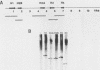
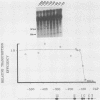
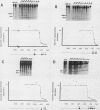
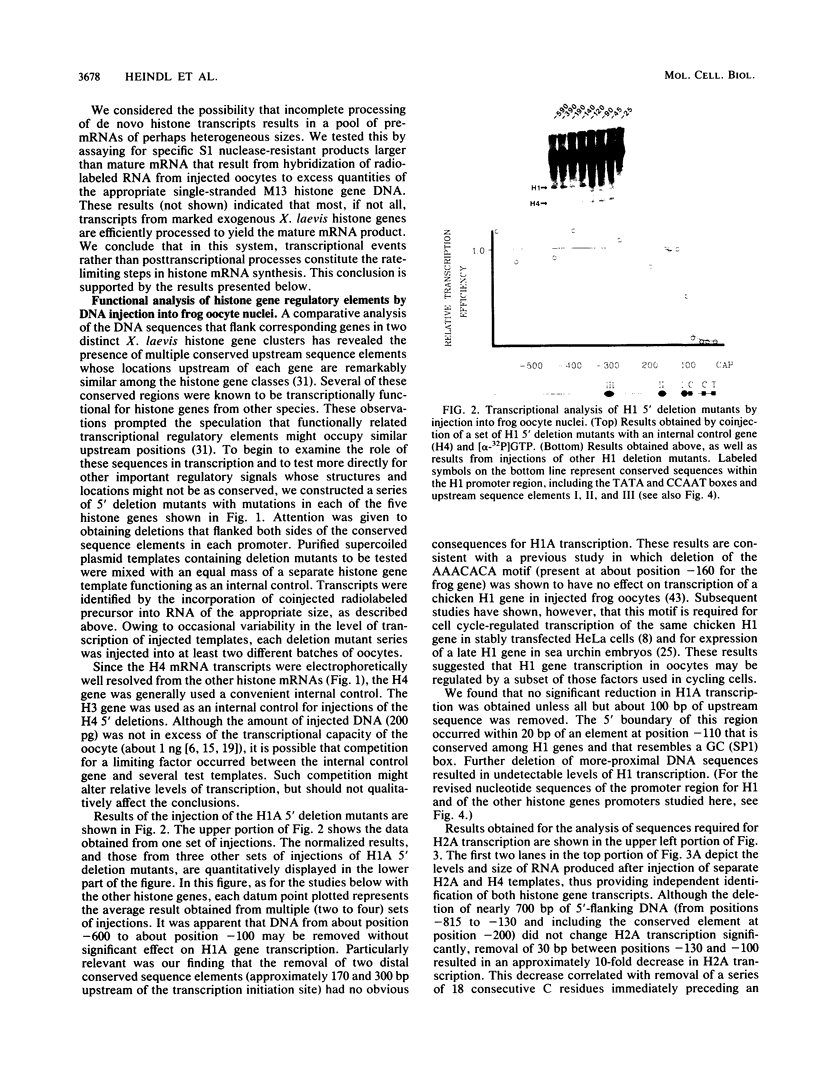
Abstract
Amphibian oogenesis is accompanied by the accumulation of histone mRNA and proteins in the absence of ongoing DNA replication. To begin an analysis of the mechanisms by which histone gene expression is regulated during frog oogenesis and embryogenesis, we used oocyte injection to examine the upstream sequences required for transcription of genes encoding each of the five histone classes. We found that sequences necessary for maximal levels of transcription are located 100 to 200 base pairs upstream of the corresponding start sites. In this region, each promoter examined contains conserved sequence elements, several of which seem to be histone gene class specific, in addition to other, more common sequence elements believed to be used by general transcription factors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artishevsky A., Wooden S., Sharma A., Resendez E., Jr, Lee A. S. Cell-cycle regulatory sequences in a hamster histone promoter and their interactions with cellular factors. 1987 Aug 27-Sep 2Nature. 328(6133):823–827. doi: 10.1038/328823a0. [DOI] [PubMed] [Google Scholar]
- Bark C., Weller P., Zabielski J., Janson L., Pettersson U. A distant enhancer element is required for polymerase III transcription of a U6 RNA gene. Nature. 1987 Jul 23;328(6128):356–359. doi: 10.1038/328356a0. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Schlissel M. S. A positive transcription factor controls the differential expression of two 5S RNA genes. Cell. 1985 Oct;42(3):759–767. doi: 10.1016/0092-8674(85)90272-7. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Clerc R. G., Bucher P., Strub K., Birnstiel M. L. Transcription of a cloned Xenopus laevis H4 histone gene in the homologous frog oocyte system depends on an evolutionary conserved sequence motif in the -50 region. Nucleic Acids Res. 1983 Dec 20;11(24):8641–8657. doi: 10.1093/nar/11.24.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey L., Hanly S. M., Roeder R. G., Heintz N. Distinct transcription factors bind specifically to two regions of the human histone H4 promoter. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7241–7245. doi: 10.1073/pnas.83.19.7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton S., Wells J. R. A gene-specific promoter element is required for optimal expression of the histone H1 gene in S-phase. EMBO J. 1988 Jan;7(1):49–56. doi: 10.1002/j.1460-2075.1988.tb02782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrée O. H., Bendig M. M., De Laaf R. T., Koster J. G. Organization of Xenopus histone gene variants within clusters and their transcriptional expression. Biochim Biophys Acta. 1984 Jun 16;782(2):132–141. doi: 10.1016/0167-4781(84)90016-2. [DOI] [PubMed] [Google Scholar]
- Dworkin M. B., Dawid I. B. Use of a cloned library for the study of abundant poly(A)+RNA during Xenopus laevis development. Dev Biol. 1980 May;76(2):449–464. doi: 10.1016/0012-1606(80)90393-0. [DOI] [PubMed] [Google Scholar]
- Falkner F. G., Zachau H. G. Correct transcription of an immunoglobulin kappa gene requires an upstream fragment containing conserved sequence elements. Nature. 1984 Jul 5;310(5972):71–74. doi: 10.1038/310071a0. [DOI] [PubMed] [Google Scholar]
- Fletcher C., Heintz N., Roeder R. G. Purification and characterization of OTF-1, a transcription factor regulating cell cycle expression of a human histone H2b gene. Cell. 1987 Dec 4;51(5):773–781. doi: 10.1016/0092-8674(87)90100-0. [DOI] [PubMed] [Google Scholar]
- Garabedian M. J., Hung M. C., Wensink P. C. Independent control elements that determine yolk protein gene expression in alternative Drosophila tissues. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1396–1400. doi: 10.1073/pnas.82.5.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian M. J., Shepherd B. M., Wensink P. C. A tissue-specific transcription enhancer from the Drosophila yolk protein 1 gene. Cell. 1986 Jun 20;45(6):859–867. doi: 10.1016/0092-8674(86)90560-x. [DOI] [PubMed] [Google Scholar]
- Gargiulo G., Razvi F., Ruberti I., Mohr I., Worcel A. Chromatin-specific hypersensitive sites are assembled on a Xenopus histone gene injected into Xenopus oocytes. J Mol Biol. 1985 Feb 5;181(3):333–349. doi: 10.1016/0022-2836(85)90223-2. [DOI] [PubMed] [Google Scholar]
- Georgiev O., Birnstiel M. L. The conserved CAAGAAAGA spacer sequence is an essential element for the formation of 3' termini of the sea urchin H3 histone mRNA by RNA processing. EMBO J. 1985 Feb;4(2):481–489. doi: 10.1002/j.1460-2075.1985.tb03654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Delimitation of far upstream sequences required for maximal in vitro transcription of an H2A histone gene. Proc Natl Acad Sci U S A. 1982 Jan;79(2):297–301. doi: 10.1073/pnas.79.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Spacer DNA sequences upstream of the T-A-T-A-A-A-T-A sequence are essential for promotion of H2A histone gene transcription in vivo. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7102–7106. doi: 10.1073/pnas.77.12.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B., Wickens M. P. The use of Xenopus oocytes for the expression of cloned genes. Methods Enzymol. 1983;101:370–386. doi: 10.1016/0076-6879(83)01028-9. [DOI] [PubMed] [Google Scholar]
- Hanly S. M., Bleecker G. C., Heintz N. Identification of promoter elements necessary for transcriptional regulation of a human histone H4 gene in vitro. Mol Cell Biol. 1985 Feb;5(2):380–389. doi: 10.1128/mcb.5.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel C. C., Birnstiel M. L. The organization and expression of histone gene families. Cell. 1981 Aug;25(2):301–313. doi: 10.1016/0092-8674(81)90048-9. [DOI] [PubMed] [Google Scholar]
- Hentschel C., Probst E., Birnstiel M. L. Transcriptional fidelity of histone genes injected into Xenopus oocyte nuclei. Nature. 1980 Nov 6;288(5786):100–102. doi: 10.1038/288100a0. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Gerring S. L., Corces V. G. The ovarian, ecdysterone, and heat-shock-responsive promoters of the Drosophila melanogaster hsp27 gene react very differently to perturbations of DNA sequence. Mol Cell Biol. 1987 Mar;7(3):973–981. doi: 10.1128/mcb.7.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBella F., Sive H. L., Roeder R. G., Heintz N. Cell-cycle regulation of a human histone H2b gene is mediated by the H2b subtype-specific consensus element. Genes Dev. 1988 Jan;2(1):32–39. doi: 10.1101/gad.2.1.32. [DOI] [PubMed] [Google Scholar]
- Lai Z. C., Maxson R., Childs G. Both basal and ontogenic promoter elements affect the timing and level of expression of a sea urchin H1 gene during early embryogenesis. Genes Dev. 1988 Feb;2(2):173–183. doi: 10.1101/gad.2.2.173. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Maxson R., Cohn R., Kedes L., Mohun T. Expression and organization of histone genes. Annu Rev Genet. 1983;17:239–277. doi: 10.1146/annurev.ge.17.120183.001323. [DOI] [PubMed] [Google Scholar]
- Old R. W., Sheikh S. A., Chambers A., Newton C. A., Mohammed A., Aldridge T. C. Individual Xenopus histone genes are replication-independent in oocytes and replication-dependent in Xenopus or mouse somatic cells. Nucleic Acids Res. 1985 Oct 25;13(20):7341–7358. doi: 10.1093/nar/13.20.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old R. W., Woodland H. R., Ballantine J. E., Aldridge T. C., Newton C. A., Bains W. A., Turner P. C. Organization and expression of cloned histone gene clusters from Xenopus laevis and X. borealis. Nucleic Acids Res. 1982 Dec 11;10(23):7561–7580. doi: 10.1093/nar/10.23.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osley M. A., Gould J., Kim S., Kane M. Y., Hereford L. Identification of sequences in a yeast histone promoter involved in periodic transcription. Cell. 1986 May 23;45(4):537–544. doi: 10.1016/0092-8674(86)90285-0. [DOI] [PubMed] [Google Scholar]
- Perry M., Thomsen G. H., Roeder R. G. Genomic organization and nucleotide sequence of two distinct histone gene clusters from Xenopus laevis. Identification of novel conserved upstream sequence elements. J Mol Biol. 1985 Oct 5;185(3):479–499. doi: 10.1016/0022-2836(85)90065-8. [DOI] [PubMed] [Google Scholar]
- Perry M., Thomsen G. H., Roeder R. G. Major transitions in histone gene expression do not occur during development in Xenopus laevis. Dev Biol. 1986 Aug;116(2):532–538. doi: 10.1016/0012-1606(86)90154-5. [DOI] [PubMed] [Google Scholar]
- Probst E., Kressmann A., Birnstiel M. L. Expression of sea urchin histone genes in the oocyte of Xenopus laevis. J Mol Biol. 1979 Dec 15;135(3):709–732. doi: 10.1016/0022-2836(79)90173-6. [DOI] [PubMed] [Google Scholar]
- Pruijn G. J., van Driel W., van der Vliet P. C. Nuclear factor III, a novel sequence-specific DNA-binding protein from HeLa cells stimulating adenovirus DNA replication. Nature. 1986 Aug 14;322(6080):656–659. doi: 10.1038/322656a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P. J., O'Neill E. A., Wides R. J., Kelly T. J. Sequence-specific interactions between cellular DNA-binding proteins and the adenovirus origin of DNA replication. Mol Cell Biol. 1987 Feb;7(2):875–886. doi: 10.1128/mcb.7.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastry B. S., Honda B. M., Roeder R. G. Altered levels of a 5 S gene-specific transcription factor (TFIIIA) during oogenesis and embryonic development of Xenopus laevis. J Biol Chem. 1984 Sep 25;259(18):11373–11382. [PubMed] [Google Scholar]
- Sive H. L., Heintz N., Roeder R. G. Multiple sequence elements are required for maximal in vitro transcription of a human histone H2B gene. Mol Cell Biol. 1986 Oct;6(10):3329–3340. doi: 10.1128/mcb.6.10.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strub K., Galli G., Busslinger M., Birnstiel M. L. The cDNA sequences of the sea urchin U7 small nuclear RNA suggest specific contacts between histone mRNA precursor and U7 RNA during RNA processing. EMBO J. 1984 Dec 1;3(12):2801–2807. doi: 10.1002/j.1460-2075.1984.tb02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen W. M., Moorman A. F., Destrée O. H. Histone gene expression in early development of Xenopus laevis. Analysis of histone mRNA in oocytes and embryos by blot-hybridization and cell-free translation. Differentiation. 1983;24(3):226–233. doi: 10.1111/j.1432-0436.1983.tb01324.x. [DOI] [PubMed] [Google Scholar]
- Woodland H. R. Histone synthesis during the development of Xenopus. FEBS Lett. 1980 Nov 17;121(1):1–10. doi: 10.1016/0014-5793(80)81252-x. [DOI] [PubMed] [Google Scholar]
- Younghusband H. B., Sturm R., Wells J. R. Mutagenesis of conserved 5' elements and transcription of a chicken H1 histone gene. Nucleic Acids Res. 1986 Jan 24;14(2):635–644. doi: 10.1093/nar/14.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]