Summary
Osmosensing and osmoregulatory processes undertaken in gills of euryhaline fish are coordinated by integrative actions of various signaling molecules/transcriptional factors. Considerable numbers of studies report the hyper- and hypo-osmoregulatory functions of fish gills, by illustrating the process of gill cell remodeling and the modulation of the expression of ion channels/transporters. Comparatively mechanistic information relayed from signal integration to transcriptional regulation in mediating gill cell functions has not yet been elucidated. In this study we demonstrate the functional links from cortisol stimulation, to Akt activation, to the expression of the transcriptional factor, Ostf1. Using the synthetic glucocorticoid receptor agonist, dexamethasone (DEX), Ostf1 expression is found to be activated via glucocorticoid receptor (GR) and mediated by the Akt-GSK3β signaling pathway. Pharmacological experiments using kinase inhibitors reveal that the expression of Ostf1 is negatively regulated by Akt activation. The inhibition of PI3K or Akt activities, by the specific kinase inhibitors (wortmannin, LY294002 or SH6), stimulates Ostf1 expression, while a reduction of GSK3β activity by LiCl reduces Ostf1 expression. Collectively, our report for the first time indicates that DEX can induce Ostf1 via GR, with the involvement of the Akt-GSK3β signaling pathway in primary eel gill cell cultures. The data also suggest that Ostf1 may play different roles in gill cell survival during seawater acclimation.
Key words: Akt signaling, GSK3β, Dexamethasone, Eel gill cell culture, Osmotic stress transcription factor 1
Introduction
Osmosensing is a fundamental process that is known to be mediated by a complex signaling and regulatory mechanism (Wehner et al., 2003). In mammals, most cells are bathed in extracellular fluid with well-controlled osmolarity. Notable exceptions include renal medullary cells and intestinal cells. Under hyperosmotic challenge, numerous studies have highlighted the involvement of the transcription factor, tonicity-responsive enhancer binding protein (TonEBP/NFAT5) to promote the synthesis and uptake of organic osmolytes to protect renal cells from the damaging effects of osmotic stress (Burg et al., 2007). The importance of the transcriptional regulation to intracellular osmotic adjustment has been demonstrated (Han et al., 2004).
In fish, the identification of the Osmotic stress transcription factor 1 (Ostf1) and its early response to hyperosmotic stress, have relayed its role in osmosensing and transcriptional regulation during salinity adaptation (Choi and An, 2008; Fiol and Kültz, 2005; Fiol and Kültz, 2007; Tse et al., 2008). The closest mammalian homolog of Ostf1 is the splice variant of transforming growth factor-β-stimulated clone-22 (TSC-22)/glucocorticoid-induced leucine zipper (GILZ) protein family (Fiol et al., 2007), which is known to be important for the regulation of epithelia sodium channel (ENaC) activity in mammalian kidney epithelial cells (Soundararajan et al., 2005). In view of the sequence similarity to the mammalian GILZ, an assumption of the involvement of the “seawater-adapting hormone”, cortisol, in Ostf1 regulation in fish has been suggested. The effect of the synthetic glucocorticoid receptor agonist, dexamethasone (DEX) on Ostf1 expression levels in primary gill cell culture of tilapia was therefore tested (Fiol et al., 2006), but with no observable effects. However, in a follow-up study on the intraperitoneal injection of cortisol in both freshwater and seawater tilapia, McGuire and co-workers have reported a significant induction in branchial Ostf1 mRNA expression (McGuire et al., 2010). Nevertheless, to date, the transcriptional relationship between cortisol and Ostf1 is not yet clear, although both are known to be involved in hypo-osmoregulation. Moreover, our present understanding of the regulation of Ostf1 expression is mainly conferred by anisosmotic signals. The cross-talking regulation involving osmoregulatory hormones, as well as osmotic stress-elicited signaling molecules, have not been identified.
Different osmoregulatory hormones (i.e. growth hormone, prolactin (PRL), natriuretic peptides and cortisol) and signaling pathways (i.e. mitogen-activated protein kinase (MAPK)) are important in the regulation of hyper- and/or hypo-osmotic adaption. Previous studies in our laboratory using eel gill primary culture have showed the modulatory effects of cortisol, PRL, the insulin-like growth factor-1 (IGF-1) on the expression levels of different ion-transporters (Tse et al., 2007). A recent study has demonstrated that the hyperosmolality-induced signaling molecules, MAPK and myosin light chain kinase (MLCK), were involved in the regulation of osmolyte transporters (Chow and Wong, 2011); however, the study also reported that the activation of MAPK- and MLCK-signaling pathways was not associated to hyperosmolality-activated Ostf1 expression. Nevertheless, other than MAPK and MLCK pathways, a growing body of evidence suggests the involvement of the key survival factor, Akt (protein kinase B) for the protection of mammalian lymphocytes from apoptosis upon hyperosmotic challenge (Bortner et al., 2012). Pisitkun and co-workers have shown that the Akt pathway can be regulated by the osmoregulatory hormone vasopressin (Pisitkun et al., 2008). In view of the importance of cortisol in gill cell remodeling during hyperosmotic acclimation, the observation of hyperosmolality-induced Ostf1 expression (Fiol and Kültz, 2005; Tse et al., 2008; Tse et al., 2012) and cellular apoptosis (Inokuchi and Kaneko, 2012), in this study we have aimed to demonstrate the role of DEX and Akt-glycogen synthase kinase 3β (GSK3β)-signaling on the regulation of Ostf1 expression in primary gill cell culture of Japanese eels.
Materials and Methods
Animals and primary gill cell culture
All animals were housed and handled in accordance with the guidelines and regulations of the Hong Kong Baptist University. Japanese eels (Anguilla japonica) weighing between 500 and 600 grams were reared in fiberglass tanks supplied with charcoal-filtering aerated tap water at 18–20°C under a 12 hour:12 hour L:D photoperiod for a minimum of 3 weeks. For primary gill cell culture preparation, fish were anesthetized in sodium-bicarbonate-buffered 0.1% MS222 (Sigma) and perfused with PBS (pH 7.7) to remove branchial blood cells. The fish was then decapitated and the gill arches were excised and washed. Gill tissues were cut into small fragments and were subjected to two cycles of tryptic digestion (0.5% trypsin + 5.3 mmol l−1 EDTA) (Sigma) for 20 minutes at room temperature in a rotator (300 rpm). Cell suspension was filtered through stainless steel mesh (104 and 73.7 µm, Sigma), washed in PBS and finally resuspended in Leibovitz's L-15 medium (Gibco, Invitrogen) supplemented with 10% fetal bovine serum (FBS, HyClone), 1% penicillin/streptomycin, 0.5% fungizone (Gibco, Invitrogen), and seeded at a density of 2×106 cells cm−2 onto collagen-coated culture plates (Iwaki). The culture was incubated at 22°C in a growth chamber with humidified air.
Experiments on the primary gill cell culture
One day after seeding, each culture well was rinsed with PBS to remove mucous and unattached cells. The attached gill cells were then exposed to isotonic or hypertonic medium. Hypertonic medium was prepared by an addition of 90 mM NaCl (Sigma) to the Leibovitz's L-15 medium (320 mosmol l−1), making its osmolarity to 500 mOsmol l−1. The osmolarity of the prepared medium was measured by a vapour pressure osmometer (Wescor 5500XR). To investigate the effect of DEX on Ostf expression, the cells were subjected to 1 µM DEX, 10 µM RU486 (glucocorticoid receptor antagonist) and/or 10 µM spironolactone (mineralocorticoid receptor antagonist) (Sigma) in isotonic medium (Kelly and Chasiotis, 2011).
In other experiments the cells were treated with either (i) 200 nM wortmannin, (ii) 25 µM LY294002, (iii) 5 µM SH6, or (iv) 15 mM LiCl (Calbiochem) in both iso- and hypertonic media. Total RNA and protein samples were collected after 6 hours of the treatment. Total RNA was extracted by TRIZOL solution (Invitrogen) for the measurement of the transcript levels of several targeted genes using real-time PCR. The protein lysates were extracted by RIPA buffer (50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM EDTA, 1% NP-40 alternative (Calbiochem) and 0.1% SDS) for Western blotting.
Real-time PCR analysis
Total RNA with an A260/A280 ratio of 1.8–2.0 was used. To briefly summarize our experiment, 0.5 µg of total cellular RNA was reverse transcribed by the high capacity RNA-to-cDNA kit (Applied Biosystems). PCR reactions were conducted with the StepOneTM real-time PCR system using power SYBR® green PCR master mix (Applied Biosystems). Verified gene-specific primers for Japanese eel were used for real-time PCR analysis (gapdh-F: 5′-GCGCCAGCCAGAACATCATC-3′; gapdh-R: 5′-CGTTAAGCTCGGGGATGACC-3′; ostf1-F: 5′-TCCGCCAGCTCCTTGATTTG-3′; Ostf1-R: 5′-AGCAGGCAATGGATCTTGTGAA-3′) (Chow et al., 2009; Chow and Wong, 2011). The copy number of the transcripts for each sample was calculated with reference to a parallel amplification of known concentrations of the respective cloned PCR fragments. The occurrence of primer-dimers and secondary products was inspected using melting curve analysis. Our data have indicated that the amplification was specific for each individual set of primers. Control amplification was done either without reverse transcriptase or without RNA. The relative expression ratio of Ostf1/gapdh was calculated in accordance with the method described (Pfaffl, 2001):
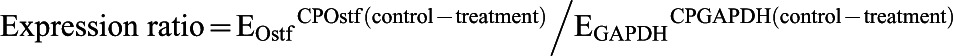 |
where E = 10(−1/slope) and CP is the crossing point at which fluorescence rises above the background.
Western blotting analysis
The protein samples were subjected to electrophoresis in 10% polyacrylamide gels. The gels were then blotted onto PVDF membranes (Bio-Rad). Western blotting was conducted using rabbit polyclonal anti-GilZ/TilZ antibody (1:1000) (Abcam) (Tse et al., 2012), anti-phospho-Akt, anti-total Akt, anti-phospho-GSK3β or anti-total GSK3β (1:1000) (Cell signaling), followed by an incubation with horseradish peroxidase-conjugated goat anti-rabbit antibody (1:4000) (Bio-Rad). Specific bands were visualized using Western Lightening Plus chemiluminescent reagent (PerkinElmer Life Sciences). The blots were next washed in PBS with 0.5% Tween 20 and re-probed with mouse anti-actin serum (1:100; JLA20, Developmental Studies Hybridoma Bank, the University of Iowa, USA).
Statistical analysis
Drug treatments were performed in triplicate in each experiment and every experiment was repeated at least three times. All data are represented as mean±s.e.m. Statistical significance was assessed with Student's t-test or one-way analysis of variance (ANOVA) followed by Duncan's multiple range tests. Groups were considered significantly different if P<0.05.
Results
Effects of the synthetic glucocorticoid dexamethasone (DEX) on Akt-GSK3β signaling and Ostf1 expression
The treatment of the cultured gill cells with 1 µM DEX in the isotonic medium caused an induction of Ostf1 expression (using rabbit polyclonal anti-GilZ/TilZ antibody) as revealed by Western blotting (Fig. 1A). The induction was associated with a transient increase in the level of phosphorylated Akt (p-Akt) but also with a decreased level of phosphorylated GSK3β (p-GSK3β). The observation of the DEX-elicited Ostf1 protein expression was supported by the real-time PCR analysis of Ostf1 transcript level (Fig. 1B). A time-dependent induction of Ostf1 mRNA expression was observed.
Fig. 1. Effects of dexamethasone (DEX) on Ostf1 expression and Akt-GSK3β signaling in Japanese eel primary gill cell cultures.
(A) A representative Western blot shows that DEX treatment for 6 hours induced the Ostf1 protein expression level. A transient increase of Akt and GSK3β phosphorylation was observed followed by a decrease of the phosphorylated levels. (B) Real-time PCR analysis of time-dependent Ostf1 mRNA expression levels in the DEX-treated cells. *P<0.05 compared between the control and the DEX-treated cells.
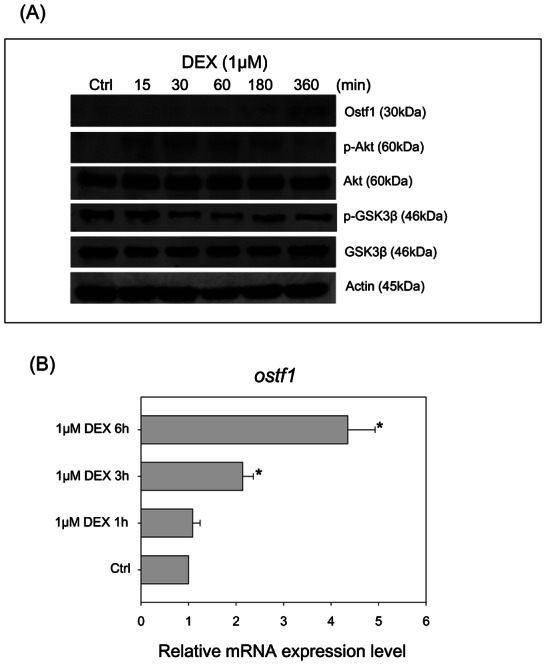
To determine whether the action of DEX on Ostf1 expression was receptor-mediated, a competitive antagonist for glucocorticoid receptor (GR) RU486 was used for the co-treatment study. Fig. 2A indicates that the co-treatment of the cells with RU486 significantly reduces the protein expression levels of DEX-stimulated Ostf1 in the cells. The inhibitory effect was consistently demonstrated in the measurement of mRNA expression levels of Ostf1 (Fig. 2B). The use of a competitive antagonist for mineralocorticoid receptor (MR), spironolactone in DEX co-treatment did not exhibit noticeable effects on DEX-induced Ostf1 expression. The treatment with either antagonist alone had no observable effect on the basal expression level of Ostf1 mRNA. Our data have therefore confirmed that the action of DEX on Ostf1 expression was mediated through the GR.
Fig. 2. Dexamethasone (DEX) induced Ostf1 via glucocorticoid receptor (GR) in Japanese eel primary gill cell culture.
(A) A representative Western blot shows the protein expression levels of Ostf1 in the cells treated with DEX, competitive GR antagonist RU486 or DEX+RU486. (B) Real-time PCR analysis of Ostf1 mRNA expression level in the cells treated with DEX and/or GR antagonist RU486/mineralocorticoid receptor inhibitor, spironolactone. The data indicated that DEX-activated Ostf1 expression was significantly inhibited by RU486. Significant effect of spironolactone on DEX-elicited Ostf1 expression was not detected. *P<0.05 compared between the DEX-treated and the DEX/antagonist co-treated cells.
Effects of Akt-GSK3β signaling on Ostf1 expression
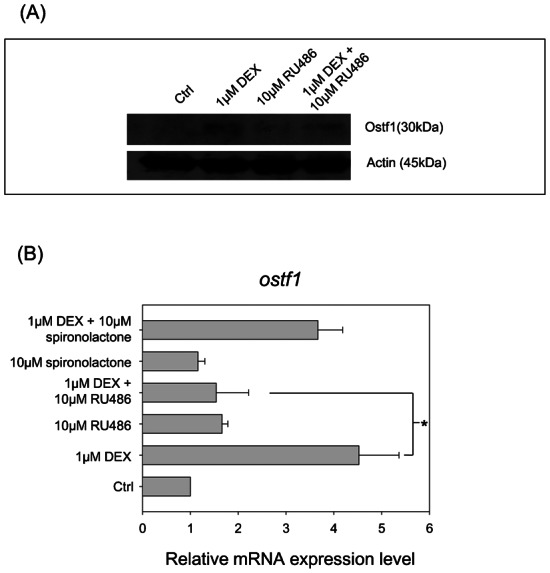
In our previous report of hyperosmotic treatment on primary gill cell cultures, the induction of MAPK and MLCK signaling pathways were found to have no noticeable effects on Ostf1 mRNA expression (Chow and Wong, 2011). As shown in Fig. 1A, the DEX treatment modulated Akt-GSK3β signaling in the gill cell culture. This prompted us to investigate if the hyperosmotic treatment also stimulated the Akt signaling pathway. A representative Western blot showed the effects of hyperosmotic treatment (500 mOsmol l−1) on Ostf1 expression and the Akt-GSK3β-signaling pathway (Fig. 3). The increased Ostf1 protein expression (Fig. 3A) was verified by a time-dependent induction of Ostf1 mRNA expression detected within the 6 hours of the treatment (Fig. 3B). Our data also showed that the treatment provoked a transient increase of Akt phosphorylation, but a consequent decrease of p-GSK3β (Fig. 3A).
Fig. 3. Hyperosmolality (500 mosmol l−1) activated Akt-GSK3β signaling and Ostf1 expression in Japanese eel primary gill cell culture.
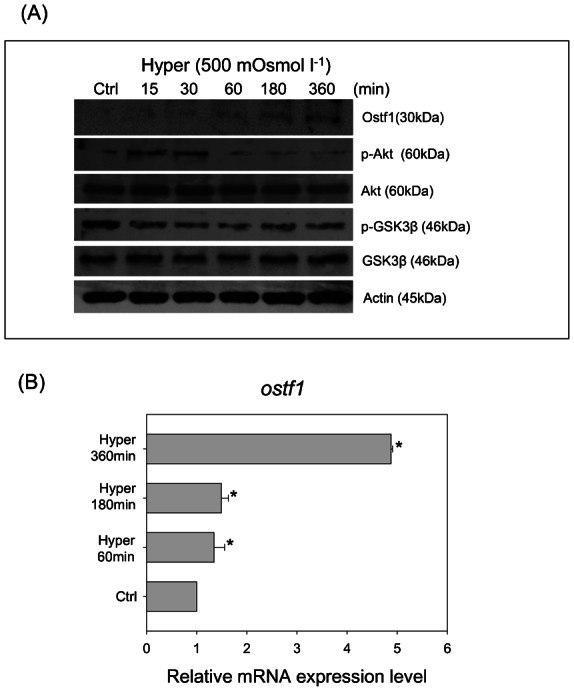
(A) A representative Western blot shows the effects of hyperosmolality on a transient increase of Akt and GSK3β phosphorylation, followed by a decrease of the phosphorylated levels. Ostf1 expression level was increased in a time-dependent manner after hyperosmotic treatment. (B) Real-time PCR analysis revealed the hyperosmolality induced Ostf1 mRNA expression. *P<0.05 compared between the control and the hyperosmolality-treated cells.
To delineate the regulatory function of Akt-GSK3β signaling on Ostf1 expression, four kinase inhibitors (i.e. wortmannin and LY294002 for phosphoinositide-3 kinase (PI3K), SH6 for p-Akt and LiCl for GSK3β) were used to treat the cells in a follow-up study. The suppressive effects of the kinase inhibitors on p-Akt levels were outlined in Fig. 4A, indicating the specific action of the inhibitors. The reduction of p-Akt was found to be inversely related to an increase of Ostf1 protein level (Fig. 4A). The use of the selective GSK3β inhibitor LiCl, increased p-GSK3β levels but reduced Ostf1 expression (Fig. 5A). A reduction of Ostf1 transcript levels was consistently demonstrated in the LiCl treatment (Fig. 5B).
Fig. 4. Effects of the specific kinase inhibitors on Ostf1 expression in Japanese eel primary gill culture.
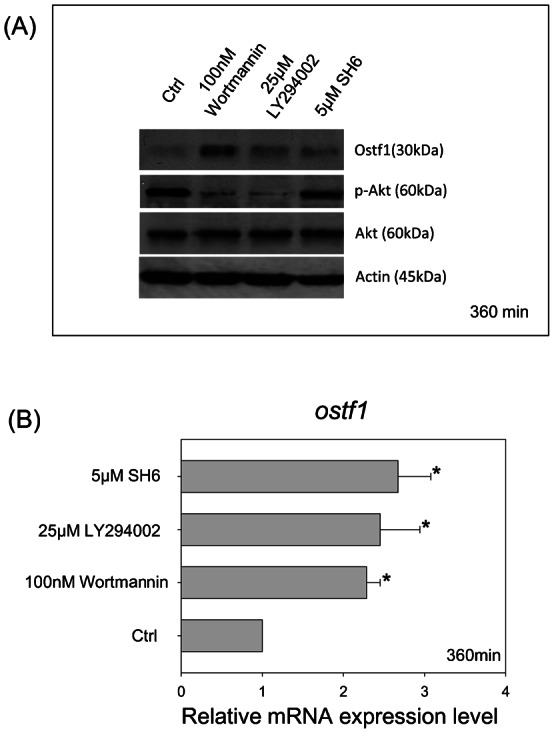
(A) A representative Western blot shows the inhibitory or stimulatory effects of the kinase inhibitors (i.e. wortmannin, LY294002 or SH6), respectively, on Akt phosphorylation or Ostf1 protein expression levels. (B) Real-time PCR analysis revealed the stimulatory effects of the kinase inhibitors on Ostf1 mRNA expression levels. *P<0.05 compared between the control and the kinase inhibitor-treated cells.
Fig. 5. Effects of GSK3β inhibitor, LiCl, on Ostf1 expression in Japanese eel primary gill culture.
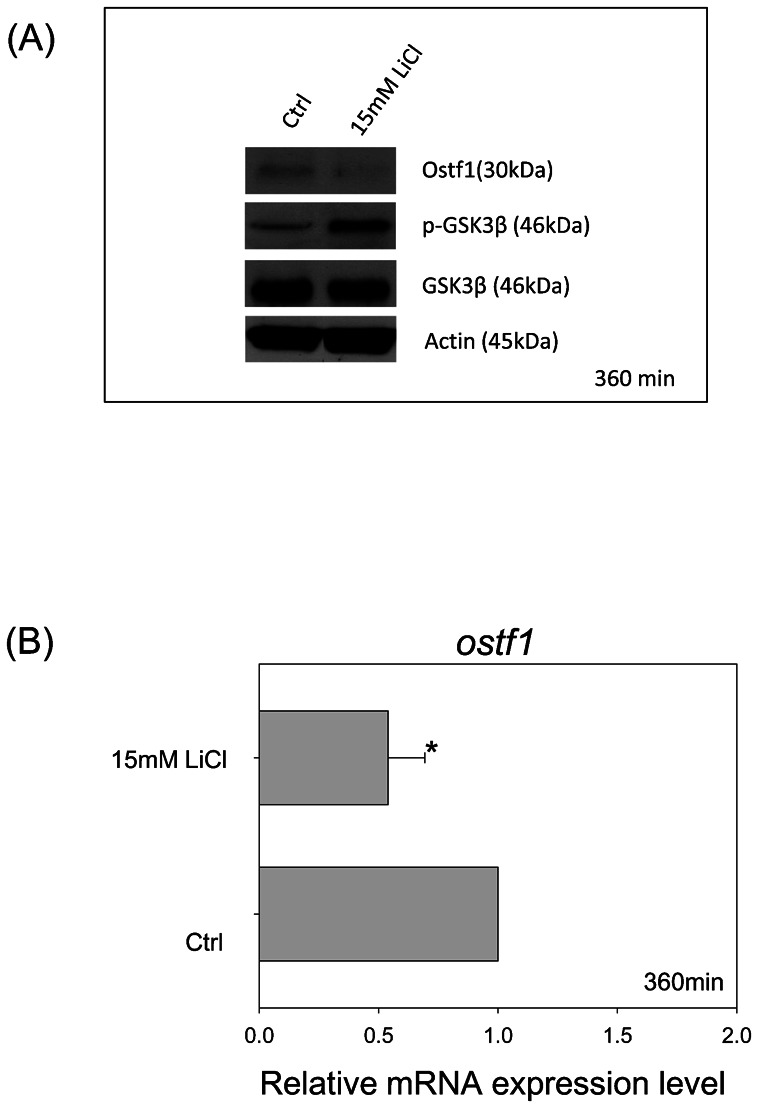
(A) A representative Western blot shows the inhibitory effects of LiCl on GSK3β activity (as shown by an increase in the level of GSK3β phosphorylation) and Ostf1 expression level. (B) Real-time PCR analysis revealed the inhibitory effect of LiCl on Ostf1 mRNA expression level. *P<0.05 compared between the control and the LiCl-treated cells.
Discussion
Hormones are fundamental in mediating osmoregulatory responses against anisosmotic challenges (Wehner et al., 2003; Hoffmann et al., 2007; Hoffmann et al., 2009). In fish, several osmoregulatory hormones such as cortisol, growth hormone and PRL are known to play important roles in mediating osmoregulatory acclimation in waters of different salinities (McCormick and Bradshaw, 2006). Although the general physiological functions of these hormones have been identified, their fundamental mechanistic action to cross-talk with anisosmolality-elicited signaling cascade has not yet been elucidated. A considerable numbers of studies using mammalian cell models have reported the involvement of various signaling molecules in the regulation to combat osmotic stress (Schliess et al., 2007). However, a similar analytic approach of study using the fish model has, to date, been inadequate (Evans, 2010), thereby highlighting the need to discover the empirical mechanism of osmosensing and osmoregulation. In this study using primary gill cell cultures, we have demonstrated for the first time the regulatory relationship from cortisol stimulation to Akt/GSK3β-signaling to the activation of Ostf1 expression.
We have reported an increase of Ostf1 mRNA level after 6 hours of hyperosmotic treatment (500 mOsmol l−1) in the primary gill cell culture, an observation consistent with our previous and other studies (Choi and An, 2008; Fiol and Kültz, 2005; Tse et al., 2008). Using an antibody tagging the closest mammalian ortholog of Ostf1-GILZ protein, hyperosmolality-activated Ostf1 protein levels were consistently demonstrated. The fish Ostf1 and mammalian GILZ both consist of a conserved transforming growth factor-β-stimulated clone-22 domain (TSC-22D). Nine distinct TSC-22D transcript variants have been identified in mammals and are found to be expressed in the murine renal cortex, medulla and papilla (Fiol et al., 2007). In the Japanese eel, the increase of Ostf1 protein expression was found to be localized in gill mitochondrion-rich cells (MRCs) (Tse et al., 2012), which have been identified as the targeted cells regulated by cortisol during seawater acclimation (Wong and Chan, 2001). With the benefit of hindsight, it is reasonable to assume that the fish Ostf1 could be regulated by cortisol.
Given the fact that DEX can bind specifically to GR in fish (Allison and Omeljaniuk, 1998; Arterbery et al., 2011), a synthetic GR agonist DEX was used to treat the gill cell culture. Our results have confirmed a significant increase in the mRNA and protein expression levels of Ostf1. The use of GR antagonist further supports this observation and indicates that the DEX-activated Ostf1 expression was made manifest via GR. It is noteworthy that our data were different from the observation reported by Fiol's group using tilapia gill cell culture, of which the DEX treatment did not affect the expression level of Ostf1 (Fiol et al., 2006). The discrepancy in the effect may be attributed to the variation in the cellular responsiveness between the eel and tilapia gill cell cultures to DEX treatment. Nevertheless, in other experiments using whole animal studies in tilapia, the stimulatory effect of cortisol on gill Ostf1 mRNA expression was observed (McGuire et al., 2010). Taken together, the effects of DEX or hyperosmolality on the activation of Ostf1 expression have been demonstrated, but the signaling cascade involved in the regulation is still not known. Therefore, in the second part of the experiment, we investigated the involvement of Akt-GSK3β signaling pathway in the regulation.
In the primary gill cell culture treated with DEX or exposed to hyperosmolality, accompanied by Ostf1 activation, a transient activation of Akt-signaling was observed. To test this assumption and address the possible involvement of the Akt pathway on Ostf1 expression, we adopted a pharmacological approach to modulate this signaling pathway. It is largely understood that the Akt signaling involves the upstream regulation by PI3K and plays key roles in cell survival (Conery et al., 2004). By applying specific PI3K (i.e. wortmannin, LY294002) (Powis et al., 1994; Gharbi et al., 2007) and Akt (i.e. SH-6) (Kozikowski et al., 2003) inhibitors in the gill cell culture, Ostf1 expression was increased. The observation mirrored the mammalian study showing an upregulation of GILZ expression when the PI3-kinase/Akt pathway was inhibited (Grugan et al., 2008). To confirm our observation, we targeted the Akt downstream molecule GSK3β, which is negatively regulated by greater Akt activity (increased level of p-Akt). Our data showed that the inhibition of GSK3β activity (increased level of p-GSK3β) by LiCl (Oreña et al., 2000), in fact decreased Ostf1 expression.
In the present paper we have shown that hyperosmolality or DEX treatment stimulates Ostf1 expression and modulates the Akt/GSK3β signaling pathway in primary eel gill cell culture. The stimulation was GR dependent and negatively regulated by the Akt signaling pathway. Although Ostf1 is not found in mammals, its closest mammalian homolog (GILZ) has been identified as involved in cell proliferation and apoptosis (Ayroldi and Riccardi, 2009), while the Akt-signaling pathway is known to be fundamental to cell survival. Given that seawater acclimation experiments have induced gill cell remodeling (i.e. cell proliferation and apoptosis) (Inokuchi and Kaneko, 2012; Wong and Chan, 1999), cortisol stimulation of gill MRCs (Wong and Chan, 2001), and yielded findings in the localization of Ostf1 in gill MRCs of seawater acclimating eels (Tse et al., 2012), the possible role of Ostf1 in the regulation of freshwater and seawater MRC population is worthy of further study.
Acknowledgments
This work was supported by the General Research Fund (HKBU 261610), Hong Kong.
Footnotes
Competing interests: The authors have no competing interests to declare.
References
- Allison C. M., Omeljaniuk R. J. (1998). Specific binding sites for [3H]dexamethasone in the hypothalamus of juvenile rainbow trout, Oncorhynchus mykiss. Gen. Comp. Endocrinol. 110, 2–10 10.1006/gcen.1997.7043 [DOI] [PubMed] [Google Scholar]
- Arterbery A. S., Fergus D. J., Fogarty E. A., Mayberry J., Deitcher D. L., Lee Kraus W., Bass A. H. (2011). Evolution of ligand specificity in vertebrate corticosteroid receptors. BMC Evol. Biol. 11, 14 10.1186/1471-2148-11-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayroldi E., Riccardi C. (2009). Glucocorticoid-induced leucine zipper (GILZ): a new important mediator of glucocorticoid action. FASEB J. 23, 3649–3658 10.1096/fj.09-134684 [DOI] [PubMed] [Google Scholar]
- Bortner C. D., Scoltock A. B., Sifre M. I., Cidlowski J. A. (2012). Osmotic stress resistance imparts acquired anti-apoptotic mechanisms in lymphocytes. J. Biol. Chem. 287, 6284–6295 10.1074/jbc.M111.293001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M. B., Ferraris J. D., Dmitrieva N. I. (2007). Cellular response to hyperosmotic stresses. Physiol. Rev. 87, 1441–1474 10.1152/physrev.00056.2006 [DOI] [PubMed] [Google Scholar]
- Choi C. Y., An K. W. (2008). Cloning and expression of Na+/K+-ATPase and osmotic stress transcription factor 1 mRNA in black porgy, Acanthopagrus schlegeli during osmotic stress. Comp. Biochem. Physiol. 149B, 91–100 10.1016/j.cbpb.2007.08.009 [DOI] [PubMed] [Google Scholar]
- Chow S. C., Wong C. K. (2011). Regulatory function of hyperosmotic stress-induced signaling cascades in the expression of transcription factors and osmolyte transporters in freshwater Japanese eel primary gill cell culture. J. Exp. Biol. 214, 1264–1270 10.1242/jeb.050435 [DOI] [PubMed] [Google Scholar]
- Chow S. C., Ching L. Y., Wong A. M., Wong C. K. (2009). Cloning and regulation of expression of the Na+-Cl−-taurine transporter in gill cells of freshwater Japanese eels. J. Exp. Biol. 212, 3205–3210 10.1242/jeb.031302 [DOI] [PubMed] [Google Scholar]
- Conery A. R., Cao Y., Thompson E. A., Townsend C. M., Jr, Ko T. C., Luo K. (2004). Akt interacts directly with Smad3 to regulate the sensitivity to TGF-β-induced apoptosis. Nat. Cell Biol. 6, 366–372 10.1038/ncb1117 [DOI] [PubMed] [Google Scholar]
- Evans T. G. (2010). Co-ordination of osmotic stress responses through osmosensing and signal transduction events in fishes. J. Fish Biol. 76, 1903–1925 10.1111/j.1095-8649.2010.02590.x [DOI] [PubMed] [Google Scholar]
- Fiol D. F., Kültz D. (2005). Rapid hyperosmotic coinduction of two tilapia (Oreochromis mossambicus) transcription factors in gill cells. Proc. Natl. Acad. Sci. USA 102, 927–932 10.1073/pnas.0408956102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiol D. F., Kültz D. (2007). Osmotic stress sensing and signaling in fishes. FEBS J. 274, 5790–5798 10.1111/j.1742-4658.2007.06099.x [DOI] [PubMed] [Google Scholar]
- Fiol D. F., Chan S. Y., Kültz D. (2006). Regulation of osmotic stress transcription factor 1 (Ostf1) in tilapia (Oreochromis mossambicus) gill epithelium during salinity stress. J. Exp. Biol. 209, 3257–3265 10.1242/jeb.02352 [DOI] [PubMed] [Google Scholar]
- Fiol D. F., Mak S. K., Kültz D. (2007). Specific TSC22 domain transcripts are hypertonically induced and alternatively spliced to protect mouse kidney cells during osmotic stress. FEBS J. 274, 109–124 10.1111/j.1742-4658.2006.05569.x [DOI] [PubMed] [Google Scholar]
- Gharbi S. I., Zvelebil M. J., Shuttleworth S. J., Hancox T., Saghir N., Timms J. F., Waterfield M. D. (2007). Exploring the specificity of the PI3K family inhibitor LY294002. Biochem. J. 404, 15–21 10.1042/BJ20061489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grugan K. D., Ma C., Singhal S., Krett N. L., Rosen S. T. (2008). Dual regulation of glucocorticoid-induced leucine zipper (GILZ) by the glucocorticoid receptor and the PI3-kinase/AKT pathways in multiple myeloma. J. Steroid Biochem. Mol. Biol. 110, 244–254 10.1016/j.jsbmb.2007.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K. H., Woo S. K., Kim W. Y., Park S. H., Cha J. H., Kim J., Kwon H. M. (2004). Maturation of TonEBP expression in developing rat kidney. Am. J. Physiol. 287, F878–F885 10.1152/ajprenal.00047.2004 [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Schettino T., Marshall W. S. (2007). The role of volume-sensitive ion transport systems in regulation of epithelial transport. Comp. Biochem. Physiol. 148A, 29–43 10.1016/j.cbpa.2006.11.023 [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Lambert I. H., Pedersen S. F. (2009). Physiology of cell volume regulation in vertebrates. Physiol. Rev. 89, 193–277 10.1152/physrev.00037.2007 [DOI] [PubMed] [Google Scholar]
- Inokuchi M., Kaneko T. (2012). Recruitment and degeneration of mitochondrion-rich cells in the gills of Mozambique tilapia Oreochromis mossambicus during adaptation to a hyperosmotic environment. Comp. Biochem. Physiol. 162A, 245–251 10.1016/j.cbpa.2012.03.018 [DOI] [PubMed] [Google Scholar]
- Kelly S. P., Chasiotis H. (2011). Glucocorticoid and mineralocorticoid receptors regulate paracellular permeability in a primary cultured gill epithelium. J. Exp. Biol. 214, 2308–2318 10.1242/jeb.055962 [DOI] [PubMed] [Google Scholar]
- Kozikowski A. P., Sun H., Brognard J., Dennis P. A. (2003). Novel PI analogues selectively block activation of the pro-survival serine/threonine kinase Akt. J. Am. Chem. Soc. 125, 1144–1145 10.1021/ja0285159 [DOI] [PubMed] [Google Scholar]
- McCormick S. D., Bradshaw D. (2006). Hormonal control of salt and water balance in vertebrates. Gen. Comp. Endocrinol. 147, 3–8 10.1016/j.ygcen.2005.12.009 [DOI] [PubMed] [Google Scholar]
- McGuire A., Aluru N., Takemura A., Weil R., Wilson J. M., Vijayan M. M. (2010). Hyperosmotic shock adaptation by cortisol involves upregulation of branchial osmotic stress transcription factor 1 gene expression in Mozambique Tilapia. Gen. Comp. Endocrinol. 165, 321–329 10.1016/j.ygcen.2009.07.016 [DOI] [PubMed] [Google Scholar]
- Oreña S. J., Torchia A. J., Garofalo R. S. (2000). Inhibition of glycogen-synthase kinase 3 stimulates glycogen synthase and glucose transport by distinct mechanisms in 3T3-L1 adipocytes. J. Biol. Chem. 275, 15765–15772 10.1074/jbc.M910002199 [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisitkun T., Jacob V., Schleicher S. M., Chou C. L., Yu M. J., Knepper M. A. (2008). Akt and ERK1/2 pathways are components of the vasopressin signaling network in rat native IMCD. Am. J. Physiol. 295, F1030–F1043 10.1152/ajprenal.90339.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis G., Bonjouklian R., Berggren M. M., Gallegos A., Abraham R., Ashendel C., Zalkow L., Matter W. F., Dodge J., Grindey G.et al. (1994). Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 54, 2419–2423. [PubMed] [Google Scholar]
- Schliess F., Reinehr R., Häussinger D. (2007). Osmosensing and signaling in the regulation of mammalian cell function. FEBS J. 274, 5799–5803 10.1111/j.1742-4658.2007.06100.x [DOI] [PubMed] [Google Scholar]
- Soundararajan R., Zhang T. T., Wang J., Vandewalle A., Pearce D. (2005). A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. J. Biol. Chem. 280, 39970–39981 10.1074/jbc.M508658200 [DOI] [PubMed] [Google Scholar]
- Tse W. K., Au D. W., Wong C. K. (2007). Effect of osmotic shrinkage and hormones on the expression of Na+/H+ exchanger-1, Na+/K+/2Cl− cotransporter and Na+/K+-ATPase in gill pavement cells of freshwater adapted Japanese eel, Anguilla japonica. J. Exp. Biol. 210, 2113–2120 10.1242/jeb.004101 [DOI] [PubMed] [Google Scholar]
- Tse W. K., Chow S. C., Wong C. K. (2008). The cloning of eel osmotic stress transcription factor and the regulation of its expression in primary gill cell culture. J. Exp. Biol. 211, 1964–1968 10.1242/jeb.017368 [DOI] [PubMed] [Google Scholar]
- Tse W. K., Chow S. C., Wong C. K. (2012). Eel osmotic stress transcriptional factor 1 (Ostf1) is highly expressed in gill mitochondria-rich cells, where ERK phosphorylated. Front. Zool. 9, 3 10.1186/1742-9994-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner F., Olsen H., Tinel H., Kinne–Saffran E., Kinne R. K. (2003). Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev. Physiol. Biochem. Pharmacol. 148, 1–80 10.1007/s10254-003-0009-x [DOI] [PubMed] [Google Scholar]
- Wong C. K., Chan D. K. (1999). Chloride cell subtypes in the gill epithelium of Japanese eel Anguilla japonica. Am. J. Physiol. 277, R517–R522. [DOI] [PubMed] [Google Scholar]
- Wong C. K., Chan D. K. (2001). Effects of cortisol on chloride cells in the gill epithelium of Japanese eel, Anguilla japonica. J. Endocrinol. 168, 185–192 10.1677/joe.0.1680185 [DOI] [PubMed] [Google Scholar]


