Abstract
OBJECTIVE:
Patients with stage I non-small cell lung cancer who have undergone complete surgical resection harbor a 30% risk for tumor recurrence. Thus, the identification of factors that are predictive for tumor recurrence is urgently needed. The aim of this study was to test the prognostic value of serum albumin levels on tumor recurrence in patients with stage I non-small cell lung cancer.
METHODS:
Stage I non-small cell lung cancer patients who underwent complete surgical resection of the primary tumor at Zhejiang Hospital were analyzed in this study. Serum albumin levels were measured before surgery and once again after surgery in 101 histologically diagnosed non-small cell lung cancer patients. Correlations between the pre- and post-operative serum albumin levels and various clinical demographics and recurrence-free survival rates were analyzed.
RESULTS:
Patients with pre-operative hypoalbuminemia (<3.5 g/dl) had a significantly worse survival rate than patients with normal pre-operative serum albumin levels (≥3.5 g/dl) (p = 0.008). Patients with post-operative hypoalbuminemia had a worse survival rate when compared with patients with normal post-operative serum albumin levels (p = 0.001). Cox multivariate analysis identified pre-operative hypoalbuminemia, post-operative hypoalbuminemia and tumor size over 3 cm as independent negative prognostic factors for recurrence.
CONCLUSION:
Serum albumin levels appear to be a significant independent prognostic factor for tumor recurrence in patients with stage I non-small cell lung cancer who have undergone complete resection. Patient pre-treatment and post-treatment serum albumin levels provide an easy and early means of discrimination between patients with a higher risk for recurrence and patients with a low risk of recurrence.
Keywords: Prognostic Impact, Recurrence-Free Survival, Stage I Non-Small Cell Lung Cancer, Serum Albumin Level
INTRODUCTION
According to global cancer statistics, 10.9 million new cancer cases are diagnosed each year, of which 1.35 million are lung cancers. Approximately 6.7 million patients die of cancer every year, and lung cancers are responsible for 1.18 million deaths annually (1). In 2012, lung cancer was the most common cause of malignancy-related mortality worldwide, and non-small cell lung cancer (NSCLC) accounted for nearly 80% of all lung cancer deaths (2). Stage I tumors have become more easily detected due to recent advances in diagnostic techniques (3). However, despite the success of surgical resection, more than one-third of patients with stage I NSCLC die of disease recurrence within 5 years of tumor resection (4). In addition, while adjuvant chemotherapy administered after resection of stage II-IIIA NSCLC is the standard of care, the survival benefit of adjuvant chemotherapy for stage I NSCLC patients remains controversial (5–8). Despite the high recurrence rate, patients with stage I NSCLC are not routinely recommended to undergo adjuvant chemotherapy outside of clinical trials.
Based on the uncertainty regarding the value of adjuvant chemotherapy treatment for stage I NSCLC patients after undergoing surgical resection, risk factors for tumor recurrence must be identified to guide patient management. The identification of risk factors for recurrence in stage I NSCLC patients who have undergone resection may provide a more appropriate estimation of individual outcomes and allow the optimization of patient stratification in clinical trials, leading to more meaningful assessments in these studies. The 7th edition of the TNM classification (TNM7) for lung cancer was published in 2009 (9). Until recently, few studies investigated patient survival and patterns of recurrence in stage I NSCLC patients who have undergone resection (TNM7) (10–14).
Malnutrition commonly occurs in cancer patients who are receiving treatment. Serum albumin is an objective parameter that closely correlates with the degree of malnutrition and is a regularly used biomarker for long-term nutrition status (15,16). Albumin is produced by the liver and maintains intravascular oncotic pressure, facilitates transport of substances and acts as a free radical scavenger. In adult humans, the normal range of serum albumin is 3.5–5.0 g/dl, and patients with serum albumin levels <3.5 g/dl are considered to be hypoalbuminemic (17,18). Accumulating data has highlighted a correlation between hypoalbuminemia and survival in cancer patients. A prospective study conducted by the British United Provident Association demonstrated that a decline in serum albumin levels could be a manifestation of the disease process of preclinical cancer (19). In other cohort studies, investigators have reported additional examples of a prognostic role for serum albumin levels in various kinds of malignancies. Hypoalbuminemia has been reported as a negative prognostic factor for survival in colorectal carcinoma (20,21), gastric carcinoma (22), and breast cancer (23), among other types of cancer.
Espinosa E et al. (24) also reported that in patients with advanced NSCLC, low serum albumin levels correlate with poor survival. However, to the best of our knowledge, previous studies have not explored the prognostic value of serum albumin levels on tumor recurrence in stage I NSCLC patients who have undergone complete resection.
In this study, for the first time, we investigated the predictive value of serum albumin for tumor recurrence in stage I NSCLC patients who have undergone complete resection. Pre-operative and post-operative serum albumin levels were measured, and recurrence-free survival (RFS) was considered to be the primary endpoint.
PATIENTS AND METHODS
Inclusion criteria and enrollment
We conducted a retrospective study using in-patient medical records of patients with stage I NSCLC who were treated at Zhejiang Hospital in China. Our study protocol was approved by the Ethics Committee of Zhejiang Hospital, and all experiments were conducted in accordance with the Declaration of Helsinki. The requirement for informed consent was waived by the committee, as our study was a retrospective review of patient records.
The records of 256 patients with stage I NSCLC admitted to Zhejiang Hospital between January 2002 and December 2011 were reviewed based on the following inclusion criteria: (i) patient was pathologically diagnosed with stage I NSCLC (TNM 7); (ii) patient underwent pulmonary lobectomy and systematic lymph node dissection; (iii) patient displayed good performance status (ECOG score≤2); (iv) patient displayed normal renal, cardiac and liver functions; (v) patient had complete pre-operative and post-operative serum albumin level records; and (vi) patient had complete follow-up data. Exclusion criteria consisted of the following: (i) patient underwent neoadjuvant chemotherapy or radiation therapy; (ii) patient underwent adjuvant chemotherapy or radiation after the initial surgical resection; (iii) patient post-operative death occurred within 30 days of surgery; and (iv) patient death was not caused by cancer progression.
Definitions
Local recurrence was defined as tumor recurrence in contiguous anatomical sites, including the ipsilateral hemithorax and mediastinum, after the initial surgical resection. Distant recurrence was defined as tumor recurrence in the contralateral lung or outside of the hemithorax and mediastinum after the initial surgical resection. Cancer recurrence was confirmed histologically or radiographically. Pattern of recurrence was defined as all recurrences discovered between the initial operation and death or the final follow-up on record. RFS was defined as the duration from the date of surgical resection to the date of the first recurrence. Patients without recurrence at the last follow-up or death were classified as censored observations. Body mass index (BMI) was calculated as weight (kg)/height2 (m2). Patients with a BMI<20 are considered to be underweight; therefore, the BMI cut-off value was set at 20.
Serum albumin was estimated by employing the optimized standard method recommended by Peters T Jr. (25). Serum albumin levels ≥3.5 g/dl were considered to be normal because this value corresponds to the lower extreme range that is observed in a normal population using the methods described above. Patients with serum albumin levels <3.5 g/dl were considered to be hypoalbuminemic.
Follow-up
Post-operatively, patients were examined at 3-month intervals for the first year, at 6-month intervals for the second year and annually thereafter on an outpatient basis. The follow-up evaluation included a physical examination, chest radiography and blood analysis. Whenever symptoms or signs of recurrence were detected, further evaluations were performed, which included computed tomography scans of the chest and abdomen, brain magnetic resonance imaging and bone scintigraphy. All patients were monitored through March 2012.
Statistical Analysis
Statistical analysis was performed using the SPSS 17.0 software package. Survival rates were analyzed using the Kaplan–Meier method and were compared using the log-rank test. P-values for differences between groups of patients were calculated using Fisher's exact test (two-tailed). Correlations between serum albumin levels and different variables were assessed using the product moment correlation coefficient (r). Univariate and multivariate analyses were performed using the Cox proportion-hazards model. Statistical significance was defined as p<0.05.
RESULTS
Descriptive characteristics
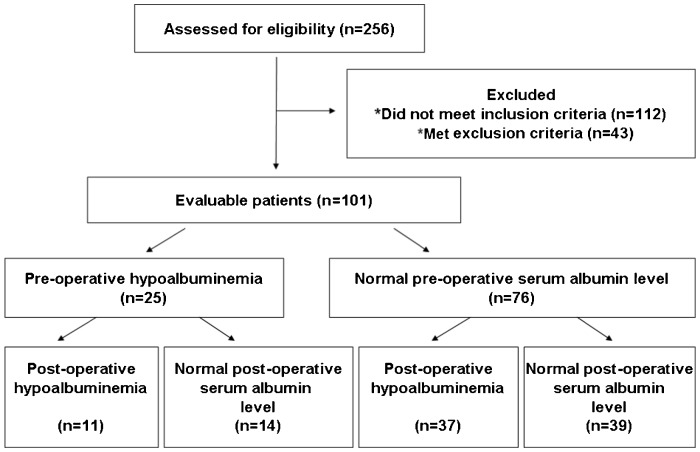
We analyzed data from 101 stage I NSCLC patients (Figure 1). All patients were of Chinese ethnicity, and most of the patients were male (78.2%). The mean age of the patients was 62.6±1.37 years (ranging from 31 to 83 years). Approximately two-thirds of the patients had a history of smoking. Approximately half of the patients were histologically diagnosed with adenocarcinoma, and 37 (36.6%) patients were diagnosed with squamous cell carcinoma.
Figure 1.
Enrollment procedure in the study.
Twenty-five (24.8%) patients presented with pre-operative hypoalbuminemia. Gender, age, ECOG score, BMI, smoking history, histological type, and tumor grade did not significantly influence the pretreatment serum albumin levels observed in the patients. The presence of hypoalbuminemia significantly correlated with larger tumor size (p = 0.026, r = −0.221) and visceral pleural invasion (p<0.001, r = 0.385). Serum albumin levels did not correlate with the presence of lymphatic permeation and intratumoral vascular invasion (Table 1).
Table 1.
Associations between clinicopathological characteristics and hypoalbuminemia in 101 stage I NSCLC patients who underwent complete surgical resection.
| No. of cases | Hypoalbuminemia | % | r-value | p-value*) | |
| Gender | |||||
| Male | 79 | 23 | 29.1 | 0.192 | 0.055 |
| Female | 22 | 2 | 9.1 | ||
| Age | |||||
| <65 years | 33 | 7 | 21.2 | 0.057 | 0.568 |
| ≥65 years | 68 | 18 | 26.5 | ||
| ECOG | |||||
| 0 or 1 | 80 | 17 | 21.3 | 0.158 | 0.114 |
| 2 | 21 | 8 | 38.1 | ||
| BMI (kg/m2) | |||||
| <20 | 29 | 7 | 24.1 | −0.009 | 0.929 |
| ≥20 | 72 | 18 | 25.0 | ||
| Smoking history | |||||
| Smoker | 62 | 19 | 30.6 | 0.172 | 0.085 |
| Non-smoker | 39 | 6 | 15.4 | ||
| Tumor size | |||||
| ≤2.0cm | 29 | 3 | 10.3 | −0.221 | 0.026 |
| >2.0cm ≤3.0cm | 31 | 8 | 25.8 | ||
| >3.0cm | 41 | 14 | 34.1 | ||
| Visceral pleural invasion | |||||
| Present | 21 | 12 | 57.1 | 0.385 | <0.001 |
| Absent | 80 | 13 | 16.3 | ||
| Lymphatic permeation | |||||
| Present | 19 | 6 | 31.6 | 0.076 | 0.449 |
| Absent | 82 | 19 | 23.2 | ||
| Intratumoral vascular invasion | |||||
| Present | 33 | 12 | 36.4 | 0.187 | 0.061 |
| Absent | 68 | 13 | 19.1 | ||
| Histological type | |||||
| Squamous cell carcinoma | 37 | 10 | 27.0 | 0.041 | 0.686 |
| Adenocarcinoma | 55 | 13 | 23.6 | ||
| Other | 9 | 2 | 22.2 | ||
| Tumor grade | |||||
| G1 | 26 | 7 | 26.9 | 0.015 | 0.880 |
| G2 | 47 | 11 | 23.4 | ||
| G3 | 28 | 7 | 25.0 |
Associations between clinicopathological characteristics and hypoalbuminemia were assessed using the product moment correlation coefficient (r). P-values<0.05 were defined as indicative of statistical significance.
Correlation between serum albumin and RFS
The median follow-up was 48 months (ranging from 3 to 120 months). Twenty-two documented recurrences (21.8%) were observed, with a median time to recurrence of 24 months (ranging from 3 to 64 months). Eleven (45.8%) of the recurrences were distant, 10 (41.7%) of the recurrences were locoregional and 3 (12.5%) were distant and locoregional.
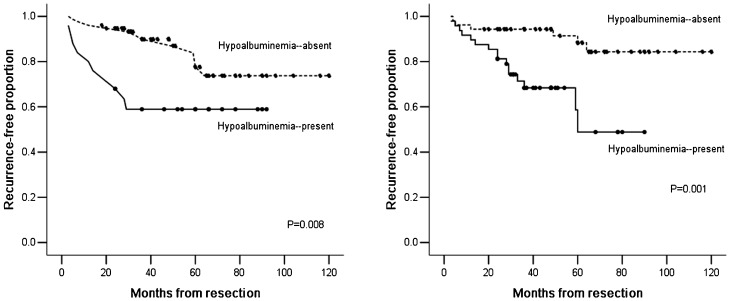
The mean RFS in patients with a normal pre-operative serum albumin level was significantly longer than the mean RFS in patients with pre-operative hypoalbuminemia (100.2±5.1, 95%CI: 90.1–110.2 vs. 60.5±7.8, 95%CI: 45.3–75.7, p = 0.008). The 5-year recurrence-free proportion (RFP) for patients with normal pre-operative serum albumin levels was significantly higher than the 5-year RFP in patients with pre-operative hypoalbuminemia (85% vs. 59%) (Figure 2A).
Figure 2.
A) Kaplan-Meier recurrence-free survival curves for 101 patients with stage I NSCLC are shown according to pre-operative serum albumin levels (p = 0.008). B) Kaplan-Meier recurrence-free survival curves for 101 patients with stage I NSCLC are shown according to post-operative serum albumin levels (p = 0.001).
Similar results were obtained regarding the relationship between post-operative serum albumin levels and RFS. The mean RFS in patients with normal post-operative serum albumin levels was significantly longer than the mean RFS in patients with post-operative hypoalbuminemia (107.4±4.8, 95%CI: 98.0–116.8 vs. 62.1±5.6, 95%CI: 51.1–73.0, p = 0.001). The 5-year RFP for patients with normal post-operative serum albumin levels was significantly higher than the 5-year RFP for patients with post-operative hypoalbuminemia (91% vs. 62%) (Figure 2B).
Patients were divided into four subgroups according to pre-surgical and post-surgical serum albumin levels: 1) pre-treatment hypoalbuminemia and post-treatment hypoalbuminemia (11 patients); 2) normal pre-treatment serum albumin levels and post-treatment hypoalbuminemia (37 patients); 3) pre-treatment hypoalbuminemia and normal post-treatment serum albumin levels (14 patients); and 4) normal pre-treatment serum albumin levels and normal post-treatment serum albumin levels (39 patients). The mean RFS durations for these four subgroups were 31.2, 80, 69.4 and 108.4 months, respectively (p<0.001).
Univariate and multivariate analysis of factors associated with prognosis
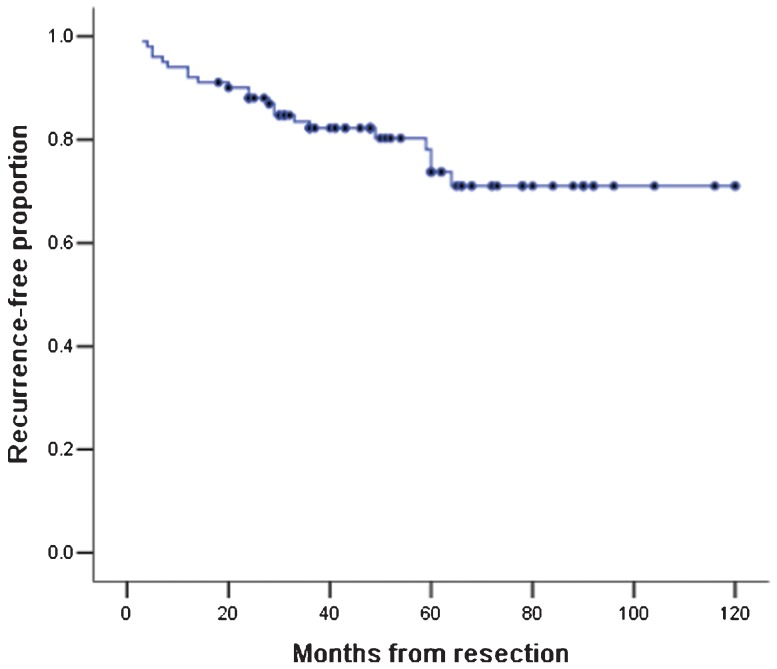
The median follow-up period was 49 months (ranging from 3 to 120 months). RFS for the entire cohort is shown in Fig. 3). Univariate analysis identified 5 significant risk factors for recurrence: large tumor size (≥3 cm), visceral pleural invasion, intratumoral vascular invasion, pre-operative hypoalbuminemia, and post-operative hypoalbuminemia (Table 2). Multivariate analysis using the Cox regression model indicated that pre-operative hypoalbuminemia (HR = 0.361, p = 0.028), post-operative hypoalbuminemia (HR = 0.221, p = 0.005) and large tumor size (HR = 2.059, p = 0.038) were independent prognostic factors for tumor recurrence (Table 3). Among the factors identified as significant predictors of tumor recurrence, post-operative hypoalbuminemia carried the highest degree of significance.
Figure 3.
Kaplan-Meier recurrence-free survival curve for 101 patients with stage I NSCLC.
Table 2.
Univariate analysis of risk factors for recurrence.
| Factors | No. of patients | Recurrence-free proportion at 5 years (%) | p-value*) | |
| Gender | ||||
| Male | 79 | 76 | 0.153 | |
| Female | 22 | 85 | ||
| Age | ||||
| <65 years | 33 | 88 | 0.175 | |
| ≥65 years | 68 | 74 | ||
| ECOG | ||||
| 0 or 1 | 80 | 81 | 0.365 | |
| 2 | 21 | 68 | ||
| BMI (kg/m2) | ||||
| <20 | 29 | 77 | 0.618 | |
| ≥20 | 72 | 82 | ||
| Smoking history | ||||
| Smoker | 62 | 76 | 0.223 | |
| Non-smoker | 39 | 82 | ||
| Tumor size | ||||
| ≤2.0cm | 29 | 93 | 0.012 | |
| >2.0cm≤3.0cm | 31 | 78 | ||
| >3.0cm | 41 | 68 | ||
| Visceral pleural invasion | ||||
| Present | 21 | 61 | 0.006 | |
| Absent | 80 | 83 | ||
| Lymphatic permeation | ||||
| Present | 19 | 77 | 0.943 | |
| Absent | 82 | 84 | ||
| Intratumoral vascular invasion | ||||
| Present | 33 | 70 | 0.027 | |
| Absent | 68 | 83 | ||
| Histological type | ||||
| Squamous cell carcinoma | 37 | 75 | 0.521 | |
| Adenocarcinoma | 55 | 79 | ||
| Other | 9 | 86 | ||
| Tumor grade | ||||
| G1 | 26 | 75 | 0.244 | |
| G2 | 47 | 78 | ||
| G3 | 28 | 88 | ||
| Pre-operative hypoalbuminemia | ||||
| Present | 25 | 59 | 0.008 | |
| Absent | 76 | 85 | ||
| Post-operative hypoalbuminemia | ||||
| Present | 48 | 62 | 0.001 | |
| Absent | 53 | 91 |
p<0.05 was defined as indicative of statistical significance.
Table 3.
Multivariate analysis of risk factors for recurrence.
| Factors | Unfavorable | Favorable | Hazard ratio (95%CI) | p-value*) |
| Tumor size | ≤2.0cm | >3.0 cm | 2.059 (1.042–4.068) | 0.038 |
| Visceral pleural invasion | Present | Absent | 0.492 (0.187–1.294) | 0.151 |
| Intratumoral vascular invasion | Present | Absent | 0.715 (0.277–1.841) | 0.486 |
| Pre-operative hypoalbuminemia | Present | Absent | 0.361 (0.146–0.894) | 0.028 |
| Post-operative hypoalbuminemia | Present | Absent | 0.221 (0.077–0.634) | 0.005 |
p<0.05 was defined as indicative of statistical significance.
95%CI: 95% confidence interval.
Subgroup analysis combining serum albumin levels and other risk factors
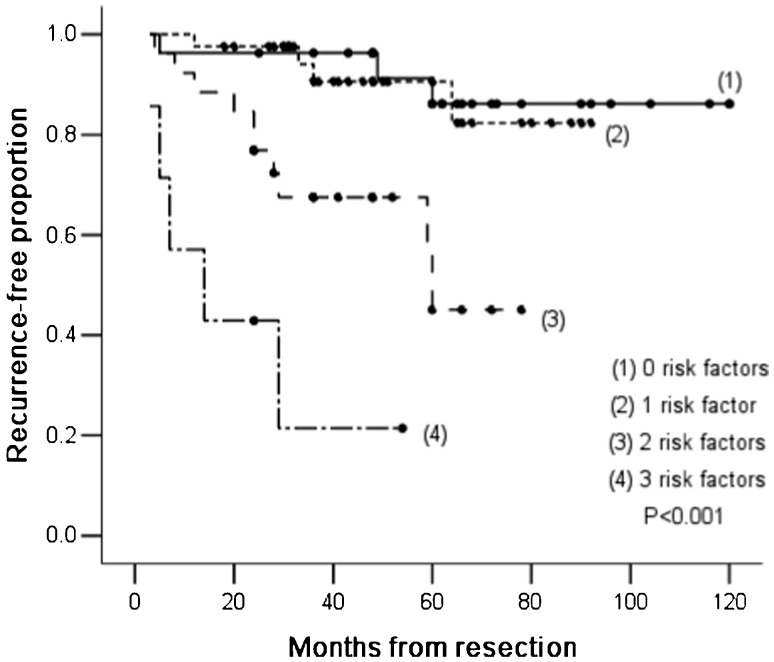
Patients were divided into four subgroups according to pre-operative serum albumin levels, post-operative serum albumin levels and tumor size in the following manner: 1) patients with 0 risks (27 patients); 2) patients with 1 risk (41 patients); 3) patients with 2 risks (26 patients); and 4) patients with 3 risks (7 patients). The mean RFS durations for these four subgroups were 109.1±5.9 (0 risks, 95%CI: 97.4–120.7), 83.7±3.8 (1 risk, 95%CI: 76.2–91.2), 54.7±5.6 (2 risks, 95%CI: 43.7–65.7) and 21.9±7.5 (3 risks, 95%CI: 7.2–36.7) months (p<0.001). The median overall survival (OS) durations were 26.5 (0 risks), 21.0 (1 risk), 15.0 (2 risks) and 8.5 months (3 risks) (p<0.001). The 5-year RFPs for the four subgroups were 92% (0 risks), 91% (1 risk), 59% (2 risks) and 26% (3 risks) (Figure 4).
Figure 4.
Kaplan-Meier recurrence-free survival curves are shown according to the number of independent risk factors.
DISCUSSION
Surgical resection remains the most effective treatment for patients with early-stage NSCLC. However, the reported 5-year survival rates of patients with stage I disease are between 60% and 90% (26,27). The rate of recurrence in patients with stage I NSCLC suggests that systemic tumor cell dissemination occurs before tumor resection but remains undetectable by current clinical techniques (28). Based on the high frequency of recurrence in patients with stage I NSCLC, which is presumed to be a localized disease, several clinical trials have been conducted to analyze the benefit of adjuvant chemotherapy for stage I NSCLC patients who have undergone complete resection, although the results are conflicting. A retrospective analysis performed by the Cancer and Leukemia Group B (CALGB) 9633, which randomly assigned patients with stage IB NSCLC to receive adjuvant chemotherapy or no additional treatment, demonstrated a possible benefit for patients with tumors larger than 4 cm but not for patients with smaller primary tumors (29). Meta-analysis of the Lung Adjuvant Cisplatin Evaluation (LACE) suggested a poorer outcome for patients with stage IA tumors who received adjuvant chemotherapy when compared with patients with stage IA tumors who did not receive treatment (30). Thus, the current standard of care for stage I NSCLC patients who have undergone resection remains post-operative surveillance regardless of the possibility of occult “micrometastatic” disease. If appropriate methods were available to determine which stage I NSCLC patients would benefit from post-operative chemotherapy, then patients with an increased risk for recurrence could be selected for therapy and would be expected to experience substantial clinical benefit from adjuvant systemic chemotherapy. Therefore, methods to definitively select stage I NSCLC patients are urgently needed to help identify patients that are more likely to relapse.
Serum albumin is the most abundant protein in human blood plasma and constitutes approximately half of the blood serum protein. Albumin transports hormones, fatty acids and other compounds; buffers blood pH; and maintains osmotic pressure among other functions. Serum albumin provides a simple method for estimating visceral protein function. Malnutrition and inflammation suppress albumin synthesis (31). In the hospital setting, serum albumin is used to assess patient nutritional status, disease severity, disease progression and prognosis. Serum albumin has also been described as an independent prognostic factor for survival in various malignancies, such as colorectal, gastric, breast and lung cancers (20–24).
Until recently, the clinical application of serum albumin in the context of tumor recurrence in stage I NSCLC patients who have undergone complete resection has rarely been studied. This study is the first to report the prognostic value of serum albumin levels in stage I NSCLC patients who have undergone complete resection.
Remarkably, the presence of pre-operative hypoalbuminemia significantly correlated with larger tumor size and visceral pleural invasion. Similarly, Lohsiriwat et al. (32) reported that in patients with rectal cancer who underwent surgical excision, hypoalbuminemia correlated with larger tumor size. Asher V et al. also demonstrated that pre-operative hypoalbuminemia was associated with larger tumor size in ovarian cancer patients (33). A mechanistic explanation for these associations remains to be determined. One possible explanation is that large tumors and visceral pleural invasion cause more cancer-related symptoms, which leads to poor food intake and protein loss through pleural effusion. However, further studies are required to clarify the mechanism between tumor recurrence and serum albumin levels.
Because surgery is the standard treatment for stage I NSCLC, we found that both pre-operative and post-operative serum albumin levels could serve as markers that reflect the risk of recurrence after complete resection in patients with stage I NSCLC. The results of our study revealed that the 5-year RFP for stage I NSCLC patients with normal pre-operative serum albumin levels was significantly higher than the 5-year RFP for stage I NSCLC patients with hypoalbuminemia. Similar results were obtained when comparing post-operative serum albumin levels and RFP. Remarkably, patients with normal pre-operative and post-operative serum albumin levels had the most favorable prognoses. In addition, patients with both pre-operative hypoalbuminemia and post-operative hypoalbuminemia had worse prognoses. Using the Cox proportional-hazards regression model for the multivariate analysis, pre-operative and post-operative serum albumin retained significance as independent prognostic factors for RFS and tumor size. Several studies have demonstrated the prognostic value of serum albumin on the OS of patients with NSCLC. In a study analyzing prognostic factors in patients with potentially curable lung cancer, Win T et al. (34) reported that hypoalbuminemia is associated with poor OS. Another study analyzing prognostic factors in patients with advanced NSCLC conducted by the Okayama Lung Cancer Study Group also revealed that the serum albumin level is an independent prognostic factor (35). In addition, Fujii T et al. (36) reported that serum albumin levels could be used for predicting short-term recurrence in patients with operable colorectal cancer. Although the association between hypoalbuminemia and tumor progression remains unclear, this association may be partially explained by the fact that tumors interact directly and indirectly with host inflammatory cells. As part of the systemic inflammatory response to the tumor, pro-inflammatory cytokines and growth factors are released, which have profound catabolic effects on the host metabolism. The lower serum albumin concentrations may be due to the production of cytokines such as interleukin-6, which modulates the production of albumin by hepatocytes. The presence of micrometastatic tumor cells in the liver may induce Kupffer cells to produce a variety of cytokines, which could modulate hepatocyte albumin synthesis (37–39).
After determining the prognostic value of pre- and post-operative serum albumin levels for recurrence and tumor size, we further separated the patients into four subgroups with different survival outcomes. Our results demonstrated that the combination of pre- and post-operative serum albumin levels offered a means by which to discriminate between stage I NSCLC patients with a higher risk for recurrence and stage I NSCLC patients with a lower risk for recurrence.
Limitations of our study include the relatively small study population, which was recruited at a single institution. However, our patient population is uniform, as the study cohort consisted of only stage I NSCLC patients who underwent complete resection.
Generally speaking, this study suggests that serum albumin is a useful marker for the prediction of RFS for patients with stage I NSCLC. The use of serum albumin levels as a means to evaluate risk for tumor recurrence could easily be implemented, as the test is inexpensive, rapid and widely available in hospitals.
ACKNOWLEDGMENTS
This study was supported by the Zhejiang Provincial Health Bureau Foundation (Grant No. 2011KYA024).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012 CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Seki N, Eguchi K, Kaneko M, Ohmatsu H, Kakinuma R, Matsui E, et al. The adenocarcinoma-specific stage shift in the Anti-lung Cancer Association project: significance of repeated screening for lung cancer for more than 5 years with low-dose helical computed tomography in a high-risk cohort. Lung Cancer. 2010;67(3):318–24. doi: 10.1016/j.lungcan.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Martini N, Bains MS, Burt ME, Zakowski MF, McCormack P, Rusch VW, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109(1):120–9. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- 5.Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzales-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(9):719–27. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 6.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–60. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 7.Lin ZZ, Shau WY, Shao YY, Yang YY, Kuo RNC, Yang JCH, et al. Survival Following Surgery with or without Adjuvant Chemotherapy for Stage I-IIIA Non-Small Cell Lung Cancer: An East Asian Population-Based Study. Oncologist. 2012;17(10):1294–302. doi: 10.1634/theoncologist.2012-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuboi M, Ohira T, Saji H, Miyajima K, Kajiwara N, Uchida O, et al. The present status of postoperative adjuvant chemotherapy for completely resected non-small cell lung cancer. Ann Thorac Cardiovasc Surg. 2007;13(2):73–7. [PubMed] [Google Scholar]
- 9.Diederich S. Lung cancer staging update: the revised TNM classification. Cancer Imaging. 2010:S134–5. doi: 10.1102/1470-7330.2010.9022. 10 Spec no A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patnaik SK, Kannisto E, Knudsen S, Yendamuri S. Evaluation of MicroRNA Expression Profiles That May Predict Recurrence of Localized Stage I Non-Small Cell Lung Cancer after Surgical Resection. Cancer Res. 2010;70(1):36–45. doi: 10.1158/0008-5472.CAN-09-3153. [DOI] [PubMed] [Google Scholar]
- 11.Starnes SL, Pathrose P, Wang J, Succop P, Morris JC, Bridges J, et al. Clinical and Molecular Predictors of Recurrence in Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg. 2012;93(5):1606–13. doi: 10.1016/j.athoracsur.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Wang L, Liu PY, Yang P, You M. Gene-Expression Signature Predicts Postoperative Recurrence in Stage I Non-Small Cell Lung Cancer Patients. Plos One. 2012;7(1):e30880. doi: 10.1371/journal.pone.0030880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rinewalt D, Shersher DD, Daly S, Fhied C, Basu S, Mahon B, et al. Development of a serum biomarker panel predicting recurrence in stage I non-small cell lung cancer patients. J Thorac Cardiov Sur. 2012;144(6):1344–51. doi: 10.1016/j.jtcvs.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Wagner KW, Ye YQ, Lin J, Vaporciyan AA, Roth JA, Wu XF. Genetic Variations in Epigenetic Genes Are Predictors of Recurrence in Stage I or II Non-Small Cell Lung Cancer Patients. Clin Cancer Res. 2012;18(2):585–92. doi: 10.1158/1078-0432.CCR-11-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laky B, Janda M, Bauer J, Vavra C, Cleghorn G, Obermair A. Malnutrition among gynaecological cancer patients. Eur J Clin Nutr. 2007;61(5):642–6. doi: 10.1038/sj.ejcn.1602540. [DOI] [PubMed] [Google Scholar]
- 16.Fuhrman P, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004;104(8):1258–64. doi: 10.1016/j.jada.2004.05.213. [DOI] [PubMed] [Google Scholar]
- 17.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franch-Arcas G. The meaning of hypoalbuminaemia in clinical practice. Clin Nutr. 2001;20(3):265–9. doi: 10.1054/clnu.2001.0438. [DOI] [PubMed] [Google Scholar]
- 19.Law MR, Morris JK, Wald NJ, Hale AK. Serum-Albumin and Mortality in the Bupa Study. Int J Epidemiol. 1994;23(1):38–41. doi: 10.1093/ije/23.1.38. [DOI] [PubMed] [Google Scholar]
- 20.Cengiz O, Kocer B, Surmeli S, Santicky MJ, Soran A. Are pretreatment serum albumin and cholesterol levels prognostic tools in patients with colorectal carcinoma. Med Sci Monitor. 2006;12(6):Cr240–Cr7. [PubMed] [Google Scholar]
- 21.Boonpipattanapong T, Chewatanakornkul S. Preoperative carcinoembryonic antigen and albumin in predicting survival in patients with colon and rectal carcinomas. J Clin Gastroenterol. 2006;40(7):592–5. doi: 10.1097/00004836-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Onate-Ocana LF, Aiello-Crocifoglio V, Gallardo-Rincon D, Herrera-Goepfert R, Brom-Valladares R, Carrillo JF, et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14(2):381–9. doi: 10.1245/s10434-006-9093-x. [DOI] [PubMed] [Google Scholar]
- 23.Lis CG, Grutsch JF, Vashi PG, Lammersfeld CA. Is serum albumin an independent predictor of survival in patients with breast cancer. Jpen-Parenter Enter. 2003;27(1):10–5. doi: 10.1177/014860710302700110. [DOI] [PubMed] [Google Scholar]
- 24.Espinosa E, Feliu J, Zamora P, Baron MG, Sanchez JJ, Ordonez A, et al. Serum-Albumin and Other Prognostic Factors Related to Response and Survival in Patients with Advanced Nonsmall Cell Lung-Cancer. Lung Cancer. 1995;12(1-2):67–76. doi: 10.1016/0169-5002(95)00407-r. [DOI] [PubMed] [Google Scholar]
- 25.Peters T. Serum-Albumin. Adv Protein Chem. 1985;37:161–245. doi: 10.1016/s0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- 26.Hung JJ, Jeng WJ, Hsu WH, Huang BS, Wu YC. Time Trends of Overall Survival and Survival after Recurrence in Completely Resected Stage I Non-small Cell Lung Cancer. J Thorac Oncol. 2012;7(2):397–405. doi: 10.1097/JTO.0b013e31823b564a. [DOI] [PubMed] [Google Scholar]
- 27.Varlotto JM, Recht A, Flickinger JC, Medford-Davis LN, Dyer AM, DeCamp MM. Varying Recurrence Rates and Risk Factors Associated With Different Definitions of Local Recurrence in Patients With Surgically Resected, Stage I Nonsmall Cell Lung Cancer. Cancer-Am Cancer Soc. 2010;116(10):2390–400. doi: 10.1002/cncr.25047. [DOI] [PubMed] [Google Scholar]
- 28.Flores RM, Ihekweazu UN, Rizk N, Dycoco J, Bains MS, Downey RJ, et al. Patterns of recurrence and incidence of second primary tumors after lobectomy by means of video-assisted thoracoscopic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiov Sur. 2011;141(1):59–64. doi: 10.1016/j.jtcvs.2010.08.062. [DOI] [PubMed] [Google Scholar]
- 29.Strauss GM, Herndon JE, Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, et al. Adjuvant Paclitaxel Plus Carboplatin Compared With Observation in Stage IB Non-Small-Cell Lung Cancer: CALGB 9633 With the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26(31):5043–51. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung Adjuvant Cisplatin Evaluation: A pooled analysis by the LACE collaborative group. J Clin Oncol. 2008;26(21):3552–9. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 31.Yeun JY, Kaysen GA. Factors influencing serum albumin in dialysis patients. Am J Kidney Dis. 1998;32(6(Suppl 4):S118–25. doi: 10.1016/s0272-6386(98)70174-x. [DOI] [PubMed] [Google Scholar]
- 32.Lohsiriwat V, Lohsiriwat D, Boonnuch W, Chinswangwatanakul V, Akaraviputh T, Lert-Akayamanee N. Pre-operative hypoalbuminemia is a major risk factor for postoperative complications following rectal cancer surgery. World J Gastroentero. 2008;14(8):1248–51. doi: 10.3748/wjg.14.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asher V, Lee J, Bali A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med Oncol. 2012;29(3):2005–9. doi: 10.1007/s12032-011-0019-5. [DOI] [PubMed] [Google Scholar]
- 34.Win T, Sharples L, Groves AM, Ritchie AJ, Wells FC, Laroche CM. Predicting survival in potentially curable lung cancer patients. Lung. 2008;186(2):97–102. doi: 10.1007/s00408-007-9067-1. [DOI] [PubMed] [Google Scholar]
- 35.Maeda T, Ueoka H, Tabata M, Kiura K, Shibayama T, Gemba K, et al. Prognostic factors in advanced non-small cell lung cancer: Elevated serum levels of neuron specific enolase indicate poor prognosis. Jpn J Clin Oncol. 2000;30(12):534–41. doi: 10.1093/jjco/hyd139. [DOI] [PubMed] [Google Scholar]
- 36.Fujii T, Sutoh T, Morita H, Katoh T, Yajima R, Tsutsumi S, et al. Serum Albumin Is Superior to Prealbumin for Predicting Short-Term Recurrence in Patients with Operable Colorectal Cancer. Nutr Cancer. 2012;64(8):1169–73. doi: 10.1080/01635581.2012.718034. [DOI] [PubMed] [Google Scholar]
- 37.O'Gorman P, McMillan DC, McArdle CS. Impact of weight loss, appetite, and the inflammatory response on quality of life in gastrointestinal cancer patients. Nutr Cancer. 1998;32(2):76–80. doi: 10.1080/01635589809514722. [DOI] [PubMed] [Google Scholar]
- 38.Barber MD, Ross JA, Fearon KCH. Changes in nutritional, functional, and inflammatory markers in advanced pancreatic cancer. Nutr Cancer. 1999;35(2):106–10. doi: 10.1207/S15327914NC352_2. [DOI] [PubMed] [Google Scholar]
- 39.McMillan DC, Watson WS, O'Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39(2):210–3. doi: 10.1207/S15327914nc392_8. [DOI] [PubMed] [Google Scholar]