Abstract
Heart rates during ischaemia and reperfusion are possible determinants of reperfusion arrhythmias. We used ivabradine, a selective If current inhibitor, to assess the effects of heart rate reduction (HRR) during ischaemia–reperfusion on reperfusion ventricular arrhythmias and assessed potential anti-arrhythmic mechanisms by optical mapping. Five groups of rat hearts were subjected to regional ischaemia by left anterior descending artery occlusion for 8 min followed by 10 min of reperfusion: (1) Control n = 10; (2) 1 μM of ivabradine perfusion n = 10; (3) 1 μM of ivabradine + 5 Hz atrial pacing throughout ischaemia–reperfusion n = 5; (4) 1 μM of ivabradine + 5 Hz pacing only at reperfusion; (5) 100 μM of ivabradine was used as a 1 ml bolus upon reperfusion. For optical mapping, 10 hearts (ivabradine n = 5; 5 Hz pacing n = 5) were subjected to global ischaemia whilst transmembrane voltage transients were recorded. Epicardial activation was mapped, and the rate of development of ischaemia-induced electrophysiological changes was assessed. HRR observed in the ivabradine group during both ischaemia (195 ± 11 bpm vs. control 272 ± 14 bpm, p < 0.05) and at reperfusion (168 ± 13 bpm vs. 276 ± 14 bpm, p < 0.05) was associated with reduced reperfusion ventricular fibrillation (VF) incidence (20% vs. 90%, p < 0.05). Pacing throughout ischaemia–reperfusion abolished the protective effects of ivabradine (100% VF), whereas pacing at reperfusion only partially attenuated this effect (40% VF). Ivabradine, given as a bolus at reperfusion, did not significantly affect VF incidence (80% VF). Optical mapping experiments showed a delay to ischaemia-induced conduction slowing (time to 50% conduction slowing: 10.2 ± 1.3 min vs. 5.1 ± 0.7 min, p < 0.05) and to loss of electrical excitability in ivabradine-perfused hearts (27.7 ± 4.3 min vs. 14.5 ± 0.6 min, p < 0.05). Ivabradine administered throughout ischaemia and reperfusion reduced reperfusion VF incidence through HRR. Heart rate during ischaemia is a major determinant of reperfusion arrhythmias. Heart rate at reperfusion alone was not a determinant of reperfusion VF, as neither a bolus of ivabradine nor pacing immediately prior to reperfusion significantly altered reperfusion VF incidence. This anti-arrhythmic effect of heart rate reduction during ischaemia may reflect slower development of ischaemia-induced electrophysiological changes.
Keywords: Ischaemia–reperfusion, Reperfusion arrhythmias, Ventricular arrhythmias, Ivabradine, Heart rate reduction
Highlights
► Ivabradine treatment reduced the incidence of reperfusion ventricular arrhythmia. ► The anti-arrhythmic effects of ivabradine were due to heart rate reduction. ► Mean heart rate during ischaemia is a determinant of reperfusion arrhythmias. ► Heart rate reduction delayed onset of ischaemia-induced electrophysiological changes. ► Selective heart rate lowering is a potential anti-arrhythmic therapeutic strategy.
1. Introduction
Ventricular arrhythmias, can occur within seconds of restoration of blood flow to previously ischaemic myocardium, as initially shown by Tennant and Wiggers just under a century ago [1]. Reperfusion after brief periods of ischaemia, lasting seconds to minutes, occurs in the context of coronary artery vasospasm, or unstable angina, and is associated with reperfusion ventricular arrhythmias [2]. Reperfusion after longer periods of ischaemia lasting several hours occurs during percutaneous coronary intervention for patients with acute myocardial infarction, which is also associated with reperfusion arrhythmias [3].
The arrhythmogenic mechanisms that underlie reperfusion ventricular tachycardia (VT) and ventricular fibrillation (VF) are thought to relate to the abrupt electrophysiological and biochemical changes brought about by the restoration of blood flow [4]. The reperfusion of ischaemic myocardium triggers the rapid and heterogeneous restoration of action potentials towards that of pre-ischaemic levels [5], and the marked spatial heterogeneity in action potentials immediately after reperfusion can predispose to re-entry [6]. Reperfusion can also lead to intracellular calcium overloading [7], which then increases the electrogenic forward mode activity of the sarcolemmal sodium–calcium exchanger, and can cause Phase 4 delayed after depolarisations and triggered action potentials.
It has been shown in experimental studies that heart rate during acute ischaemia–reperfusion is an important determinant of susceptibility to reperfusion arrhythmias [8–10], with higher heart rates predisposing to arrhythmias. These data suggest that a clinical therapeutic strategy of heart rate reduction during ischaemia–reperfusion may reduce the incidence of reperfusion arrhythmias. However, it remains unclear if the heart rate during ischaemia or during reperfusion is more important in determining reperfusion arrhythmia susceptibility, and conflicting experimental evidence exists in support of either hypothesis [8,11].
Ivabradine (IVA) is a selective If current blocker with heart rate lowering effects [12], clinically licensed for chronic stable angina and chronic heart failure. Ivabradine treatment has also been shown to reduce the incidence of cardiovascular death and hospital admissions in a heart failure population in the SHIFT study [13], and it reduced the number of coronary events in a subgroup of chronic stable angina patients with heart rates of 70 beats per minute or greater in the BEAUTIFUL study [14,15]. Based on preclinical data showing that reduced heart rates beneficially impacted on reperfusion arrhythmia risk [8–11], we hypothesised that ivabradine treatment may provide anti-arrhythmic protection against reperfusion arrhythmias.
We assessed if heart rate lowering with ivabradine can reduce the incidence of reperfusion arrhythmias in an ex vivo rat model of acute regional ischaemia–reperfusion. We also sought to clarify if the heart rate during ischaemia or during reperfusion was more important in determining reperfusion arrhythmia susceptibility, as this will influence the timing of ivabradine administration in any potential clinical therapeutic strategy. We performed studies using ivabradine and atrial pacing in varying combinations to address these questions. We then performed optical mapping of transmembrane voltage to investigate the mechanism by which heart rate may influence reperfusion arrhythmogenesis.
2. Materials and methods
2.1. Ethical approval
This work was performed in accordance with standards set out in the UK Animals (Scientific Procedures) Act 1986.
2.2. Acute ischaemia–reperfusion studies
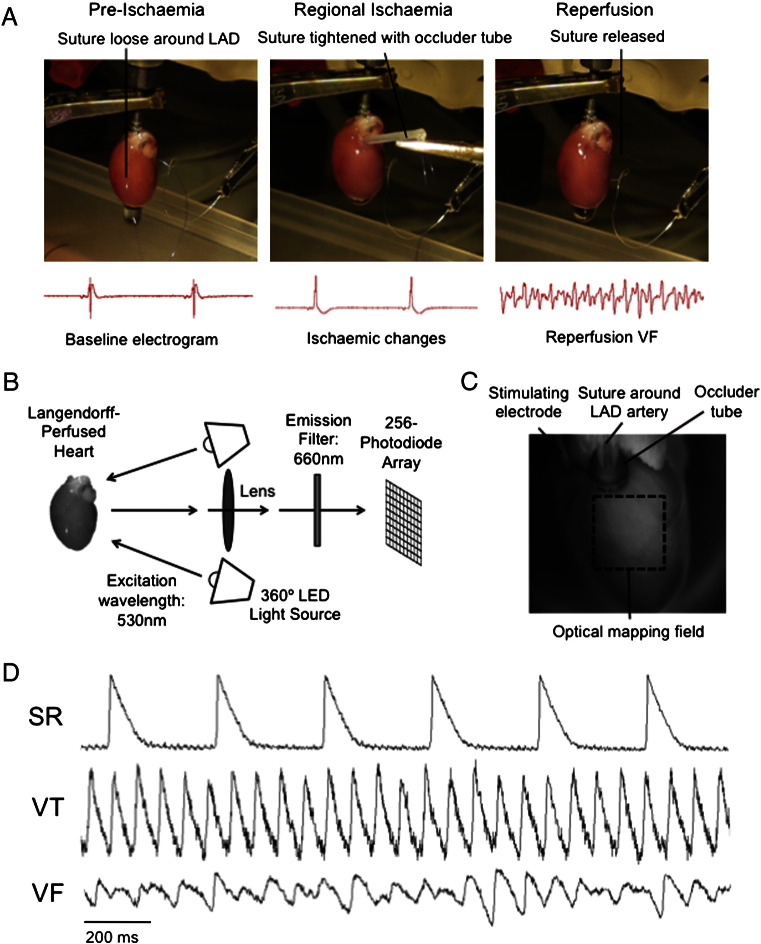
Thirty-five adult male Sprague–Dawley rats (weight 250–300 g) were sacrificed, and hearts were rapidly excised and retrogradely perfused via the aorta with modified Krebs–Henseleit solution (in mmol/l: NaCl 118.5, CaCl2 1.85, KCl 4.5, glucose 11.1, NaHCO3 25, MgSO4 2.5, NaH2PO4 1.4) gassed with 95% O2/5% CO2 at 37 °C ± 0.5 °C and pH 7.35 ± 0.05. Perfusion pressure through the aorta was constant and maintained between 90 and 100 cm H2O. Unipolar electrograms were recorded at a sampling frequency of 1 kHz using a silver electrode placed at the left ventricular anterolateral wall and a reference electrode attached to the aortic cannula. Electrodes were connected to a Bioamplifier and a PowerLab data acquisition system (AD Instruments, Sydney, Australia). During the stabilisation phase, a 6.0 Ethicon prolene ligature (Johnson & Johnson Ethicon, Livingston, UK) was placed around the left anterior descending (LAD) artery 1–2 mm distal from where it emerges beneath the left atrium, initially without tension (Fig. 1A).
Fig. 1.
Acute ischaemia–reperfusion and optical mapping experiments: (A) Regional ischaemia–reperfusion experiments. The left anterior descending (LAD) artery was temporarily occluded to generate ischaemia and then released for reperfusion. (B) Experimental set-up for optical mapping experiments. (C) Heart in optical mapping chamber, with the optical mapping field shown on the anterior surface of the left ventricle. (D) Representative ventricular epicardial optical recordings of transmembrane voltage (Vm) during sinus rhythm (SR), ventricular tachycardia (VT) and ventricular fibrillation (VF).
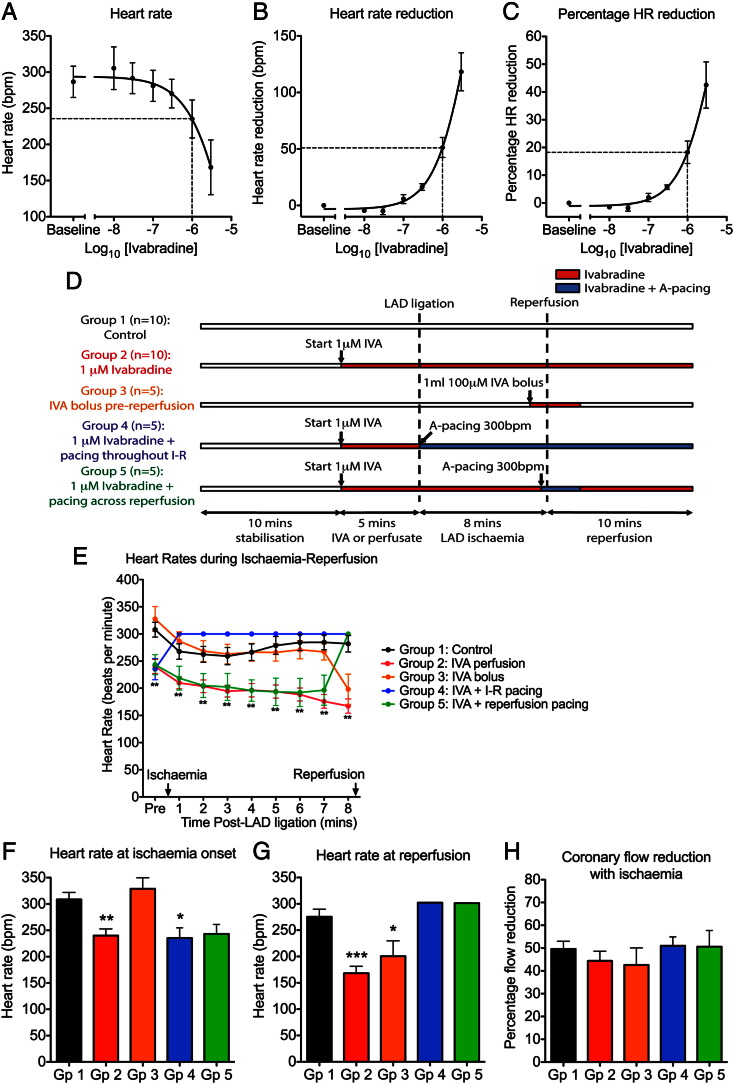
The hearts were stabilised for 15 min before being randomly allocated to one of five treatment groups (Fig. 2D). Atrial pacing and ivabradine (IVA) were used to control heart rates during the experiments. Right atrial pacing was used and ventricular pacing avoided as ventricular pacing per se has been shown to facilitate the development of reperfusion arrhythmias [16]. An ivabradine concentration of 1 μM was selected for the acute ischaemia–reperfusion experiments based on concentration–response studies showing an 18 ± 4% reduction in sinus heart rate with this concentration (see Figs. 2A–C and Data supplement), a similar magnitude to the clinical heart rate reduction (HRR) reported in the SHIFT trial (~ 16% HRR) [13]. Ivabradine was kindly provided by Servier Laboratories, France.
Group 1 — Control (n = 10): Hearts were subjected to 8 min of ischaemia followed by 10 min of reperfusion, without pacing or ivabradine.
Group 2 — 1 μM of ivabradine (IVA, n = 10): Hearts were perfused with 1 μM of ivabradine, starting 5 min before ischaemia onset and then throughout the experiment. This group had reduced heart rates at ischaemia onset, throughout the duration of ischaemia and at reperfusion.
Group 3 — Ivabradine bolus at reperfusion (n = 5): Hearts were perfused with a 1 ml bolus of 100 μM of ivabradine 1 min prior to reperfusion. This group had reduced heart rates at reperfusion only.
Group 4 — 1 μM of ivabradine + atrial pacing during ischaemia and reperfusion (n = 5): Hearts were perfused with 1 μM of ivabradine, starting 5 min before ischaemia onset and throughout the experiment, matching Group 2. In addition, hearts were paced from the right atrium at 300 beats per minute (bpm) from the onset of ischaemia until the end of the experiment.
Group 5 — 1 μM of ivabradine + atrial pacing across reperfusion (n = 5): Hearts were perfused with 1 μM of ivabradine, starting 5 min before ischaemia onset and throughout the experiment, matching Groups 2 and 4, and were paced at 300 bpm starting 30 s before reperfusion and for a further minute after onset of reperfusion.
Fig. 2.
Ivabradine reduced sinus heart rate, with reversal by atrial pacing: (A–C) Ivabradine concentration–response studies: (A) Sinus heart rate, (B) absolute heart rate reduction and (C) percentage heart rate (HR) reduction with increasing concentrations of ivabradine (n = 3). Ivabradine at a concentration of 1 μM reduced heart rate by 51 ± 9 bpm (18 ± 4%). (D) Study protocol for acute ischaemia–reperfusion experiments showing the five groups used to assess the effects of heart rate during ischaemia and reperfusion on reperfusion arrhythmia incidence. (E) Heart rate pre-ischaemia and during regional ischaemia were different for the five study groups. (F) Heart rate at onset of ischaemia. (G) Heart rate at reperfusion. (H) Percentage coronary flow reduction with regional ischaemia (*p < 0.05, **p < 0.01, ***p < 0.001 vs. control).
To generate regional ischaemia, the prolene ligature placed earlier around the LAD artery was tightened using a polythene occluder tube placed over the suture, thereby occluding flow through the artery (Fig. 1A). Successful occlusion of the artery was confirmed by a reduction in coronary flow rate (CFR) of > 30% as assessed by measuring the coronary effluent, in conjunction with an alteration in the axis and morphology of the unipolar electrogram. Hearts were subjected to 8 min of regional ischaemia, before the suture was released to restore flow across the LAD artery. We selected 8 min of ischaemia because the relationship between ischaemia duration and reperfusion VT/VF incidence is “bell-shaped” [8], with our data showing that 8 min of ischaemia is on the steep part of this bell-shaped relationship (see Data supplement). Reperfusion was confirmed by an increase in CFR towards that of pre-ischaemic values. The reperfusion phase was continued for 10 min with continuous electrogram monitoring for all 5 groups. The incidence of ventricular arrhythmias during the ischaemia and reperfusion were recorded. Ventricular arrhythmias were classified according to the Lambeth Convention guidelines [17]. The primary endpoints were reperfusion VT incidence and reperfusion VF incidence.
2.3. Optical mapping studies during global no-flow ischaemia
In order to assess the effects of heart rate on the rate of development of ischaemia-induced electrophysiological changes, optical mapping experiments (Fig. 1B) were performed in a global no-flow ischaemia model. Ten hearts were perfused at fixed coronary flow rates (15 ml/min) inside a perspex optical mapping chamber (Cairn Research, Faversham, UK) (Fig. 1C). Following a 15-minute stabilisation period, the hearts were stained with RH237, a potentiometric dye (25 μl of 1 mg/ml RH237 in dimethyl sulfoxide; Invitrogen, UK), and perfused with an excitation–contraction uncoupler, 10 μM of blebbistatin (Sigma-Aldrich, UK), to eliminate motion artefact [18].
To record optical action potentials, the hearts were excited with light-emitting diodes (excitation wavelength 530 nm), and the emitted fluorescent light was collected and split with a dichroic mirror at 630 nm. The shorter wavelength light portion was focused onto a charge-coupled device camera to obtain plain images, whilst the longer wavelength portion was passed through an emission filter (660 nm) and detected using a Hamamatsu 256 (16 × 16) photo-diode array detector (Cairn Research, Faversham, UK), at sampling rate of 1 kH with a spatial resolution of 1 mm. Signals were recorded during pacing from the left ventricular free wall (cycle length 200 ms, stimulus 1 mA).
The hearts were randomised to one of the following two groups, chosen to produce two extremes of heart rates during global no-flow ischaemia:
Group 1 — Ivabradine (IVA): Hearts were perfused with 1 μM of ivabradine, starting 5 min before the onset of global ischaemia, to reduce heart rate during global ischaemia (n = 5).
Group 2 — Pacing at 300 bpm: Hearts were paced at 300 beats per minute (bpm) from the onset of ischaemia through to the end of the experiment, to increase heart rate during global ischaemia (n = 5).
The hearts were then subjected to global no-flow ischaemia and transmembrane voltage (Vm) transients were recorded every 30 s during ventricular pacing to study the changes induced by ischaemia. The primary endpoint was the time to loss of electrical excitability with ventricular pacing. The secondary endpoints were the conduction velocity after 10 min of global ischaemia and time to 50% reduction in CV.
2.4. Optical mapping data analysis
Optical mapping data were acquired using QRecord software and analysed off-line using Optiq software (Cairn Research Ltd, Kent, UK). Activation maps were generated, and local conduction velocities were derived, using MATLAB R2010a software (MathWorks, Massachusetts, USA), using methods previously described [19].
2.5. Statistical analysis
Statistical analyses were performed using Prism 5.0 software (GraphPad Software, California, USA). The logrank test was used to compare freedom from reperfusion arrhythmias between groups. Analysis of variance (ANOVA) tests were performed to compare means between multiple groups and post-hoc Tukey's test was used if ANOVA was significant. Pearson's correlation was used to correlate mean heart rates with reperfusion arrhythmia incidence. A p-value of < 0.05 was considered statistically significant. All values shown are mean ± S.E.M., unless otherwise stated.
3. Results
3.1. Acute ischaemia–reperfusion studies
3.1.1. Ivabradine reduced sinus heart rate, with reversal by atrial pacing
Figs. 2E–G and Table 1 show the heart rates for these five study groups. Group 1 was the control group, and had heart rate governed by sinus rhythm. Group 2 was perfused with ivabradine and, compared with control, had reduced heart rates at ischaemia onset (p < 0.05), during ischaemia (p < 0.05) and at reperfusion (p < 0.05). Group 3 had boluses of ivabradine at reperfusion only, with significantly slower heart rates limited to the reperfusion phase compared to the control (p < 0.05). Group 4 had ivabradine perfusion with simultaneous atrial pacing throughout I–R, so compared with Group 2 (ivabradine only), had increased heart rates during ischaemia (p < 0.05) and at reperfusion (p < 0.05). Group 5 had ivabradine perfusion with pacing across reperfusion, and compared with Group 2, had increased heart rates restricted to the reperfusion phase (p < 0.05).
Table 1.
Differences in heart rates between the five study groups: Heart rate (HR) at ischaemia onset, mean heart rate during ischaemia and heart rate at reperfusion for the five groups (⁎p < 0.05, ⁎⁎p < 0.01, ⁎⁎⁎p < 0.001 vs. Group 1 — Control; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. Group 2 — Ivabradine).
| HR at ischaemia onset | Mean HR during ischaemia | HR at reperfusion | |
|---|---|---|---|
| Group 1 | 309 ± 13 | 273 ± 14 | 276 ± 14 |
| Group 2 | 240 ± 13⁎⁎ | 191 ± 11⁎⁎⁎ | 168 ± 13⁎⁎⁎ |
| Group 3 | 329 ± 21## | 261 ± 20# | 201 ± 29⁎ |
| Group 4 | 235 ± 20⁎ | 300 ± 0### | 302 ± 0### |
| Group 5 | 243 ± 18 | 213 ± 20 | 301 ± 0### |
3.1.2. Ivabradine reduced reperfusion VT and VF via selective heart rate reduction
These differences in heart rates during ischaemia and at reperfusion resulted in varying incidences of reperfusion arrhythmias across the groups, as shown in Fig. 3. The percentage reduction in coronary flow rate, which is a measure of the size of the ischaemic zone and a potential confounding variable, was not different between groups (Fig. 2H).
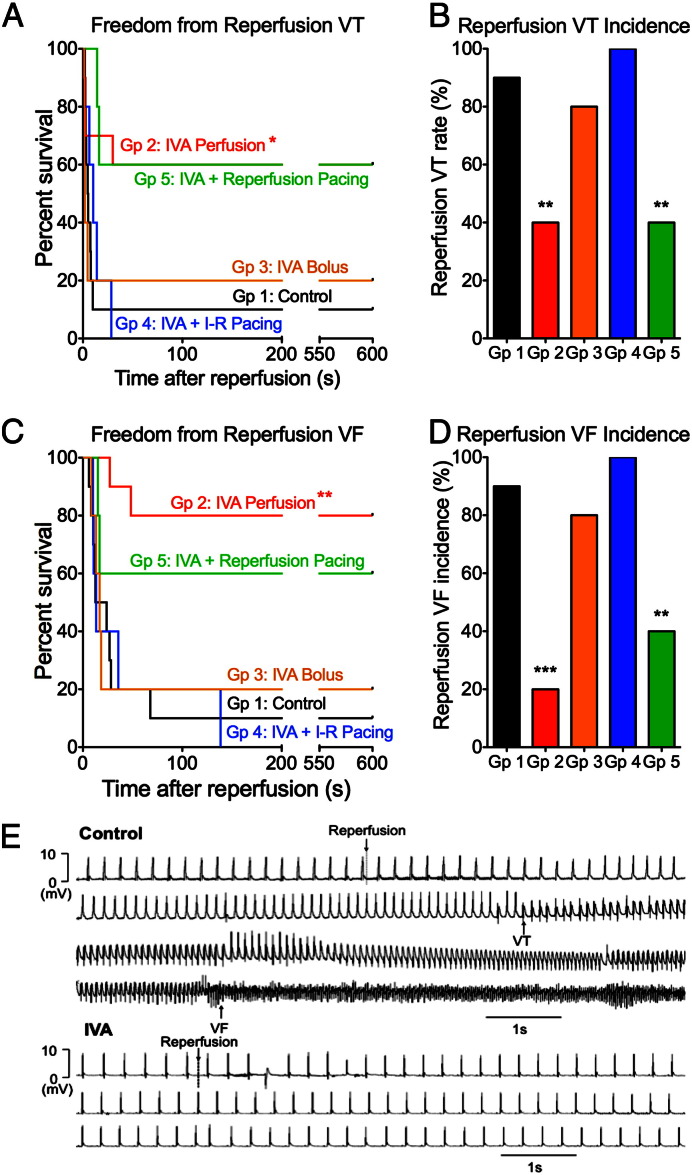
Fig. 3.
Ivabradine reduced the incidence of reperfusion VT and VF via heart rate reduction: (A to D) Freedom from reperfusion VT, reperfusion VT incidence, freedom from reperfusion VF and reperfusion VF incidence for the five groups: Ivabradine (Group 2) improved freedom from reperfusion VT/VF and reduced reperfusion VT/VF incidence compared to control (Group 1). Atrial pacing (Group 4) abolished ivabradine's protective effects. An ivabradine bolus at reperfusion (Group 3) did not significantly alter arrhythmia incidence compared to control. Atrial pacing only at reperfusion in addition to IVA (Group 4) did not significantly alter arrhythmia incidence compared to IVA perfusion (Group 2) (*p < 0.05, **p < 0.01, ***p < 0.001 vs. control) (Group 1: n = 10, Group 2: n = 10, Group 3: n = 5, Group 4: n = 5, Group 5: n = 5). (E) Top: Electrogram trace showing the development of VT and VF following reperfusion in a control heart. Bottom: Epicardial electrogram trace from an ivabradine-treated heart, showing slower heart rate before reperfusion, a single ventricular ectopic following reperfusion, and the absence of reperfusion VT/VF.
Ivabradine perfusion to lower heart rate throughout ischaemia and reperfusion (Group 2) reduced reperfusion VF incidence to 20% compared to 90% in control hearts (Group 1) (p < 0.05), and reduced reperfusion VT incidence from 90% to 40% (p < 0.05) (Figs. 3A–D). Fig. 3E shows an example of a control heart developing reperfusion VT and VF several seconds after reperfusion, and an ivabradine-treated heart with slower heart rate pre-reperfusion developing a single ventricular premature beat without VT or VF following reperfusion.
In order to investigate if the anti-arrhythmic effects of ivabradine (Group 2) were due to its heart rate reduction effects or due to another mechanism independent of heart rate, we compared the arrhythmia incidence with that of Group 4, where atrial pacing was delivered at 300 bpm in addition to ivabradine perfusion. In this group, atrial pacing with concomitant ivabradine administration abolished ivabradine's anti-arrhythmic effect (100% VT, 100% VF). These data suggest that the anti-arrhythmic effects of ivabradine in this context can be attributed solely to its heart rate lowering effect.
3.1.3. Heart rate during reperfusion was not a determinant of reperfusion arrhythmia susceptibility
Having established that ivabradine protected against reperfusion arrhythmias through heart rate reduction, we investigated if heart rate during reperfusion was important in determining reperfusion arrhythmia susceptibility using Groups 3 and 5. A bolus of ivabradine just before reperfusion to lower heart rate at reperfusion (Group 3) did not significantly reduce reperfusion VF incidence compared to controls (80% vs. 90%) (Figs. 3A–D). Similarly, ivabradine perfusion plus atrial pacing at reperfusion only to increase heart rate at reperfusion (Group 5) did not significantly increase reperfusion VF incidence compared to ivabradine alone (Group 2) (40% vs. 20%). The arrhythmia incidence in these groups suggests that heart rate at reperfusion was not the critical variable accounting for the anti-arrhythmic effect of ivabradine, as either increasing or reducing the heart rate at reperfusion failed to significantly alter reperfusion VT/VF incidence.
3.1.4. Mean heart rate during ischaemia is a determinant of reperfusion arrhythmia susceptibility
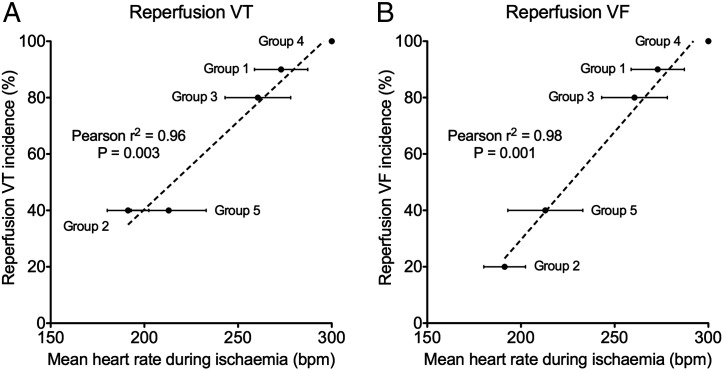
We then investigated if the mean heart rate during ischaemia is important in determining reperfusion arrhythmia susceptibility. We plotted mean heart rates over the course of 8 min of regional ischaemia for the five groups against reperfusion VT and VF incidence and found excellent linear correlations between mean heart rate during ischaemia and the subsequent reperfusion arrhythmia incidence for both VT and VF, as shown in Fig. 4 (reperfusion VT: Pearson's r2 = 0.96, p < 0.05; reperfusion VF: Pearson's r2 = 0.98, p < 0.05), suggesting that mean heart rate during ischaemia is an important determinant of reperfusion arrhythmogenesis.
Fig. 4.
Mean heart rate during ischaemia is the main determinant of reperfusion arrhythmias: (A) Reperfusion VT incidence and (B) reperfusion VF incidence plotted on the y-axes against the mean heart rates during ischaemia (standard errors bars shown) for the 5 experimental groups on the x-axes. There were excellent correlations between reperfusion VT and VF incidence with mean heart rate during ischaemia (Pearson's r2 = 0.96, p = 0.003 and Pearson's r2 = 0.98, p = 0.001 for reperfusion VT and VF respectively).
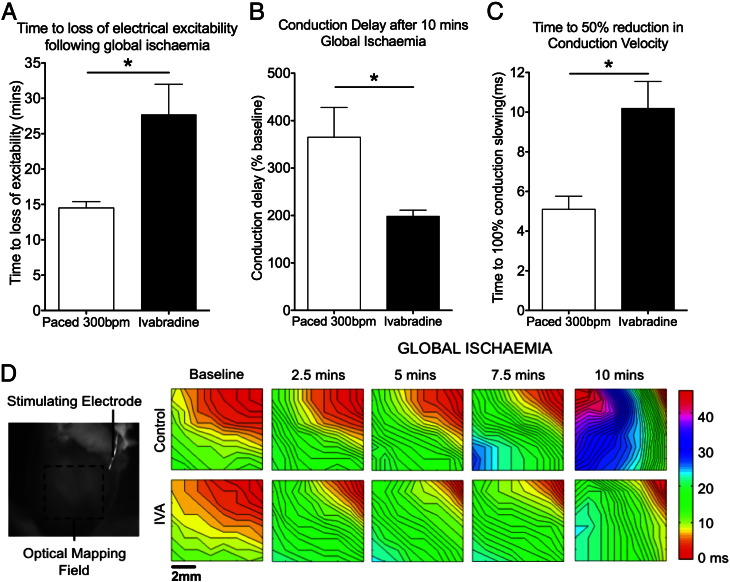
3.2. Heart rate reduction slowed the development of ischaemia-induced electrophysiological changes
Optical mapping studies were then performed to assess possible mechanisms by which a reduction in mean heart rate during ischaemia can protect against reperfusion arrhythmias.
The optical mapping study was designed to create two groups with different heart rates during global ischaemia. Heart rate was reduced in the ivabradine group (223 ± 12 bpm) compared to paced hearts (300 bpm) at onset of ischaemia (p < 0.05). Heart rate slowed progressively in the ivabradine group after onset of global ischaemia, until a cessation of spontaneous activity by 5 min of global ischaemia, whilst heart rate was maintained at 300 bpm throughout ischaemia in the paced group.
These global no-flow ischaemia experiments showed that heart rate was an important determinant of the rate of onset and development of ischaemia-induced electrophysiological changes, as shown in Fig. 5. Hearts in the ivabradine group, which had significantly slower heart rates, had delayed onset of loss of electrical excitability compared to paced hearts (p < 0.05; Fig. 5A). The development of conduction slowing during global ischaemia was also slower in the ivabradine group compared to the paced group. Following 10 min of global ischaemia, there was less conduction slowing in ivabradine-perfused hearts compared to paced hearts (p < 0.05; Fig. 5B). The time to a 50% reduction in conduction velocity was twice as long in the ivabradine group compared to the paced group (p < 0.05; Fig. 5C). The slower development of conduction slowing in the ivabradine group can be seen in the series of activation maps in Fig. 5D.
Fig. 5.
Ivabradine slowed the onset of acute ischaemia-induced electrophysiological changes: (A) There was a delay in time to loss of electrical excitability in the ivabradine group compared to the paced group. (n = 5 in each group, *p < 0.05). (B) The degree of conduction slowing was reduced in the ivabradine group after 10 min of global ischaemia. (C) There was a delay in the time to a 50% reduction in conduction velocity in the ivabradine group. (D) Isochronal activation maps during ventricular pacing showing progressive conduction slowing with global ischaemia. Conduction velocity slowing was faster in the paced group compared to the ivabradine group (red is early activation, blue-violet is late activation).
4. Discussion
In this study, our four main findings were: (1) ivabradine reduced the incidence of reperfusion VT and VF following regional ischaemia, (2) ivabradine's anti-arrhythmic effects were due to its selective heart rate lowering effects, (3) the mean heart rate during ischaemia was an important determinant of reperfusion arrhythmia susceptibility, and (4) slower heart rates during ischaemia delayed the development of ischaemia-induced electrophysiological changes.
4.1. Ivabradine reduced the incidence of reperfusion VT and VF
Our results showed that the administration of ivabradine significantly lowered the incidence of reperfusion VT and VF following a period of regional ischaemia, with a 50% absolute reduction in VT incidence and a 70% reduction in VF incidence. Our data support existing studies in the literature showing that reduced heart rates during acute ischaemia–reperfusion were associated with reductions in reperfusion arrhythmias [8–11]. The results from our experiments also fit with recent studies suggesting that ivabradine can protect against acute ischaemia-induced arrhythmias [20,21]. Vaillant et al. demonstrated that ivabradine reduced heart rate by 31% and increased VF thresholds by 2.9-fold in pigs subjected to coronary artery occlusion [20]. The protective effect of HRR against ischaemia-induced arrhythmias was also previously shown in studies using propranolol [22,23]. However the anti-arrhythmic effects of propranolol are more complex than HRR alone, and include reduced β1-Gs-cyclic adenosine monophosphate (cAMP)-mediated cytosolic calcium and reactive oxygen species overload, and β2-Gi stimulus trafficking [24]. We have shown that, in addition to protecting against ischaemia-induced arrhythmia, ivabradine can also reduce arrhythmias during the subsequent reperfusion phase. Taken together, these results point towards a potential role for ivabradine as an anti-arrhythmic agent in preventing arrhythmias during the different phases of acute ischaemia–reperfusion.
4.2. The anti-arrhythmic effects of ivabradine were due to heart rate reduction
In our studies, the anti-arrhythmic protection conferred by ivabradine treatment was completely abolished when atrial pacing was delivered to maintain heart rates similar to those in the control group. This was similarly observed by Vaillant et al., where the protective effects of ivabradine against ischaemia-induced arrhythmias were also mainly due to heart rate reduction [20]. In those experiments, there was only a small increase in VF thresholds if pacing was employed to maintain heart rate during acute ischaemia. However, Heusch et al. demonstrated that ivabradine's protection during acute ischaemia–reperfusion can go beyond heart rate reduction [25]. In a porcine model of ischaemia–reperfusion, they showed that ivabradine can significantly reduce infarct size, and that although the benefit of ivabradine on myocardial flow and function were eliminated by atrial pacing, part of the reduction in infarct size by ivabradine was unaffected by atrial pacing. Our data would suggest that although the reduction in infarct size by ivabradine may be due to multiple mechanisms not restricted to its effects on heart rate, its anti-arrhythmic effects are predominantly due to heart rate reduction.
4.3. Mean heart rate during ischaemia is a determinant of reperfusion arrhythmias
We showed that heart rate during ischaemia is more important in determining reperfusion arrhythmias susceptibility compared with heart rate at reperfusion. It was previously unclear if the heart rate during ischaemia or at reperfusion was more important and experimental studies had thus far produced conflicting results. Studies in isolated rat hearts showed that atrial pacing during acute regional ischaemia caused a rate-related increase in reperfusion arrhythmia incidence, suggesting that heart rate during ischaemia was more important [8]. This was supported by studies showing that diltiazem and metoprolol can protect against reperfusion arrhythmias due to its negative chronotropic effects during ischaemia [9,10]. On the contrary, Zuanetti et al. presented evidence suggesting that it may be the heart rate at reperfusion that is more important in determining reperfusion arrhythmia susceptibility [11]. They showed that vagal stimulation to reduce heart rate at reperfusion reduced reperfusion VF, whilst concomitant pacing to maintain heart rate during vagal stimulation significantly attenuated the observed anti-arrhythmic effect.
The discrepancies in these studies have been attributed to the differences in species, duration of ischaemia and in the magnitude of heart rate reduction. Another possible reason is that the interventions used to lower heart rate in these studies, β-adrenoreceptor blockers and vagal stimulation, have multiple other effects in addition to their negative chronotropic effects. Their broad actions make it difficult to discern if their anti-arrhythmic effects in these studies were due to the lowering of heart rate or to another effect. Using ivabradine, a selective heart rate lowering agent, we were able to dissect out the relative importance of heart rates during ischaemia and at reperfusion on reperfusion arrhythmia incidence, and we demonstrated that heart rate during ischaemia was the important determinant of reperfusion arrhythmias by showing an excellent correlation between mean heart rate during ischaemia with reperfusion arrhythmia incidence. This was again in slight divergence from the study by Heusch et al. [25], where they showed that ivabradine administration just before reperfusion can still reduce infarct size. This difference may be explained by the difference between the mechanism of protecting against reperfusion arrhythmias and that of reducing infarct size, and the timescale of both complications of reperfusion. Arrhythmias usually occur within the first few seconds of reperfusion, whereas reperfusion-induced cardiomyocyte injury, apoptosis and/or necrosis occur on a timescale of minutes to hours, allowing time for ivabradine to exert a protective effect not possible in the narrow time window of ventricular arrhythmia initiation. Our findings highlight the importance of the timing of administration of ivabradine if this therapeutic strategy is to be translated into clinical practice.
4.4. Heart rate reduction during ischaemia delayed the onset of ischaemia-induced electrophysiological changes
The concept that heart rate reduction is beneficial during ischaemia is often based on the hypothesis that lowering heart rate causes a reduction in myocardial energy consumption and can attenuate the severity of ischaemia [8]. Ceconi et al. demonstrated that heart rate reduction with ivabradine significantly reduced cardiac energy consumption, preserved redox potentials during ischaemia, and enhanced recovery at reperfusion [26].
We performed optical mapping experiments to investigate the potential mechanisms through which heart rate reduction during ischaemia with ivabradine protected against reperfusion arrhythmias. Having established the anti-arrhythmic benefits of HRR in a model of regional ischaemia, we wanted to investigate how HRR affected the myocardial ischaemia-induced changes in general. A global ischaemia model was selected because the uniform ischaemia throughout the heart in a global ischaemia model facilitated the study of ischaemia-induced electrophysiogical changes such as conduction slowing, although it does not recapitulate the reperfusion–arrhythmias associated with regional ischaemia–reperfusion.
Our optical mapping experiments showed that increased heart rates were associated with accelerated onset of ischaemia-induced changes, including earlier loss of electrical excitability and greater degrees of conduction slowing. These results provide a possible mechanism for the observation that reduced heart rates during ischaemia were associated with reduced reperfusion arrhythmias. Acute ischaemia is known to cause progressive electrophysiological changes, including a reduction in action potential duration, a reduction in membrane excitability and the slowing of conduction [27,28]. Slowing the development of ischaemia-induced electrophysiological changes by lowering heart rate with ivabradine would be expected to lead to smaller magnitudes of changes in action potentials and conduction velocities with reperfusion, which would be expected to be anti-arrhythmic [4]. Although not directly studied in our optical mapping experiments, the delay in ischaemia-induced changes may lead to delayed generation of reactive-oxygen species and reduced calcium overload, which can then reduce the likelihood of triggered arrhythmias. The delayed generation of reactive oxygen species may also reduce the opening of both the inner mitochondrial anion channel (IMAC) and the mitochondrial permeability transition pore (mPTP), both key players in reperfusion injury [29,30], which may then produce an anti-arrhythmic effect.
4.5. Selective heart rate lowering with ivabradine as a therapeutic strategy to reduce reperfusion arrhythmias
The concentration of ivabradine we chose in our experiments produced similar mean heart rate reductions at baseline to that observed of clinical trials. Our findings suggest that ivabradine may be potentially useful clinically to reduce the incidence of reperfusion arrhythmias following regional ischaemia–reperfusion. We also showed that the timing of ivabradine administration is critically important as it would be necessary to administer the drug early enough during acute ischaemia to lower the mean heart rate over the ischaemic period to have an impact on reperfusion arrhythmia incidence. In the context of primary percutaneous coronary interventions (PCI) for myocardial infarction, a possible scenario would be to administer ivabradine as a bolus in the ambulance before arrival at the hospital to reduce heart rate before the primary PCI procedure.
In cases of unstable angina, patients may be treated with ivabradine upon admission to hospital to reduce heart rates in advance of potential acute ischaemic episodes, whereas patients with arrhythmias due to stable angina or coronary vasospasm may benefit from long-term ivabradine administration. Stable patients with coronary artery disease on ivabradine may also benefit from reduced arrhythmic risk during acute episodes of ischaemia–reperfusion as a result of chronically reduced heart rates, though it has to be noted that no significant reductions in sudden cardiac death (SCD) were reported in the BEAUTIFUL and SHIFT trials [13–15]. This may be because these trials were underpowered to detect differences in SCD rates, and also because SCD in heart failure is due to multiple causes, not restricted to ischaemia–reperfusion.
4.6. Study limitations
In our experiments, we selected the ischaemia duration of 8 min prior to reperfusion for reasons described above. This duration is brief compared to the durations of ischaemia seen in cases of myocardial infarction before primary PCI, and it remains to be fully determined if the strategy of heart rate reduction is anti-arrhythmic in settings of longer ischaemia durations. Our findings may be more directly applicable to unstable angina and intermittent stable angina or coronary vasospasm, where the durations of ischaemia are brief.
Our optical mapping studies were performed in the global ischaemia–reperfusion model, which therefore did not allow detailed evaluation of the mechanism(s) of reperfusion arrhythmias generated after regional ischaemia–reperfusion.
4.7. Conclusions
We demonstrated that reducing heart rate during acute ischaemia reduced the incidence of reperfusion arrhythmias, and we propose that the anti-arrhythmic effects of ivabradine may be mediated in part by slowing the development of ischaemia-induced electrophysiological changes. Ivabradine, which is licensed for chronic stable angina and chronic heart failure, may be useful in the clinical setting to prevent reperfusion arrhythmias if given early enough during the course of acute ischaemia-infarction before primary PCI, and may also be protective against reperfusion arrhythmias in unstable angina and coronary vasospasm.
Sources of funding
FSN was supported by a Medical Research Council Clinical Research Training Fellowship (G0900396). ARL is supported by a British Heart Foundation Intermediate Research Fellowship (FS/11/67/28954) and the National Institute for Health Research-funded Cardiovascular Biomedical Research Unit at the Royal Brompton Hospital. This research was also supported by a British Heart Foundation Programme grant (RG/10/11/28457) to NSP.
Disclosures
ARL has received educational grants, research support and honoraria from Servier Laboratories. No conflicts of interest, financial or otherwise, are declared by FSN, ITS and NSP.
Acknowledgments
The authors would like to thank Servier Laboratories (France) for their kind gift of the ivabradine.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.yjmcc.2013.02.001.
Appendix A. Supplementary data
Supplementary materials.
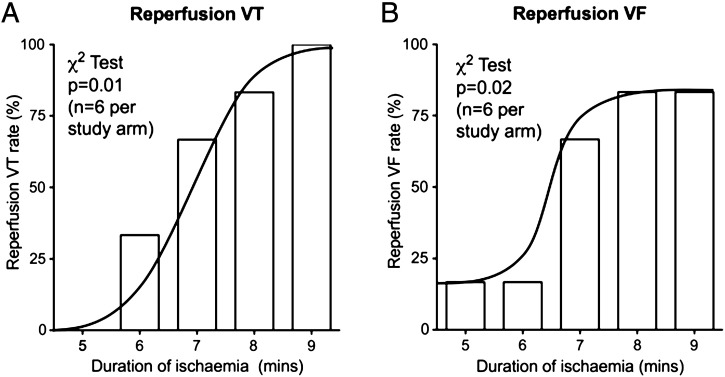
Fig. S1.
Incidence of reperfusion ventricular tachycardia (VT) and ventricular fibrillation (VF) is dependent on duration of regional ischaemia: (A) Reperfusion VT incidence and (B) Reperfusion VF incidence for the 5 different durations of ischaemia (n = 6 per study arm). The incidence of VT and VF were different between groups (p = 0.01 and p = 0.02 respectively). The ascending part of this bell-shaped relationship was between 5 and 9 minutes of ischaemia.
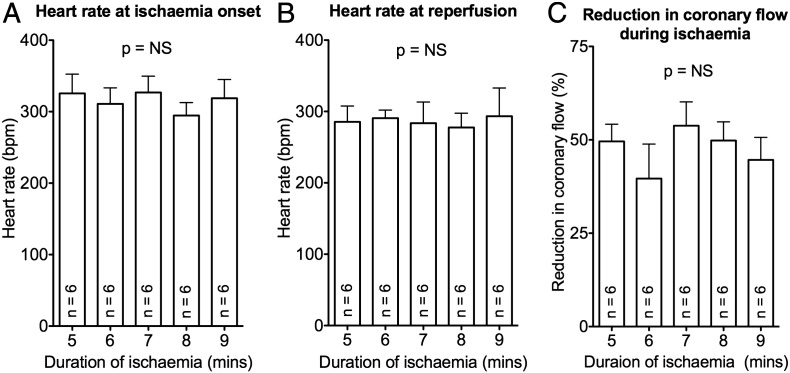
Fig. S2.
Heart rates and coronary flow reductions were similar between groups: (A) Heart rate at ischaemia onset, (B) Heart rate at reperfusion, (C) Reduction in coronary flow rate with regional ischaemia. These were not statistically different between groups (n = 6 per study arm).
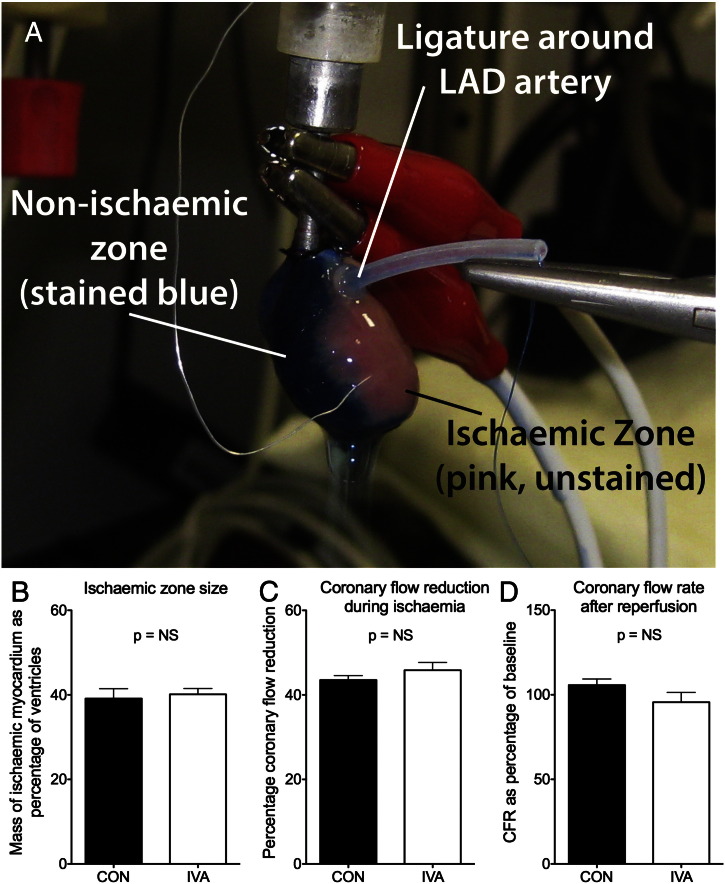
Fig. S3.
Ischaemic zone size was not affected by ivabradine: (A) Heart at the end of an ischaemia-reperfusion experiment following addition of Evans Blue into the perfusate, with the non-ischaemic zone stained blue, and the ischaemic zone unstained. (B) The mass of the ischaemic zone expressed as percentage of total ventricular mass was not different between control and ivabradine groups. (C) The percentage coronary flow reduction, a surrogate of ischaemic zone size, was also not different between groups. (D) Coronary flow recovery with reperfusion was similar between groups (CFR: coronary flow rate).
References
- 1.Tennant R., Wiggers C. The effect of coronary occlusion on myocardial contraction. J Physiol. 1935;112:351–361. [Google Scholar]
- 2.Tzivoni D., Keren A., Granot H., Gottlieb S., Benhorin J., Stern S. Ventricular fibrillation caused by myocardial reperfusion in Prinzmetal's angina. Am Heart J. 1983;105:323–325. doi: 10.1016/0002-8703(83)90534-3. [DOI] [PubMed] [Google Scholar]
- 3.Mehta R., Harjai K., Grines L., Stone G., Boura J., Cox D. Sustained ventricular tachycardia or fibrillation in the cardiac catheterization laboratory among patients receiving primary percutaneous coronary intervention: incidence, predictors, and outcomes. J Am Coll Cardiol. 2004;43:1765–1772. doi: 10.1016/j.jacc.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 4.Wit A., Janse M. Reperfusion arrhythmias and sudden cardiac death: a century of progress toward an understanding of the mechanisms. Circ Res. 2001;89:741–743. [PubMed] [Google Scholar]
- 5.Kaplinsky E., Ogawa S., Michelson E., Dreifus L. Instantaneous and delayed ventricular arrhythmias after reperfusion of acutely ischemic myocardium: evidence for multiple mechanisms. Circulation. 1981;63:333–340. doi: 10.1161/01.cir.63.2.333. [DOI] [PubMed] [Google Scholar]
- 6.Corr P., Witkowski F. Potential electrophysiologic mechanisms responsible for dysrhythmias associated with reperfusion of ischemic myocardium. Circulation. 1983;68:I16–I24. [PubMed] [Google Scholar]
- 7.Shen A.C., Jennings R.B. Kinetics of calcium accumulation in acute myocardial ischemic injury. Am J Pathol. 1972;67:441–452. [PMC free article] [PubMed] [Google Scholar]
- 8.Bernier M., Curtis M., Hearse D. Ischemia-induced and reperfusion-induced arrhythmias: importance of heart rate. Am J Physiol. 1989;256:H21–H31. doi: 10.1152/ajpheart.1989.256.1.H21. [DOI] [PubMed] [Google Scholar]
- 9.Tosaki A., Szekeres L., Hearse D.J. Metoprolol reduces reperfusion-induced fibrillation in the isolated rat heart: protection is secondary to bradycardia. J Cardiovasc Pharmacol. 1987;10:489–497. doi: 10.1097/00005344-198711000-00001. [DOI] [PubMed] [Google Scholar]
- 10.van Gilst W.H., de Graeff P.A., Kingma J.H., de Langen C.D., Wesseling H. Effects of diltiazem on reperfusion-induced arrhythmias in vitro and in vivo. J Mol Cell Cardiol. 1986;18:1255–1266. doi: 10.1016/s0022-2828(86)80429-1. [DOI] [PubMed] [Google Scholar]
- 11.Zuanetti G., De Ferrari G.M., Priori S.G., Schwartz P.J. Protective effect of vagal stimulation on reperfusion arrhythmias in cats. Circ Res. 1987;61:429–435. doi: 10.1161/01.res.61.3.429. [DOI] [PubMed] [Google Scholar]
- 12.Borer J.S., Fox K., Jaillon P., Lerebours G. Ivabradine Investigators Group. Antianginal and antiischemic effects of ivabradine, an I(f) inhibitor, in stable angina: a randomized, double-blind, multicentered, placebo-controlled trial. Circulation. 2003;107:817–823. doi: 10.1161/01.cir.0000048143.25023.87. [DOI] [PubMed] [Google Scholar]
- 13.Swedberg K., Komajda M., Böhm M., Borer J.S., Ford I., Dubost-Brama A. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 14.Fox K., Ford I., Steg P.G., Tendera M., Ferrari R. BEAUTIFUL Investigators. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–816. doi: 10.1016/S0140-6736(08)61170-8. [DOI] [PubMed] [Google Scholar]
- 15.Fox K., Ford I., Steg P.G., Tendera M., Robertson M., Ferrari R. BEAUTIFUL Investigators. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–821. doi: 10.1016/S0140-6736(08)61171-X. [DOI] [PubMed] [Google Scholar]
- 16.Nakata T., Hearse D.J., Curtis M.J. Are reperfusion-induced arrhythmias caused by disinhibition of an arrhythmogenic component of ischemia? J Mol Cell Cardiol. 1990;22:843–858. doi: 10.1016/0022-2828(90)90116-j. [DOI] [PubMed] [Google Scholar]
- 17.Walker M.J.A., Curtis M.J., Hearse D.J., Campbell R.W.F., Janse M.J., Yellon D.M. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia, infarction, and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- 18.Fedorov V., Lozinsky I., Sosunov E., Anyukhovsky E., Rosen M., Balke C. Application of blebbistatin as an excitation–contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm. 2007;4:619–626. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 19.Laughner J.I., Ng F.S., Sulkin M.S., Arthur R.M., Efimov I.R. Processing and analysis of cardiac optical mapping data obtained with potentiometric dyes. Am J Physiol Heart Circ Physiol. 2012;303:H753–H765. doi: 10.1152/ajpheart.00404.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaillant F., Timour Q., Descotes J., Manati W., Belhani D., Bui-Xuan B. Ivabradine induces an increase in ventricular fibrillation threshold during acute myocardial ischemia: an experimental study. J Cardiovasc Pharmacol. 2008;52:548–554. doi: 10.1097/FJC.0b013e3181913df4. [DOI] [PubMed] [Google Scholar]
- 21.Vaillant F., Dehina L., Mazzadi A., Descotes J., Chevalier P., Tabib A. Heart rate reduction with ivabradine increases ischaemia-induced ventricular fibrillation threshold: role of myocyte structure and myocardial perfusion. Resuscitation. 2011;82:1092–1099. doi: 10.1016/j.resuscitation.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 22.Bui-Xuan B., Aupetit J.F., Freysz M., Loufoua J., Faucon G., Timour Q. Cardiac beta-adrenoreceptor activation and ventricular fibrillation under normal and ischemic conditions. Cardiovasc Res. 1996;32:1056–1063. doi: 10.1016/s0008-6363(96)00156-3. [DOI] [PubMed] [Google Scholar]
- 23.Aupetit J.F., Frassati D., Bui-Xuan B., Freysz M., Faucon G., Timour Q. Efficacy of a beta-adrenergic receptor antagonist, propranolol, in preventing ischaemic ventricular fibrillation: dependence on heart rate and ischaemia duration. Cardiovasc Res. 1998;37:646–655. doi: 10.1016/s0008-6363(97)00304-0. [DOI] [PubMed] [Google Scholar]
- 24.Paur H., Wright P.T., Sikkel M.B., Tranter M.H., Mansfield C., O'Gara P. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenoceptor/Gi-dependent manner: a new model of takotsubo cardiomyopathy. Circulation. 2012;126:697–706. doi: 10.1161/CIRCULATIONAHA.112.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heusch G., Skyschally A., Gres P., van Caster P., Schilawa D., Schulz R. Improvement of regional myocardial blood flow and function and reduction of infarct size with ivabradine: protection beyond heart rate reduction. Eur Heart J. 2008;29:2265–2275. doi: 10.1093/eurheartj/ehn337. [DOI] [PubMed] [Google Scholar]
- 26.Ceconi C., Cargnoni A., Francolini G., Parinello G., Ferrari R. Heart rate reduction with ivabradine improves energy metabolism and mechanical function of isolated ischaemic rabbit heart. Cardiovasc Res. 2009;84:72–82. doi: 10.1093/cvr/cvp158. [DOI] [PubMed] [Google Scholar]
- 27.Akar J., Akar F. Regulation of ion channels and arrhythmias in the ischemic heart. J Electrocardiol. 2007;40:S37–S41. doi: 10.1016/j.jelectrocard.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 28.Shaw R., Rudy Y. Electrophysiologic effects of acute myocardial ischemia. A mechanistic investigation of action potential conduction and conduction failure. Circ Res. 1997;80:124–138. doi: 10.1161/01.res.80.1.124. [DOI] [PubMed] [Google Scholar]
- 29.Yellon D., Hausenloy D. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 30.Akar F.G., Aon M.A., Tomaselli G.F., O'Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005;115:3527–3535. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.