Abstract
Pediatric brain tumors are often difficult to cure and involve significant morbidity when treated with traditional treatment modalities, including neurosurgery, conventional chemotherapy, and radiotherapy. During the past two decades, a clearer understanding of tumorigenesis, molecular growth pathways, and immune mechanisms in the pathogenesis of cancer has opened up promising avenues for therapy. Pediatric clinical trials with novel biologic agents are underway to treat various pediatric brain tumors, including high and low grade gliomas and embryonal tumors. As the therapeutic potential of these agents undergoes evaluation, their toxicity profiles are also becoming better understood. These agents have potentially better central nervous system penetration and lower toxicity profiles compared with conventional chemotherapy. In infants and younger children, biologic agents may prove to be of equal or greater efficacy compared with traditional chemotherapy and radiation therapy, and may reduce the deleterious side effects of traditional therapeutics on the developing brain. Molecular pathways implicated in pediatric brain tumors, agents that target these pathways, and current clinical trials are reviewed. Associated neurologic toxicities will be discussed subsequently. Considerable work is needed to establish the efficacy of these agents alone and in combination, but pediatric neurologists should be aware of these agents and their rationale.
Introduction
Central nervous system tumors are the most common form of childhood solid tumors [1], and comprise a diverse group of tumors with different histologies, arising at various sites within the central nervous system. Although advances in neurosurgery and multi-modal therapy have led to improved patient survival for pediatric central nervous system tumors as a whole, with current 5-year survival rates of 72.5% [1], these tumors and their treatment continue to involve significant morbidity, and ultimately, less than 50% of children will be cured. Conventional chemotherapy and radiotherapy, using the principle of increased sensitivity of cancer cells to cytotoxic damage, continue to be the mainstays of treatment for pediatric brain tumors that cannot be cured by surgery alone. Multiple studies of pediatric brain tumors demonstrated significant long-term neurologic sequelae from tumors and their treatment, such as: (1) cognitive dysfunction, (2) neuroendocrine dysregulation, and (3) developmental delays [2,3]. The brains of infants and very young children, in particular, are more susceptible to neurologic and neurosensory sequelae, compared with adults, upon exposure to conventional chemotherapy and radiotherapy [4,5]. Therefore, an imperative need exists to identify newer agents with a better therapeutic window that can effectively target cancer cells, while sparing normal cells in the rapidly developing pediatric brain. Furthermore, using biologic agents to delay radiotherapy in children by even a few years may reduce adverse effects of treatment.
In the past two decades, tremendous strides in the field of molecular biology have enhanced our understanding of tumori-genesis at a molecular level. A better delineation has been established for the role of proto-oncogenes and tumor suppressor genes, cell signaling, and signal transduction pathways involved in cell cycle regulation, proliferation, survival, neoplastic angiogenesis, tumor invasion, and migration. The immune mechanisms involved in tumorigenesis and in the evasion of immune surveillance have also become better understood. Key pathways for the pathogenesis of glial and embryonal tumors include: (1) Ras-mitogen-activated protein kinase; (2) phosphatidylinositol 3-kinase/protein kinase B; (3) the Janus kinase signal transducer and activator of transcription; (4) Notch; (5) the Wingless-type murine mammary virus type integration site family; and (6) Sonic Hedgehog [6–9]. (The names of the last three pathways are derived from drosophila in which the genes were first characterized.) This growing body of knowledge has resulted in a new field of targeted therapeutics with the potential for directly inhibiting the pathways responsible for the tumorigenic state, while sparing normal brain cells (Figs 1 and 2). Furthermore, many of the newer targeted agents have a low molecular weight, which enables them to overcome the blood-brain barrier that precludes many traditional chemotherapeutic agents from entering brain tumor cells after systemic administration. Although neuro-oncology researchers are optimistic about these medications, efficacy has not yet been proven, and much work remains to be done. Moreover, although these agents are being tested as monotherapy for progressive and recurrent disease, combination therapy will likely be needed to overcome alternative pathways for tumor growth, and to account for the cytostatic nature of these agents.
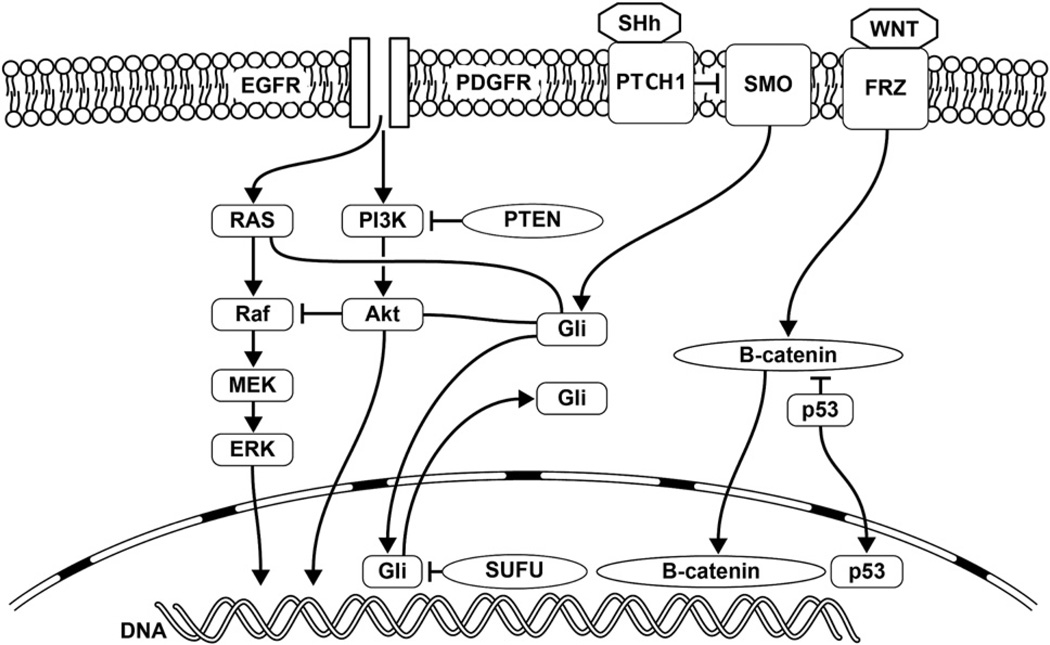
Figure 1.
Cell signaling pathways involved in brain tumorigenesis: Wingless-type murine mammary virus type integration site family (WNT)/β-catenin, sonic hedgehog (SHh), receptor tyrosine kinases, and downstream signal transduction pathways include Ras-mitogen-activated protein kinase (RAS-MAPK) and phosphatidyl 3-kinase (PI3K)/protein kinase B (Akt) pathways, with crosstalk between various pathways. EGFR, epidermal growth factor receptor; ERK, extracellular regulated mitogen-activated protein kinase; FRZ, frizzled; Gli, glioma-associated oncogene homolog 1; MEK, mitogen-activated protein kinase-extracellular regulated mitogen-activated protein kinase; PDGFR, platelet-derived growth factor receptor; PI3K, phosphatidylinositol 3-kinase; PTCH1, PATCHED1; PTEN, phosphatase and tensin homologue; SMO, smoothed; SUFU, suppressor of fused homolog.
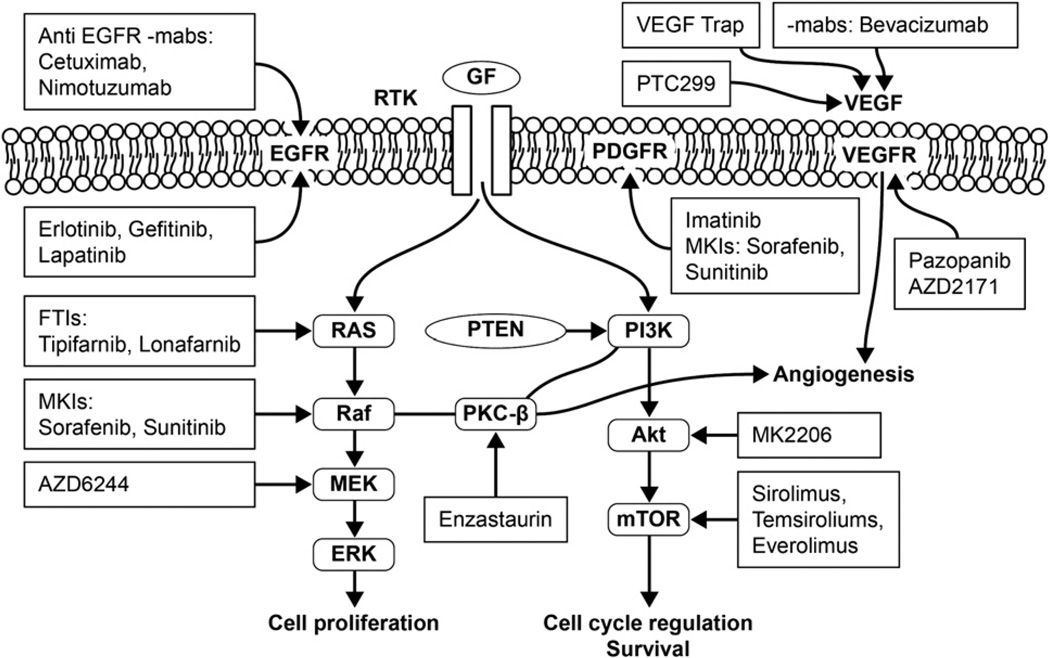
Figure 2.
Cell signaling pathways and some potential therapeutic targets and agents. Akt, protein kinase B; EGFR, epidermal growth factor receptor; ERK, extracellular regulated mitogen-activated protein kinase; FTIs, farnesyl transferase inhibitors; GF, growth factor; mabs, monoclonal antibodies; MEK, mitogen-activated protein kinase-extracellular regulated mitogen-activated protein kinase; MKIs, mulitkinase inhibitors; mTOR, mammalian target of rapamycin; PDGFR, platelet-derived growth factor receptor; PKC-β, protein kinase C-β; PTEN, phosphatase and tensin homologue; PI3K, phosphatidylinositol 3-kinase; RTK, receptor tyrosine kinase; VEGF, vascular endothelium growth factor; VEGFR, vascular endothelium growth factor receptor.
All clinicians involved in the care of children with brain tumors should be familiar with the various drug classes in our therapeutic armamentarium, and should understand their potential toxicity and value in therapy. Pediatric neurologists are especially well poised to understand and apply these drugs for rational use, because they are based on the molecular biology of the developing brain.
This review will focus on novel targeted therapeutic agents that have been in development or use for various cancers, and that are now under clinical investigation for the treatment of pediatric brain tumors (Table 1). It will describe some of the pathways targeted by these new agents and the role these pathways play in the developing brain. A companion article in this Journal will review the known neurotoxicities of these agents.
Table 1.
Biologic agents in pediatric CNS tumor trials
| Target | Drug |
|---|---|
| Epidermal growth factor receptor | Monoclonal antibodies: cetuximab and nimotuzumab |
| Small molecule inhibitors: erlotinib, gefitinib, and lapatinib | |
| Platelet-derived growth factor receptor | Imatinib and sunitinib |
| Ras/Raf/MEK/ERK pathway | Tipifarnib and lonafarnib |
| Farnesyltransferase inhibitors | Sorafenib and sunitinib (multikinase inhibitors) |
| Raf inhibitors | |
| MEK inhibitors | AZD6244 |
| Phosphatidylinositol 3-kinase/mTOR | MK-2206 |
| or Akt/mTOR pathway | Sirolimus, temsirolimus, and |
| Akt inhibitors | everolimus |
| mTOR inhibitors | |
| Angiogenesis inhibitors | Monocolonal antibody: bevacizumab |
| VEGF pathway | Small molecule inhibitors: pazopanib, |
| VEGF and basic fibroblast growth | PTC299, and AZD2171 |
| factor | VEGF Trap |
| Protein kinase C-β inhibitors | Thalidomide and lenalidomaide |
| αvβ3 and αvβ5 integrin inhibitors | Enzastaurin |
| Cilengitide | |
| Histone deactylase inhibitors | Suberoylanilide hydroxamic acid, depsipeptide, and valproic acid |
| Sonic Hedgehog pathway | GDC0449 |
| SMO inhibitor | |
| Notch signaling pathway | RO4929097 |
| Gamma-secretase inhibitor |
Abbreviations:
Akt = Protein kinase B
CNS = Central nervous system
ERK = Extracellular regulated mitogen-activated protein kinase
MEK = Mitogen-activated protein kinase-extracellular regulated mitogen-activated protein kinase
mTOR = Mammalian target of rapamycin
SMO = Smoothened
VEGF = Vascular endothelial growth factor
Growth signaling and receptor tyrosine protein kinase inhibitor
Receptor tyrosine kinases, a subclass of transmembrane receptor proteins, act as crucial regulators of critical cellular processes, such as proliferation, differentiation, cell survival and metabolism, cell migration, and cell-cycle control [10]. Different growth factor ligands bind to the extracellular component of their respective receptor tyrosine kinases, resulting in the activation and auto-phosphorylation of an intracytoplasmic tyrosine kinase domain and the subsequent activation of a host of downstream signaling molecules and transduction pathways, many of which influence the transcription of specific genes. The complexity of the developing central nervous system suggests that the specification of a neural stem cell’s fate is achieved through the temporally and spatially restricted expression of either receptor tyrosine kinases or their ligands [10,11].
A key hallmark of tumor formation involves a loss of normal cell homeostasis because of the deregulated and dysfunctional cell signaling mediated by one or more of four principal mechanisms: (1) autocrine activation, (2) chromosomal translocation, (3) the overexpression of receptor tyrosine kinases, and (4) gain of function mutations. The end result is a receptor that is constitutively active, even without the presence of a ligand [12–15]. Some of the receptor tyrosine kinases implicated in brain tumorigenesis include epidermal growth factor receptor, platelet-derived growth factor receptor, vascular endothelial growth factor receptor, and fibroblast growth factor receptor. Two major classes of newer biologic agents include monoclonal antibodies against growth factor ligands or ligand-binding sites and the small molecule inhibitors, which target the intracellular tyrosine kinase domains.
Epidermal growth factor receptor
The epidermal growth factor receptor family comprises four members: epidermal growth factor receptor, erythroblastic leukemia viral oncogene homolog 2 (erbB2), erythroblastic leukemia viral oncogene homolog 3 (erbB3), and erythroblastic leukemia viral oncogene homolog 4 (erbB4). These receptors are expressed on actively dividing cells in the brain’s neurogenic niches, such as the subventricular zone. The response of these multipotent stem and progenitor cells to epidermal growth factor ligands results in the dramatic expansion and migration of these cells to different brain regions. Recent evidence also suggests that cell diversity during development results from the asymmetric distribution of epidermal growth factor receptor in mitotic cerebral cortical precursors [16]. The withdrawal or decrease in epidermal growth factor receptor ligands promotes differentiation. Exposure of the receptor to the growth factor ligand results in the oligomerization of the monomeric receptor and the activation of downstream pathways such as the Ras/mitogen-activated protein kinase, phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin, and β-catenin pathways. The epidermal growth factor receptor also influences other signaling systems such as protein kinase C, the signal transducer and activator of transcription, integrins, and Wingless-type murine mammary virus type integration site family-associated pathways [17].
Truncations, amplifications, and the overexpression of the epidermal growth factor receptor family of receptors were reported in pediatric high grade gliomas [18–21] and brainstem gliomas [22], ependymomas [23], and medulloblastomas [24,25], making the epidermal growth factor receptor a rational therapeutic target. Monoclonal antibodies, i.e., cetuximab [26,27] and panitimumab [28], small-molecule inhibitors of the epidermal growth factor receptor tyrosine kinases, i.e., erlotinib [29,30] and gefitinib [31], and dual (combined epidermal growth factor receptor and ERBB2) inhibitors, e.g., lapatinib [32], are some of the agents that have been tested in preclinical in vitro or xenograft brain tumor models, and a few that have gone on to phase I/phase II pediatric clinical trials. In addition to their potential use in monotherapy or combination chemotherapy, these agents also demonstrate promise as radio-sensitizers [33].
Erlotinib (Tarceva; CyDex, Lenexa, KS) is an orally administered agent with activity against epidermal growth factor receptor and, to a lesser extent, erbB2 [34]. A pediatric phase I trial by the Children’s Oncology Group indicated that erolitinib in combination with temozolomide in children with refractory solid tumors was well tolerated [29]. Another recently conducted pediatric phase I trial with erlotinib monotherapy in patients with refractory or recurrent brain tumors and erlotinib in combination with radiotherapy in patients with newly diagnosed brainstem gliomas concluded that erlotinib exhibited an acceptable tolerability profile, though it carried the risk for potential intratumoral hemorrhage [30].
Gefitinib (Iressa; AstraZeneca, Wilmington, DE) is another orally administered inhibitor of the epidermal growth factor receptor. A phase II study by the Pediatric Brain Tumor Consortium indicated that gefitinib, given in combination and after radiotherapy, appears to be well tolerated in patients with newly diagnosed diffuse intrinsic pontine gliomas. Overall survival rates at 12 months and 24 months were 56.4% ± 7.6% confidence interval and 19.6% ± 5.9% confidence interval, respectively, which appear slightly superior to survival rates in other trials. Three patients with ≥36 months of follow-up remained progression-free, suggesting that molecular characterization may help identify subsets of patients who respond to treatment, and such molecular stratification may help tailor therapy [31].
Lapatinib (Tykerb; GlaxoSmithKline, Research Triangle Park, NC) is an orally administered dual epidermal growth factor receptor/erbB2 inhibitor [35]. A recently concluded phase I trial from the Pediatric Brain Tumor Consortium revealed that lapatinib in twice-daily dosing was well tolerated in children with recurrent central nervous system tumors. Some patients appeared to manifest prolonged disease stabilization. However, further studies are needed to study its effects on tumor growth and survival [32]. An ongoing phase II study of lapatinib with bevacizumab in children with recurrent ependymoma is being performed. Cetuximab (Erbitux; ImClone Systems, New York, NY and Bristol-Myers Squibb, Princeton, NJ) is a monoclonal antibody against the epidermal growth factor receptor, and a recent study indicated that cetuximab given in combination with irinotecan in refractory/recurrent solid tumors appears to be safe, warranting further phase II trials [27].
Nimotuzumab is a humanized monoclonal antibody against epidermal growth factor receptor, and its activity appears to be dependent on the density of the epidermal growth factor receptor, thereby creating a potential for sparing normal cells while targeting only tumor cells with the overexpression of epidermal growth factor receptor. A multi-institutional European study evaluating nimotuzumab with radiation in diffuse intrinsic pontine gliomas reported no survival advantage compared with previous reports. Given its favorable safety profile, investigators also performed a pilot study using nimotuzumab with vinorelbine and radiation; preliminary results demonstrated promise [36].
Platelet-derived growth factor receptor
The platelet-derived growth factor receptor family comprises two receptors, platelet-derived growth factor receptor-α and platelet-derived growth factor receptor-β, which undergo dimerization and activation on exposure to platelet-derived growth factor ligands. Platelet-derived growth factor receptor-α in the postnatal developing brain is expressed in oligodendrocyte progenitor cells and glial fibrillary acidic protein-positive type B cells in the subventricular zone. Oligodendrocyte progenitor cells are responsible for mitogenic potential and the generation of new oligodendrocyte lineage cells that constitute the white matter. Interestingly, chondroitin sulfate proteoglycan NG2-positive cells in the brain that give rise to nearly all oligodendrocytes co-express platelet-derived growth factor receptor-α. The activation of platelet-derived growth factor receptor-α is important in regulating oligodendrocyte progenitor cell migration, and the expression of this receptor decreases as brain maturity increases [37]. Platelet-derived growth factor receptor-α on glial fibrillary acidic protein-positive type B cells in the neurogenic subventricular zone cell is responsible for generating oligodendrocytes and neurons [38]. The cell types that express platelet-derived growth factor receptor-β receptor occur in vascular smooth muscles, pericytes, and neurons [39,40].
Platelet-derived growth factor receptor-mediated cell signaling pathways are involved in tumor proliferation, survival, invasion, and angiogenesis [41]. Amplifications of platelet-derived growth factor receptor-α were demonstrated in pediatric high-grade gliomas and diffuse, intrinsic brainstem gliomas [42,43]. Another study revealed that the high expression of platelet-derived growth factor receptor had a significant association with malignant histology in pediatric gliomas [44]. The expression of platelet-derived growth factor receptor-α was also demonstrated in medulloblastoma and primitive neuroectodermal tumors, and an increase in platelet-derived growth factor receptor-α gene copy numbers seems to be associated with poor survival [45].
Imatinib (Gleevac; Novartis, East Hanover, NJ) is a small molecule inhibitor of breakpoint cluster region/abelson kinase, kit oncogene, and platelet-derived growth factor receptor. Although imatinib appears safe, it has proven disappointing as a treatment agent [46,47], possibly because of its poor central nervous system penetrance. In a Canadian phase II study, although all tumors in the study demonstrated the expression of platelet-derived growth factor receptor, no objective response was evident [46]. A phase I study by the Pediatric Brain Tumor Consortium also cautioned against the increased risk of intratumoral hemorrhage with imatinib [48].
Vascular endothelial growth factor receptor signaling pathway
The vascular endothelial growth factor receptor pathway currently offers the most promising targets of neoplastic angiogenesis. The vascular endothelial growth factor receptor family comprises three receptors: vascular endothelial growth factor receptor-1, vascular endothelial growth factor receptor-2, and vascular endothelial growth factor receptor-3, which bind to vascular endothelial growth factor ligands and regulate angiogenesis [49]. In the developing brain, vascular endothelial growth factor receptor stimulation not only plays a role in cerebral angiogenesis and vascular permeability, but is also recognized for its neurotrophic and growth promoting effects [50,51]. Interestingly, the vascular endothelial growth factor gene contains a hypoxia-response element, which allows for binding of the hypoxia inducible factor. The expression of vascular endothelial growth factor and vascular endothelial growth factor receptor increases with hypoxia, thus contributing to further angiogenesis and protection in these regions. Rapidly progressive tumors in the brain exhibit lower oxygen tensions (i.e., a hypoxic state) compared with normal tissue environments, thus contributing to an increase in hypoxia inducible factor-α and vascular endothelial growth factor levels [52,53]. Inhibiting the vascular endothelial growth factor receptor pathways constitutes a promising method to prevent further tumor expansion.
Monoclonal antibodies and small molecule inhibitors of the vascular endothelial growth factor ligands and vascular endothelial growth factor receptor are in clinical development. The most extensive experience with vascular endothelial growth factor inhibitors has involved bevacizumab (Avastin; Genentech, San Francisco, CA), a monoclonal antibody against vascular endothelial growth factor, especially in combination with irinotecan. A study of bevacizumab with irinotecan in recurrent low-grade gliomas indicated that the regimen is well tolerated, and also demonstrated objective tumor response [54]. This study has paved the way for a phase II multi-institutional consortium investigation, currently underway. Although this combination has proven to be of possible clinical benefit in adult malignant glioma trials, a similar benefit has unfortunately not been observed in pediatric, recurrent, high-grade gliomas and diffuse, intrinsic pontine gliomas [55,56]. Multiple small molecule inhibitors of the vascular endothelial growth factor pathway are currently in development and in various phase I/II pediatric clinical trials. A few examples include pazopanib, PTC299, and AZD2171. The multikinase inhibitors, sorafenib and sunitinib, are also being tested, and are known to exhibit antiangiogenic activity. A phase I study of vascular endothelial growth factor Trap, a soluble decoy receptor protein that binds with vascular endothelial growth factor and thereby blocks vascular endothelial growth factor signaling, is underway. Other potential vascular endothelial growth factor signaling pathway inhibitors include protein kinase C-β inhibitors. Protein kinase C-β serine/threonine kinase is a downstream effector of the vascular endothelial growth factor signaling pathway. Enzastaurin functions as a protein kinase C-β inhibitor and as a suppressor of the phosphatidylinositol 3-kinase/protein kinase B pathway [57].
Downstream signal transduction pathways
Ras/Raf/ mitogen-activated protein kinase-extracellular regulated mitogen-activated protein kinase/extracellular regulated mitogen-activated protein kinase pathway
The Ras-mitogen activated protein kinase pathways play an important role in cell proliferation and survival. The activation of growth factor receptor leads to the activation of downstream effectors such as Ras. Ras is a small guanosine triphosphatase, and its functioning requires posttranslational modifications leading to plasma membrane localization of the Ras molecule. The farnesylation of the Ras molecule catalyzed by farnesyl-transferase is one such posttranslational modification. The activation of Ras because of the overexpression or amplification of growth factor receptors such as epidermal growth factor receptor, as well as Ras mutations, have both been implicated in Ras-induced gliomagenesis because of the resultant activation of downstream effector pathways such as the Raf-mitogen-activated protein kinase-extracellular regulated mitogen-activated protein kinase-extracellular regulated mitogen-activated protein kinase pathway [58,59]. v-raf murine sarcoma viral oncogene homolog B1 (BRAF) mutations have been implicated in pediatric low-grade gliomas [60]. Given the key role that the Ras/mitogen-activated protein kinase pathway is thought to play in oncogenesis, a major interest has arisen in the development of inhibitors targeting different steps of the cascade.
Farnesyltransferase inhibitors
Farnesyltransferase inhibitors, which inhibit the post-translational modification of Ras, have been studied as potential therapeutic agents in central nervous system tumors. However, these agents, e.g., tipifarnib (Zarnestra; Selleck Chemicals, Houston, TX) and lonafarnib (Sarasar; Kenilworth, NJ, Schering-Plough Research Institute), have so far not lived up to their promise. A phase I study by the Pediatric Brain Tumor Consortium indicated that lonafarnib was safely tolerated [61]. Two phase II studies demonstrated no benefit with the use of tipifarnib as monotherapy in recurrent central nervous system tumors [62] and when administered during radiotherapy in children with newly diagnosed intrinsic pontine gliomas [63].
Raf inhibitors
Raf is a serine/threonine kinase with three isoforms. Sorafenib (Nexavar; Bayer HealthCare Pharmaceuticals, Inc., Wayne, NJ, and Onyx Pharmaceuticals, Inc., Emeryville, CA) is a multikinase inhibitor with activity against v-raf-1 murine leukemia viral oncogene homolog 1 (CRAF) and wild-type and mutant (V600E) BRAF kinases, and also demonstrates activity against kinases involved in angiogenesis (vascular endothelial growth factor receptor-2, vascular endothelial growth factor receptor-3, platelet-derived growth factor receptor-β, c-kit, and fibroblast growth factor receptor-1) [64]. Sorafenib is also under study for children with recurrent low-grade gliomas, based primarily on data demonstrating BRAF mutations in the majority of pediatric pilocytic astrocytomas [65,66]. Sunitinib (Sutent; Pfizer, Inc., New York, NY), like sorafenib, is a multikinase inhibitor against Raf and other receptor tyrosine kinases involved in angiogenesis. Based on pediatric preclinical in vivo testing, both these agents seem to inhibit tumor growth [67,68]. With advances in the molecular characterization of brain tumors, more evidence indicates that multiple pathways may be involved in any given tumor. Therefore a shift has occurred in developmental therapeutics, with an increasing interest in agents potentially targeting multiple pathways, such as multikinase inhibitors (the so-called “dirty” kinase inhibitors). A Children’s Oncology Group phase I/II study of Sorafenib in children with recurrent solid tumors and recurrent leukemia and a multi-institutional study of children with recurrent low-grade gliomas are underway.
Mitogen-activated protein kinase-extracellular regulated mitogen-activated protein kinase inhibitors
Inhibitors of mitogen-activated protein kinase-extracellular regulated mitogen-activated protein kinase are being developed as a means of blocking the activation of extracellular regulated mitogen-activated protein kinase. Tumors with BRAF mutations were demonstrated to be very sensitive to mitogen-activated protein kinase-extracellular regulated mitogen-activated protein kinase inhibitors [69]. A mitogen-activated protein kinase-extracellular regulated mitogen-activated protein kinase inhibitor, AZD6244, was demonstrated to be active against BRAF mutant juvenile pilocytic astrocytoma xenografts in preclinical testing [70]. The Pediatric Brain Tumor Consortium is performing an ongoing phase I study of AZD6244 in recurrent and resistant low-grade gliomas.
Phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin pathway
The phosphatidylinositol 3-kinase/protein kinase B pathway is another receptor tyrosine kinase-driven downstream signaling pathway involved in cell survival, growth and metabolism. The phosphatase and tensin homologue acts as a negative regulator of the phosphatidylinositol 3-kinase pathway by inhibiting phosphatidylinositol 3-kinase. Mutations resulting in the loss of the phosphatase and tensin homologue, as observed in gliomas, result in activation of the phosphatidylinositol 3-kinase/protein kinase B pathway and tumorigenesis [71]. The activation of protein kinase B was also implicated in pediatric gliomas, and seems to be more common than loss of phosphatase and tensin homologue mutations [72]. The inhibitor of protein kinase B, MK-2206, is being tested in a phase I trial.
The mammalian target of rapamycin, a serine/threonine kinase, is a critical hub and functions as a master regulator of cell growth, receiving signals from multiple pathways including the phosphatidylinositol 3-kinase/protein kinase B and Ras/extracellular regulated mitogen-activated protein kinase pathways [73]. Although phosphatidylinositol 3-kinase inhibitors as monotherapy have not been effective, emerging evidence suggests that a combination of phosphatidylinositol 3-kinase and mammalian target of rapamycin inhibitors may induce better tumor responses [74]. This idea is based on the premise that the inhibition of phosphatidylinositol 3-kinase may activate other prosurvival pathways, which may be inhibited by the addition of mammalian target of rapamycin inhibitors. A better understanding of the molecular events involved in tumorigenesis has been accompanied by increasing evidence of crosstalk between various molecular pathways. A recent study revealed that phosphatidylinositol 3-kinase/protein kinase B pathway inhibitors can inhibit Wingless-type murine mammary virus type integration site family/β-catenin pathways via crosstalk, thus suggesting a potential role for phosphatidylinositol 3-kinase/protein kinase B inhibitors in medulloblastoma [75]. Sirolimus (Rapamycin; Wyeth Pharmaceuticals, Inc., Philadelphia, PA), temsirolimus, and everolimus comprise some of the mammalian target of rapamycin inhibitors undergoing different pediatric clinical trials. These trials involve recurrent and progressive low-grade gliomas in children with neurofibromatosis-1, and subependymomal giant cell astrocytomas in children with tuberous sclerosis. A recent study demonstrated a reduction in both tumor volume and seizure frequency in patients with subependymal giant cell astrocytomas who were treated with everolimus [76].
Neoplastic angiogenesis inhibitors
Tumorigenesis, in addition to processes involved in tumor proliferation and cell survival, requires the formation of new vasculature that provides oxygen and nutrients for continued tumor growth. The process of tumor angiogenesis is complicated and involves multiple signaling pathways mediated by cytokines. Thus far, up to 40 promoters and inhibitors of angiogenesis have been identified. Among the promoters of tumor angiogenesis, vascular endothelial growth factor (already reviewed) and basic fibroblast growth factor have been most extensively studied. The expression of angiogenic cytokines has been demonstrated in pediatric central nervous system tumors [77,78]. Receptor tyrosine kinase (platelet-derived growth factor receptor) signaling pathways are also required for angiogenic signaling. Cell adhesion molecules involved in cell-cell and cell-matrix adhesion also play a crucial role in the process of angiogenesis [49,79]. As the various signaling pathways and effector molecules are more fully mapped out, multiple agents targeting neoplastic angiogenesis have been developed. Antiangiogenic agents are also especially desirable in treating central nervous system tumors, because their primary target comprises endothelial cells within the lumen of the blood vessel, thus overcoming the need to cross the blood-brain barrier.
In addition to targeting the vascular endothelial growth factor pathway, as previously discussed, other less specific, biologically antiangiogenic drugs, such as thalidomide and its analogue lenalidomide, can be used therapeutically. Thalidomide (Thalomid; Celgene, Summit, NJ), which a few decades ago had fallen into disfavor because of its teratogenic effects, has reemerged as a potential antiangiogenic agent. It exhibits both antivascular endothelial growth factor and basic fibroblast growth factor activity, as well as immunomodulatory effects. Evidence indicates that thalidomide is effective in the treatment of some central nervous system tumors [80]. A phase II study of thalidomide with radiotherapy in children with newly diagnosed diffuse, intrinsic brainstem glioma did not demonstrate any clinical benefits [81]. Lenalidomide (Revlimid; Celgene), an analogue of thalidomide, is another immunomodulatory agent with antiangiogenic activity. A recent phase I study of lenalidomide in recurrent, refractory pediatric central nervous system tumors revealed that it is well tolerated. Lenalidomide may also exert some antitumor activity, given the observed objective responses and long-term stable disease in that study [82]. Future phase II studies should shed more light on its potential antitumor activity, as should another ongoing study using lenalidomide in combination with radiotherapy for newly diagnosed brainstem gliomas.
Cell-matrix adhesion
Integrins are mediators of cell adhesion to the extracellular matrix. In the rapidly developing embryonic and early postnatal brain, the migration of cells to their proper location is dependent on a sophisticated sequence of cellular protrusions and retractions, mainly dependent on the actin cytoskeleton [83]. Integrins play a major role in the proper migration of cells through integrating signals between the extracellular and intracellular environment. Interestingly, integrins are capable of interacting with several different receptor tyrosine kinases, such as the epidermal growth factor receptor and platelet-derived growth factor receptor, as well as cytokine receptors [84]. Integrins were demonstrated not only to potentiate signaling pathways in response to growth factor ligands such as epidermal growth factor and platelet-derived growth factor, but also partly to phosphorylate (i.e., activate) these receptors in the absence of any growth factor ligands [85]. Because of the diverse roles integrin plays in cell proliferation and migration, it is a potential target in the treatment of highly aggressive brain tumors.
The αvβ3 and αvβ5 integrins are expressed in glioblastoma multiforme cells, and are thought to play a role in tumor invasion, proliferation, and angiogenesis. Cilengitide is an inhibitor of αvβ3 and αvβ5 integrins, and preclinical animal studies demonstrated its activity against central nervous system tumors such as glioblastoma. Cilengitide is also thought to demonstrate synergistic activity when combined with radiotherapy [86]. Phase I/II adult studies of cilengitide in glioblastoma have so far been encouraging [87], and have exhibited promising activity when administered in conjunction with temozolomide chemoradiotherapy in patients with glioblastoma with O-6 methyl guanine-DNA methyltransferase promoter methylation [88]. A phase I study of children with refractory central nervous system tumors indicated that cilengitide is safely tolerated [89], and a phase II Children’s Oncology Group study was recently completed.
Sonic Hedgehog pathway
The Hedgehog pathway plays an important role in embryogenesis. Hedgehog pathway activation is PATCHED1 (PTCH1) protein-mediated. The Sonic Hedgehog ligand binds with PTCH1, thereby releasing the repression on Smoothened (SMO), which then activates downstream glioma-associated oncogene homolog 1 proteins, resulting in cell proliferation and tumor formation. Sonic Hedgehog pathway mutations have been implicated in the pathogenesis of medulloblastoma. Gorlin syndrome, which is characterized by multiple basal cell carcinomas with a predisposition for medulloblastomas, was associated with PTCH1 mutations. Since then, multiple studies indicate that PTCH1, SMO, and suppressor of fused homolog mutations are associated with medulloblastoma [90,91]. A PTCH1+/− p53−/− murine model of medulloblastoma demonstrated complete tumor response when exposed to a SMO inhibitor [92]. This finding has led to an interest in the use of Sonic Hedgehog inhibitors in the treatment of medulloblastoma. GDC0449 is a SMO inhibitor currently undergoing medulloblastoma clinical trials.
Notch signaling
The Notch signaling pathway is evolutionarily conserved, and plays a key role in cell fate determination, differentiation, and proliferation. This signaling pathway is important in mediating cell-cell signals between adjacent cells, and has demonstrated an important role in maintaining neural progenitor cells while inhibiting differentiation [93,94]. The dysregulation of Notch pathways was observed in gliomas [95], medulloblastomas, and ependymomas [8]. Activation of the Notch pathway requires cleavage of the transmembrane Notch receptors. Gamma-secretase comprises one of the main proteolytic enzymes that cleave the receptor, which is then released intracellularly, activating the Notch cascade. Gamma-secretase inhibitors are promising as therapeutic targets to inhibit the Notch cascade [96]. Gamma-secretase inhibitors such as RO4929097 are undergoing phase I/II pediatric clinical trials.
Histone deactylase inhibitors
Histone acetylation and deacetylation are among the many processes involved in chromatin remodeling. Histone deacetylation is a process by which the acetyl group is removed, resulting in chromatin condensation and epigenetic silencing. Histone deacetylase inhibitors reverse this process, resulting in gene re-expression and inducing differentiation, growth arrest, or apoptosis of various tumors [97]. Histone deacetylase inhibitors in clinical trials include vorinostat (suberoylanilide hydroxamic acid), depsipeptide, and valproic acid. Preclinical studies with vorinostat demonstrated tumor activity in medulloblastoma [98,99] and high-grade gliomas [100]. A Children’s Oncology Group Phase I study demonstrated that vorinostat is safely tolerated when used alone or in combination with 13-cis retinoic acid [101]. It is currently undergoing trials in pediatric gliomas, diffuse intrinsic pontine glioma, and medulloblastoma, in combination with radiotherapy or other chemotherapeutic agents. Valproic acid comprises another histone deacetylase inhibitor that seems to be safely tolerated, with a potential role as an adjuvant to radiochemotherapy, especially in high-grade gliomas [102–104]. A preclinical in vivo study of depsipeptide in childhood cancer models revealed that although it resulted in increased histone acetylation, it did not correlate with tumor response. Given the low objective response observed, the clinical utility of depsipeptide in pediatric tumors is questionable [105].
Immunotherapy
Tumors exhibit immune tolerance and evade immune surveillance. Inducing immunologic responses to tumors and overcoming immune tolerance, using tumor antigen-targeted monoclonal antibodies [106], cytokine and inflammatory pathway-targeted therapy [107], and tumor vaccines [108], offers the potential for effectively controlling tumors. Interleukin-2, interleukin-4, interferon-α, tumor necrosis factor-α, and other cytokines have so far not proved efficacious in treating brain tumors [109]. Promising approaches to tumor vaccines under current investigation include the use of tumor-derived heat shock protein/protein complexes and dendritic cell therapy. A recent phase II study of heat shock protein/protein complex-96 vaccine in recurrent glioblastomas revealed that it is tolerable, with a suggestion of clinical benefit (published in abstract form) [110]. Dendritic cells are antigen-presenting cells, which when loaded with tumor antigens can stimulate potent cytotoxic T-cell activity, resulting in tumor death. The glioma-associated antigens EphA2, interleukin-13Rα2, and Survivin seem to be potential targets in pediatric brainstem and nonbrainstem glioma vaccine therapy [108]. A recent phase I/II study of a novel vaccination with α-type 1 polarized dendritic cells loaded with synthetic peptides for glioma-associated antigen epitopes and the administration of polyinosinic-polycytidylic acid in human leukocyte antigen-A2 patients with recurrent malignant gliomas demonstrated that it is tolerable, and also seems to exert some clinical activity [111]. Tumor vaccines exhibit great promise in the treatment of brain tumors, and require further clinical trials to assess their therapeutic potential.
Conclusions
The characterization of molecular pathways involved in tumor growth and differentiation has ushered in a new era in cancer therapeutics. As the understanding of the molecular basis of both tumorigenesis and normal brain development and the field of biologically targeted therapeutics continue to evolve, the diagnosis and management of brain tumors may soon be based on molecular stratification, allowing for patient-tailored and tumor-tailored therapy. Although some of the biologic agents tested in pediatric clinical settings have demonstrated promise, the antitumor activity observed in preclinical testing has not yet translated into efficacy in clinical trials. However, the field is young and many of these trials were initiated very recently, and are not yet designed to demonstrate efficacy. In regard to failed efficacy in completed trials, the pursuit of highly specific targeted therapy may be somewhat misguided. Tumorigenesis involves multiple pathways with significant crosstalk between pathways, and to target multiple pathways via broader-ranging agents or combinations of agents may prove necessary. Regarding another barrier to long-term success, most of these novel biologic agents exhibit cytostatic activity, and therefore may need to be combined with conventional cytotoxic chemotherapy and radiotherapy for maximal tumor response and disease control. In addition, these agents are predominantly used in phase I/II trials of patients with recurrent and resistant disease, and additional studies will be needed to evaluate the efficacy and side effects of biologic agents administered upon initial diagnosis.
Several scientific and ethical issues remain to be resolved while moving forward with this line of research, and these issues are continuously discussed and reviewed by neuro-oncologists and safety boards. Although most of the biologic agents are well tolerated in heavily pretreated patients, combination therapy for synergistic action opens up the possibility of more short-term and long-term sequelae, which will need to be carefully studied. With proven treatments offering a 70–80% survival or higher for many brain tumors, the potential risks and benefits of unproven therapy will need to be evaluated carefully and frequently before clinical trials can proceed with agents at the time of diagnosis, to delay toxic therapy. Precedents for this approach exist, such as the multicenter trials in neuro-oncology delaying radiation in infants with medulloblastoma. Pediatric and adult neurologists who treat patients for the long-term neurologic, neurosensory, and neurocognitive sequelae associated with traditional chemotherapy and radiotherapy understand the need for equivalent or better treatments with fewer adverse effects. Moreover, understanding which neurologic and cancer cell growth pathways are targeted may lead to applications of these agents toward other neurologic conditions that affect children (as is already being undertaken with neurofibromatosis and tuberous sclerosis). The number of clinical trials for biologic agents will continue to expand. Pediatric neurologists must be familiar with their use, and can play an important role in guiding parents to seek evaluations from pediatric neurooncology centers offering clinical trials of rationally designed treatments.
Acknowledgments
This work was supported by the Zickler Family Foundation and an anonymous family from Italy.
References
- 1.Central Brain Tumor Registry of the United States. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2004–2007. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2011. Available at: www.cbtrus.org. [Google Scholar]
- 2.Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. JNCI. 2009;101:946–958. doi: 10.1093/jnci/djp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner CD, Rey-Casserly C, Liptak CC, Chordas C. Late effects of therapy for pediatric brain tumor survivors. J Child Neurol. 2009;24:1455–1463. doi: 10.1177/0883073809341709. [DOI] [PubMed] [Google Scholar]
- 4.Warren KE, Packer RJ. Current approaches to CNS tumors in infants and very young children. Expert Rev Neurother. 2004;4:681–690. doi: 10.1586/14737175.4.4.681. [DOI] [PubMed] [Google Scholar]
- 5.Merchant TE, Pollack IF, Loeffler JS. Brain tumors across the age spectrum: Biology, therapy, and late effects. Semin Radiat Oncol. 2010;20:58–66. doi: 10.1016/j.semradonc.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faury D, Nantel A, Dunn SE, et al. Molecular profiling identifies prognostic subgroups of pediatric glioblastoma and shows increased YB-1 expression in tumors. J Clin Oncol. 2007;25:1196–1208. doi: 10.1200/JCO.2006.07.8626. [DOI] [PubMed] [Google Scholar]
- 7.Lo HW. Targeting Ras-Raf-ERK and its interactive pathways as a novel therapy for malignant gliomas. Curr Cancer Drug Targets. 2010;10:840–848. doi: 10.2174/156800910793357970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bont JM, Packer RJ, Michiels EM, den Boer ML, Pieters R. Biological background of pediatric medulloblastoma and ependymoma: A review from a translational research perspective. Neurol Oncol. 2008;10:1040–1060. doi: 10.1215/15228517-2008-059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brantley EC, Benveniste EN. Signal transducer and activator of transcription-3: A molecular hub for signaling pathways in gliomas. Mol Cancer Res. 2008;6:675–684. doi: 10.1158/1541-7786.MCR-07-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiner HL. The role of growth factor receptors in central nervous system development and neoplasia. Neurosurgery. 1995;37:179–194. doi: 10.1227/00006123-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 13.Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology (Bethesda) 2010;25:85–101. doi: 10.1152/physiol.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perona R. Cell signalling: Growth factors and tyrosine kinase receptors. Clin Transl Oncol. 2006;8:77–82. doi: 10.1007/s12094-006-0162-1. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Goderie SK, Temple S. Asymmetric distribution of EGFR receptor during mitosis generates diverse CNS progenitor cells. Neuron. 2005;45:873–886. doi: 10.1016/j.neuron.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 17.Burgess AW. EGFR family: Structure physiology signalling and therapeutic targets. Growth Factors. 2008;26:263–274. doi: 10.1080/08977190802312844. [DOI] [PubMed] [Google Scholar]
- 18.Bax DA, Gaspar N, Little SE, et al. EGFRvIII deletion mutations in pediatric high-grade glioma and response to targeted therapy in pediatric glioma cell lines. Clin Cancer Res. 2009;15:5753–5761. doi: 10.1158/1078-0432.CCR-08-3210. [DOI] [PubMed] [Google Scholar]
- 19.Pollack IF, Hamilton RL, James CD, et al. Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: Results from the Children’s Cancer Group 945 cohort. J Neurosurg. 2006;105(Suppl. 5):418–424. doi: 10.3171/ped.2006.105.5.418. [DOI] [PubMed] [Google Scholar]
- 20.Suri V, Das P, Pathak P, et al. Pediatric glioblastomas: A histopathological and molecular genetic study. Neurol Oncol. 2009;11:274–280. doi: 10.1215/15228517-2008-092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura M, Shimada K, Ishida E, et al. Molecular pathogenesis of pediatric astrocytic tumors. Neurol Oncol. 2007;9:113–123. doi: 10.1215/15228517-2006-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbertson RJ, Hill DA, Hernan R, et al. ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clin Cancer Res. 2003;9:3620–3624. [PubMed] [Google Scholar]
- 23.Gilbertson RJ, Bentley L, Hernan R, et al. ERBB receptor signaling promotes ependymoma cell proliferation and represents a potential novel therapeutic target for this disease. Clin Cancer Res. 2002;8:3054–3064. [PubMed] [Google Scholar]
- 24.Gilbertson RJ, Perry RH, Kelly PJ, Pearson AD, Lunec J. Prognostic significance of HER2 and HER4 coexpression in childhood medulloblastoma. Cancer Res. 1997;57:3272–3280. [PubMed] [Google Scholar]
- 25.Gajjar A, Hernan R, Kocak M. Clinical, histopathologic, and molecular markers of prognosis: Toward a new disease risk stratification system for medulloblastoma. J Clin Oncol. 2004;22:984–993. doi: 10.1200/JCO.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 26.Eller JL, Longo SL, Kyle MM, Bassano D, Hicklin DJ, Canute GW. Antiepidermal growth factor receptor monoclonal antibody cetuximab augments radiation effects in glioblastoma multiforme in vitro and in vivo. Neurosurgery. 2005;56:155–162. doi: 10.1227/01.neu.0000145865.25689.55. [DOI] [PubMed] [Google Scholar]
- 27.Trippett TM, Herzog C, Whitlock JA, et al. Phase I and pharmacokinetic study of cetuximab and irinotecan in children with refractory solid tumors: A study of the Pediatric Oncology Experimental Therapeutic Investigators’ Consortium. J Clin Oncol. 2009;27:5102–5108. doi: 10.1200/JCO.2008.20.8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pillay V, Allaf L, Wilding AL, et al. The plasticity of oncogene addiction: Implications for targeted therapies directed to receptor tyrosine kinases. Neoplasia. 2009;11:448–458. doi: 10.1593/neo.09230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jakacki RI, Hamilton M, Gilbertson RJ, et al. Pediatric phase I and pharmacokinetic study of erlotinib followed by the combination of erlotinib and temozolomide: A Children’s Oncology Group Phase I Consortium study. J Clin Oncol. 2008;26:4921–4927. doi: 10.1200/JCO.2007.15.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geoerger B, Hargrave D, Thomas F, et al. Innovative therapies for children with cancer pediatric phase I study of erlotinib in brainstem glioma and relapsing/refractory brain tumors. Neurol Oncol. 2011;13:109–118. doi: 10.1093/neuonc/noq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollack IF, Stewart CF, Kocak M, et al. A phase II study of gefitinib and irradiation in children with newly diagnosed brainstem gliomas: A report from the Pediatric Brain Tumor Consortium. Neurol Oncol. 2011;13:290–297. doi: 10.1093/neuonc/noq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fouladi M, Stewart CF, Blaney SM, et al. Phase I trial of lapatinib in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28:4221–4227. doi: 10.1200/JCO.2010.28.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumann M, Krause M, Dikomey E, et al. EGFR-targeted anti-cancer drugs in radiotherapy: Preclinical evaluation of mechanisms. Radiother Oncol. 2007;83:238–248. doi: 10.1016/j.radonc.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Schaefer G, Shao L, Totpal K, Akita RW. Erlotinib directly inhibits HER2 kinase activation and downstream signaling events in intact cells lacking epidermal growth factor receptor expression. Cancer Res. 2007;67:1228–1238. doi: 10.1158/0008-5472.CAN-06-3493. [DOI] [PubMed] [Google Scholar]
- 35.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: A dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream ERK1/2 and Akt pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 36.Massimino M, Bode U, Biassoni V, Fleischhack G. Nimotuzumab for pediatric diffuse intrinsic pontine gliomas. Expert Opin Biol Ther. 2011;11:247–256. doi: 10.1517/14712598.2011.546341. [DOI] [PubMed] [Google Scholar]
- 37.He Y, Cai W, Wang L, Chen P. A developmental study on the expression of PDGFalphaR immunoreactive cells in the brain of postnatal rats. Neurosci Res. 2009;65:272–279. doi: 10.1016/j.neures.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, et al. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Ishii Y, Oya T, Zheng L, et al. Mouse brains deficient in neuronal PDGF receptor-β develop normally but are vulnerable to injury. J Neurochem. 2006;98:588–600. doi: 10.1111/j.1471-4159.2006.03922.x. [DOI] [PubMed] [Google Scholar]
- 40.Shih AH, Holland EC. Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett. 2006;232:139–147. doi: 10.1016/j.canlet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Ostman A, Heldin CH. PDGF receptors as targets in tumor treatment. Adv Cancer Res. 2007;97:247–274. doi: 10.1016/S0065-230X(06)97011-0. [DOI] [PubMed] [Google Scholar]
- 42.Zarghooni M, Bartels U, Lee E, et al. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol. 2010;28:1337–1344. doi: 10.1200/JCO.2009.25.5463. [DOI] [PubMed] [Google Scholar]
- 43.Paugh BS, Qu C, Jones C, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010;28:3061–3068. doi: 10.1200/JCO.2009.26.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorarinsdottir HK, Santi M, McCarter R, et al. Protein expression of plateletderived growth factor receptor correlates with malignant histology and PTEN with survival in childhood gliomas. Clin Cancer Res. 2008;14:3386–3394. doi: 10.1158/1078-0432.CCR-07-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blom T, Roselli A, Häyry V, et al. Amplification and overexpression of KIT, PDGFRA, and VEGFR2 in medulloblastomas and primitive neuroectodermal tumors. J Neurooncol. 2010;97:217–224. doi: 10.1007/s11060-009-0014-2. [DOI] [PubMed] [Google Scholar]
- 46.Baruchel S, Sharp JR, Bartels U, et al. A Canadian Paediatric Brain Tumour Consortium (CPBTC) phase II molecularly targeted study of imatinib in recurrent and refractory paediatric central nervous system tumours. Eur J Cancer. 2009;45:2352–2359. doi: 10.1016/j.ejca.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Geoerger B, Morland B, Ndiaye A, et al. Target-driven exploratory study of imatinib mesylate in children with solid malignancies by the Innovative Therapies for Children with Cancer (ITCC) European Consortium. Eur J Cancer. 2009;45:2342–2351. doi: 10.1016/j.ejca.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Pollack IF, Jakacki RI, Blaney SM, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: A Pediatric Brain Tumor Consortium report. Neurol Oncol. 2007;9:145–160. doi: 10.1215/15228517-2006-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bazzoni G. Signalling pathways and adhesion molecules as targets for anti-angiogenesis therapy in tumors. Adv Exp Med Biol. 2008;610:74–87. doi: 10.1007/978-0-387-73898-7_6. [DOI] [PubMed] [Google Scholar]
- 50.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 51.Rosenstein JM, Krum JM. New roles for VEGF in nervous tissue: Beyond blood vessels. Exp Neurol. 2004;187:246–253. doi: 10.1016/j.expneurol.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 52.Li Z, Bao S, Wu Q, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jogi A, Øra I, Nilsson H, et al. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci USA. 2002;99:7021–7026. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Packer RJ, Jakacki R, Horn M, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer. 2009;52:791–795. doi: 10.1002/pbc.21935. [DOI] [PubMed] [Google Scholar]
- 55.Narayana A, Kunnakkat S, Chacko-Mathew J, et al. Bevacizumab in recurrent high-grade pediatric gliomas. Neurol Oncol. 2010;12:985–990. doi: 10.1093/neuonc/noq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gururangan S, Chi SN, Young Poussaint T, et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: A Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28:3069–3075. doi: 10.1200/JCO.2009.26.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma S, Rosen ST. Enzastaurin. Curr Opin Oncol. 2007;19:590–595. doi: 10.1097/CCO.0b013e3282f10a00. [DOI] [PubMed] [Google Scholar]
- 58.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 59.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 60.Pfister S, Janzarik WG, Remke M, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118:1739–1749. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kieran MW, Packer RJ, Onar A, et al. Phase I and pharmacokinetic study of the oral farnesyltransferase inhibitor lonafarnib administered twice daily to pediatric patients with advanced central nervous system tumors using a modified continuous reassessment method: A Pediatric Brain Tumor Consortium study. J Clin Oncol. 2007;25:3137–3143. doi: 10.1200/JCO.2006.09.4243. [DOI] [PubMed] [Google Scholar]
- 62.Fouladi M, Nicholson HS, Zhou T, et al. A phase II study of the farnesyl transferase inhibitor, tipifarnib, in children with recurrent or progressive high-grade glioma, medulloblastoma/primitive neuroectodermal tumor, or brainstem glioma: A Children’s Oncology Group study. Cancer. 2007;110:2535–2541. doi: 10.1002/cncr.23078. [DOI] [PubMed] [Google Scholar]
- 63.Haas-Kogan DA, Banerjee A, Poussaint TY, et al. Phase II trial of tipifarnib and radiation in children with newly diagnosed diffuse intrinsic pontine gliomas. Neurol Oncol. 2011;13:298–306. doi: 10.1093/neuonc/noq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the Raf/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 65.Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol (Berl) 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 66.Gronych J, Korshunov A, Bageritz J, et al. An activated mutant BRAF kinase domain is sufficient to induce pilocytic astrocytoma in mice. J Clin Invest. 2011;121:1344–1348. doi: 10.1172/JCI44656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keir ST, Maris JM, Lock R, et al. Initial testing (stage 1) of the multi-targeted kinase inhibitor sorafenib by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;55:1126–1133. doi: 10.1002/pbc.22712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maris JM, Courtright J, Houghton PJ, et al. Initial testing (stage 1) of sunitinib by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2008;51:42–48. doi: 10.1002/pbc.21535. [DOI] [PubMed] [Google Scholar]
- 69.Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kolb EA, Gorlick R, Houghton PJ, et al. Initial testing (stage 1) of AZD6244 (ARRY-142886) by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;55:668–677. doi: 10.1002/pbc.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng CK, Fan QW, Weiss WA. PI3K signaling in glioma: Animal models and therapeutic challenges. Brain Pathol. 2009;19:112–120. doi: 10.1111/j.1750-3639.2008.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pollack IF, Hamilton RL, Burger PC, et al. Akt activation is a common event in pediatric malignant gliomas and a potential adverse prognostic marker: A report from the Children’s Oncology Group. J Neurooncol. 2010;99:155–163. doi: 10.1007/s11060-010-0297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 74.Fan QW, Knight ZA, Goldenberg DD, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baryawno N, Sveinbjörnsson B, Eksborg S, Chen CS, Kogner P, Johnsen JI. Small-molecule inhibitors of phosphatidylinositol 3-kinase/Akt signaling inhibit Wnt/beta-catenin pathway cross-talk and suppress medulloblastoma growth. Cancer Res. 2010;70:266–276. doi: 10.1158/0008-5472.CAN-09-0578. [DOI] [PubMed] [Google Scholar]
- 76.Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 77.Huber H, Eggert A, Janss AJ, et al. Angiogenic profile of childhood primitive neuroectodermal brain tumours/medulloblastomas. Eur J Cancer. 2001;37:2064–2072. doi: 10.1016/s0959-8049(01)00225-8. [DOI] [PubMed] [Google Scholar]
- 78.Sie M, de Bont ES, Scherpen FJ, Hoving EW, den Dunnen WF. Tumour vasculature and angiogenic profile of paediatric pilocytic astrocytoma: Is it much different from glioblastoma? Neuropathol Appl Neurobiol. 2010;36:636–647. doi: 10.1111/j.1365-2990.2010.01113.x. [DOI] [PubMed] [Google Scholar]
- 79.Herrington B, Kieran MW. Small molecule inhibitors in children with malignant gliomas. Pediatr Blood Cancer. 2009;53:312–317. doi: 10.1002/pbc.21950. [DOI] [PubMed] [Google Scholar]
- 80.Fine HA, Figg WD, Jaeckle K, et al. Phase II trial of the antiangiogenic agent thalidomide in patients with recurrent high-grade gliomas. J Clin Oncol. 2000;18:708–715. doi: 10.1200/JCO.2000.18.4.708. [DOI] [PubMed] [Google Scholar]
- 81.Turner CD, Chi S, Marcus KJ, et al. Phase II study of thalidomide and radiation in children with newly diagnosed brain stem gliomas and glioblastoma multiforme. J Neurooncol. 2007;82:95–101. doi: 10.1007/s11060-006-9251-9. [DOI] [PubMed] [Google Scholar]
- 82.Warren KE, Goldman S, Pollack IF, et al. Phase I trial of lenalidomide in pediatric patients with recurrent, refractory, or progressive primary CNS tumors: Pediatric Brain Tumor Consortium Study PBTC-018. J Clin Oncol. 2011;29:324–329. doi: 10.1200/JCO.2010.31.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Becchetti A, Arcangeli A. Integrins and ion channels in cell migration: Implications for neuronal development, wound healing and metastatic spread. Adv Exp Med Biol. 2010;674:107–123. doi: 10.1007/978-1-4419-6066-5_10. [DOI] [PubMed] [Google Scholar]
- 84.Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem J. 2009;418:491–506. doi: 10.1042/BJ20081948. [DOI] [PubMed] [Google Scholar]
- 85.Teodorczyk M, Martin-Villalba A. Sensing invasion: Cell surface receptors driving spreading of glioblastoma. J Cell Physiol. 2010;222:1–10. doi: 10.1002/jcp.21901. [DOI] [PubMed] [Google Scholar]
- 86.Abdollahi A, Griggs DW, Zieher H, et al. Inhibition of alpha(v)beta3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res. 2005;11:6270–6279. doi: 10.1158/1078-0432.CCR-04-1223. [DOI] [PubMed] [Google Scholar]
- 87.Reardon DA, Nabors LB, Stupp R, Mikkelsen T. Cilengitide: An integrintargeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Invest Drugs. 2008;17:1225–1235. doi: 10.1517/13543784.17.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stupp R, Hegi ME, Neyns B, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:2712–2718. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 89.MacDonald TJ, Stewart CF, Kocak M, et al. Phase I clinical trial of cilengitide in children with refractory brain tumors: Pediatric Brain Tumor Consortium Study PBTC-012. J Clin Oncol. 2008;26:919–924. doi: 10.1200/JCO.2007.14.1812. [DOI] [PubMed] [Google Scholar]
- 90.Thompson MC, Fuller C, Hogg TL, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24:1924–1931. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 91.Ellison DW, Dalton J, Kocak M, et al. Medulloblastoma: Clinicopathological correlates of SHh, Wnt, and non-SHh/Wnt molecular subgroups. Acta Neuropathol (Berl) 2011;121:381–396. doi: 10.1007/s00401-011-0800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Romer JT, Kimura H, Magdaleno S, et al. Suppression of the SHh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−) p53(−/−) mice. Cancer Cell. 2004;6:229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 93.Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: Insights from mouse mutants. Nat Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 94.Ables JL, Breunig JJ, Eisch AJ, Rakic P. Not(ch) just development: Notch signalling in the adult brain. Nat Rev Neurosci. 2011;12:269–283. doi: 10.1038/nrn3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Purow BW, Haque RM, Noel MW, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 96.Shih IM, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res. 2007;67:1879–1882. doi: 10.1158/0008-5472.CAN-06-3958. [DOI] [PubMed] [Google Scholar]
- 97.Marks PA, Xu WS. Histone deacetylase inhibitors: Potential in cancer therapy. J Cell Biochem. 2009;107:600–608. doi: 10.1002/jcb.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Furchert SE, Lanvers-Kaminsky C, Juurgens H, et al. Inhibitors of histone deacetylases as potential therapeutic tools for high-risk embryonal tumors of the nervous system of childhood. Int J Cancer. 2007;120:1787–1794. doi: 10.1002/ijc.22401. [DOI] [PubMed] [Google Scholar]
- 99.Spiller SE, Ditzler SH, Pullar BJ, Olson JM. Response of preclinical medulloblastoma models to combination therapy with 13-cis retinoic acid and suberoylanilide hydroxamic acid (SAHA) J Neurooncol. 2008;87:133–141. doi: 10.1007/s11060-007-9505-1. [DOI] [PubMed] [Google Scholar]
- 100.Yin D, Ong JM, Hu J, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: Effects on gene expression and growth of glioma cells in vitro and in vivo. Clin Cancer Res. 2007;13:1045–1052. doi: 10.1158/1078-0432.CCR-06-1261. [DOI] [PubMed] [Google Scholar]
- 101.Fouladi M, Park JR, Stewart CF, et al. Pediatric phase I trial and pharmacokinetic study of vorinostat: A Children’s Oncology Group phase I consortium report. J Clin Oncol. 2010;28:3623–3629. doi: 10.1200/JCO.2009.25.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wolff JE, Kramm C, Kortmann RD, et al. Valproic acid was well tolerated in heavily pretreated pediatric patients with high-grade glioma. J Neurooncol. 2008;90:309–314. doi: 10.1007/s11060-008-9662-x. [DOI] [PubMed] [Google Scholar]
- 103.Su JM, Li XN, Thompson P, et al. Phase 1 study of valproic acid in pediatric patients with refractory solid or CNS tumors: A Children’s Oncology Group report. Clin Cancer Res. 2011;17:589–597. doi: 10.1158/1078-0432.CCR-10-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Masoudi A, Elopre M, Amini E, et al. Influence of valproic acid on outcome of high-grade gliomas in children. Anticancer Res. 2008;28:2437–2442. [PubMed] [Google Scholar]
- 105.Graham C, Tucker C, Creech J, et al. Evaluation of the antitumor efficacy, pharmacokinetics, and pharmacodynamics of the histone deacetylase inhibitor depsipeptide in childhood cancer models in vivo. Clin Cancer Res. 2006;12:223–234. doi: 10.1158/1078-0432.CCR-05-1225. [DOI] [PubMed] [Google Scholar]
- 106.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: Clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390–4399. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu Z, Shen Z. Targeting the inflammatory pathways to enhance chemotherapy of cancer. Cancer Biol Ther. 2011;12:3. doi: 10.4161/cbt.12.2.15952. [DOI] [PubMed] [Google Scholar]
- 108.Okada H, Low KL, Kohanbash G, McDonald HA, Hamilton RL, Pollack IF. Expression of glioma-associated antigens in pediatric brain stem and non-brain stem gliomas. J Neurooncol. 2008;88:245–250. doi: 10.1007/s11060-008-9566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Curtin JF, King GD, Candolfi M, et al. Combining cytotoxic and immune-mediated gene therapy to treat brain tumors. Curr Top Med Chem. 2005;5:1151–1170. doi: 10.2174/156802605774370856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parsa A, Han CCS, Kivett V, et al. Autologous heat shock protein vaccine (HSPPC-96) for patients with recurrent glioblastoma (GBM): Results of a phase II multicenter clinical trial with immunological assessments. J Clin Oncol. 2011;29(Suppl.):2565. [Google Scholar]
- 111.Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]