4827(87)90242-4. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., van de Sande J. H., Zarling D. A., Arndt-Jovin D. J., Eckstein F., Füldner H. H., Greider C., Grieger I., Hamori E., Kalisch B. Generation of left-handed Z-DNA in solution and visualization in polytene chromosomes by immunofluorescence. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):143–154. doi: 10.1101/sqb.1983.047.01.019. [DOI] [PubMed] [Google Scholar]
- Jørgensen A. L., Jones C., Bostock C. J., Bak A. L. Different subfamilies of alphoid repetitive DNA are present on the human and chimpanzee homologous chromosomes 21 and 22. EMBO J. 1987 Jun;6(6):1691–1696. doi: 10.1002/j.1460-2075.1987.tb02419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A. Amplification and expression of sequences cotransfected with a modular dihydrofolate reductase complementary dna gene. J Mol Biol. 1982 Aug 25;159(4):601–621. doi: 10.1016/0022-2836(82)90103-6. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A., Latt S. A. Evolution of chromosomal regions containing transfected and amplified dihydrofolate reductase sequences. Mol Cell Biol. 1983 Apr;3(4):699–711. doi: 10.1128/mcb.3.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S. A. Sister chromatid exchange formation. Annu Rev Genet. 1981;15:11–55. doi: 10.1146/annurev.ge.15.120181.000303. [DOI] [PubMed] [Google Scholar]
- Müller U., Lalande M., Donlon T., Latt S. A. Moderately repeated DNA sequences specific for the short arm of the human Y chromosome are present in XX males and reduced in copy number in an XY female. Nucleic Acids Res. 1986 Feb 11;14(3):1325–1340. doi: 10.1093/nar/14.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Rich A. The sequence (dC-dA)n X (dG-dT)n forms left-handed Z-DNA in negatively supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1821–1825. doi: 10.1073/pnas.80.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue M. L., Lowenhaupt K., Rich A., Nordheim A. (dC-dA)n.(dG-dT)n sequences have evolutionarily conserved chromosomal locations in Drosophila with implications for roles in chromosome structure and function. EMBO J. 1987 Jun;6(6):1781–1789. doi: 10.1002/j.1460-2075.1987.tb02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry P., Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974 Sep 13;251(5471):156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Shyman S., Weaver S. Chromosomal rearrangements associated with LINE elements in the mouse genome. Nucleic Acids Res. 1985 Jul 25;13(14):5085–5093. doi: 10.1093/nar/13.14.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. F. Highly repeated sequences in mammalian genomes. Int Rev Cytol. 1982;76:67–112. doi: 10.1016/s0074-7696(08)61789-1. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stringer J. R. Recombination between poly[d(GT).d(CA)] sequences in simian virus 40-infected cultured cells. Mol Cell Biol. 1985 Jun;5(6):1247–1259. doi: 10.1128/mcb.5.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissel B., Choo K. H. Human alpha satellite DNA--consensus sequence and conserved regions. Nucleic Acids Res. 1987 Aug 25;15(16):6751–6752. doi: 10.1093/nar/15.16.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waye J. S., Durfy S. J., Pinkel D., Kenwrick S., Patterson M., Davies K. E., Willard H. F. Chromosome-specific alpha satellite DNA from human chromosome 1: hierarchical structure and genomic organization of a polymorphic domain spanning several hundred kilobase pairs of centromeric DNA. Genomics. 1987 Sep;1(1):43–51. doi: 10.1016/0888-7543(87)90103-0. [DOI] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Molecular analysis of a deletion polymorphism in alpha satellite of human chromosome 17: evidence for homologous unequal crossing-over and subsequent fixation. Nucleic Acids Res. 1986 Sep 11;14(17):6915–6927. doi: 10.1093/nar/14.17.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Structure, organization, and sequence of alpha satellite DNA from human chromosome 17: evidence for evolution by unequal crossing-over and an ancestral pentamer repeat shared with the human X chromosome. Mol Cell Biol. 1986 Sep;6(9):3156–3165. doi: 10.1128/mcb.6.9.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. C., Manuelidis L. Sequence definition and organization of a human repeated DNA. J Mol Biol. 1980 Sep 25;142(3):363–386. doi: 10.1016/0022-2836(80)90277-6. [DOI] [PubMed] [Google Scholar]