Abstract
OBJECTIVES:
Biological markers that predict the development of invasive breast cancer are needed to improve personalized therapy for patients diagnosed with ductal carcinoma in situ. We investigated the role of basal cytokeratin 5/6 in the risk of invasion in breast ductal carcinoma in situ.
METHODS:
We constructed tissue microarrays using 236 ductal carcinoma in situ samples: 90 pure samples (group 1) and 146 samples associated with invasive carcinoma (group 2). Both groups had similar nuclear grades and were obtained from patients of similar ages. The groups were compared in terms of estrogen (ER) and progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) expression, cytokeratin 5/6 immunostaining, human epidermal growth factor receptor 1 (EGFR) membrane staining and molecular subtype, as indicated by their immunohistochemistry profiles.
RESULTS:
ER/PR-negative status was predictive of invasion, whereas HER2 superexpression and cytokeratin 5/6-positive status were negatively associated with invasion. Among the high-grade ductal carcinoma in situ cases, a triple-positive profile (positive for estrogen receptor, progesterone receptor, and HER2) and cytokeratin 5/6 expression by neoplastic cells were negatively associated with invasion. In the low-grade ductal carcinoma in situ subgroup, only cytokeratin 5/6 expression exhibited a negative association with the probability of invasion.
CONCLUSION:
The immunohistochemical expression of cytokeratin 5/6 by ductal carcinoma in situ epithelial cells may provide clinically useful information regarding the risk of progression to invasive disease.
Keywords: Breast Cancer, Ductal Carcinoma In Situ, Immunohistochemistry, Prognosis, Basal Cytokeratin
INTRODUCTION
Ductal carcinoma in situ (DCIS) consists of a group of precursor lesions of invasive breast cancer (1). The prevalence of DCIS has been increasing over the last several decades, which is likely due to improvements in screening programs; DCIS now accounts for approximately 20–25% of all breast cancer diagnoses (2). Experience with this increasing number of DCIS diagnoses has revealed the heterogeneity of this group, particularly in terms of the risk of associated invasive disease and risk of recurrence, with most recurrences appearing as invasive cancer (3–5). Although wide surgical margins substantially reduce the risk of recurrence, the safe omission of radiotherapy in certain patient subgroups has not yet been incorporated into practical clinical use (6,7). The appropriate criteria for sentinel lymph node biopsy in DCIS, which are based on the risk of discovering invasive disease according to the final pathology report, are another source of controversy. Although a consensus is lacking, young age, the presence of palpable tumors, lesion size and histological grade are used as guidelines for sentinel lymph node biopsy (8). There is a recognized need for individualized treatment of all patients with breast cancer, including those with preinvasive lesions, who would particularly benefit from the discovery of markers of invasive risk.
The expression of basal cytokeratins (CKs) determined using immunohistochemistry, together with human epidermal growth factor receptor 1 (EGFR or HER1) expression, defines the basal-like subgroup of triple-negative (TN) invasive ductal carcinomas (9), which are correlated with a poor prognosis (10,11). The same invasive carcinoma molecular profiles have been identified in DCIS cases, albeit with a lower incidence of the basal-like phenotype (12). Although the expression of certain basal CKs, such as CK 5/6 and EGFR, has been used to define the molecular basal-like subtype of DCIS, CK 5/6 has not been explored as an independent indicator of biological behavior, particularly the risk of invasion. In this study, we compared the immunoexpression of basal CK 5/6 and other classic prognostic histological variables in DCIS samples with and without invasive components.
MATERIALS AND METHODS
Patient selection and clinicopathological parameters
This retrospective study was approved by the Scientific Committee of the Department of Pathology of the Faculdade de Medicina da Universidade de São Paulo and by the Ethics Committee for Research Projects of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (CAPPesq, process 2011/14741-7). Because the study was retrospective, informed patient consent was waived, and all patient identifiers were removed. Breast samples with confirmed diagnoses of DCIS of the breast, with and without an invasive carcinoma (IC) component, were obtained from the files of the Division of Surgical Pathology of the Faculdade de Medicina da Universidade de São Paulo between 2000 and 2009. All of the tissues had been fixed in 10% buffered formaldehyde and were embedded in paraffin. The slides were rigorously reviewed and classified by a single pathologist (FNA) with expertise in breast pathology. For cases with interpretations that differed from the original reports, a consensus was reached (by FNA and FMC) by a simultaneous examination under a dual-head microscope. Nuclear grades 1 and 2 were grouped into the low-grade category, and nuclear grade 3 was defined as the high-grade category. We selected representative areas of DCIS and invasive components for tissue microarray (TMA) construction and immunohistochemical analysis. Samples with nonductal histology, insufficient material for immunohistochemical evaluation or signs of tissue autolysis, as well as those from pregnant patients, were excluded from the study. We obtained 236 samples; 90 (38.1%) represented cases of DCIS not associated with invasive carcinoma, and 146 (61.9%) represented cases of DCIS associated with invasive carcinoma.
Tissue microarray construction
The TMA was constructed at the Laboratory of Medical Investigation (LIM-14) of the Faculdade de Medicina da Universidade de São Paulo. Representative areas were identified on hematoxylin- and eosin-stained slides and were marked on paraffin blocks. Cylindrical tissues 2.0 mm in diameter were punched from regions of DCIS and IC (at least one sample from each case) in the donor paraffin block and were mounted onto the recipient block with 1.0-mm intervals between the cores using a precision microarray instrument (Beecher Instruments, Silver Spring, MD, USA) positioned on a fixed sideboard. The cores were organized in rows and columns, with renal tissue placed in position 1A for orientation. After the final configuration of the recipient blocks, the cores were heated to 60°C for 10 minutes and were sealed for sectioning with the Paraffin Tape-Transfer System (Instrumedics, St. Louis, MO, USA). We used silanized slides (StarFrost®) and a microtome for sectioning at 3-μm intervals (Leica Instruments, Wetzlar, Germany). The first histological sections were stained with hematoxylin-eosin to identify the eventual losses of tissue.
Immunohistochemistry
The immunohistochemical detection of estrogen receptors (ER), progesterone receptors (PR), HER2 protein, basal CK 5/6 and EGFR was performed on the slides from the TMA blocks. We applied the following antibodies and dilutions: SP1 1:500 (Thermo Scientific, Waltharm, MA, USA), PgR636 1:1000 (Dako, Carpeteria, CA, USA), SP3 1:100 (Thermo Scientific), D5/16B4 1:100 (Dako) and 31G7 1:200 (Zymed, San Francisco, CA, USA). The appropriate epitope retrieval method for each specific antibody was used (a pressure cooker for ER, PR and HER2; a microwave oven for CK 5/6; and 0.1% pronase for EGFR). Bound antibodies were detected using Novolink® (Leica, Bannockburn, IL, USA). Nuclear positivity was assessed for ER and PR, membranous positivity for HER2 and EGFR and membranous-cytoplasmic positivity for CK 5/6. Tumors with staining in at least 1% of the cells were considered positive for ER, PR or CK 5/6 expression. We employed external positive and negative controls, as well as internal positive controls. We considered samples positive for HER2 and EGFR only if they scored 3+ according to the guidelines of the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP) (13).
The DCIS samples were classified into molecular subtypes according to the following surrogate criteria: luminal A samples exhibited HER2-negative lesions with at least 50% ER- and/or PR-positive neoplastic cells; luminal B samples exhibited HER2-negative lesions with less than 50% ER- and/or RP-positive cells; triple-positive samples were HER2-positive and ER- and/or PR-positive; HER2-enriched samples were HER2-positive and ER- and/or PR-negative; and TN samples were ER-, PR- and HER2-negative. The luminal A and B subtypes were later combined into a single category, “luminal”, because only three cases expressed less than 50% of the evaluated hormonal receptors.
Statistical analysis
A t-test was used to compare the ages of the patients in group 1 (DCIS without invasion) and group 2 (DCIS with invasion) after the normal distribution of the data was confirmed using the Kolmogorov-Smirnov test. A chi-square test was used to evaluate the relationship between the presence of invasion and the categorical variables (i.e., nuclear grade, ER/RP status, HER2 expression, molecular profile, CK 5/6 expression and EGFR expression). Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for these variables. For the multivariate analysis, the variables that were significant in the univariate evaluation were analyzed with logistic regression using the stepwise method. The statistical analyses were performed using MedCalc software for Windows (version 11.5.0.0; MedCalc Software, Mariakerke, Belgium), and p-values smaller than 0.05 were considered significant.
RESULTS
The distributions of age and the morphological and immunohistochemical characteristics of group 1 (DCIS without invasion) and group 2 (DCIS with IBC) are summarized in Table 1). There were no significant differences regarding age, nuclear grade or EGFR expression between the two groups. A larger number of TN cases were identified in group 2 (14.4%) compared with group 1 (4.4%) (p = 0.01). In contrast, group 1 exhibited more ER/PR-positive lesions (84.4% vs. 71.2%, p = 0.03) and more cases with CK 5/6 expression (30.6% vs. 11.3%; p = 0.0007). According to the multivariate analysis, ER/PR-negative status (p = 0.001) was a predictor of associated invasive carcinoma, whereas HER2-positive status (p = 0.33) and CK 5/6-positive status were negatively associated with the presence of invasion (p = 0.0001) (Table 2).
Table 1.
Distributions of age and morphological variables among 236 cases of ductal carcinoma in situ (DCIS) classified as group 1 (pure DCIS without invasive carcinoma) and group 2 (DCIS with invasive breast carcinoma, a.k.a. DCIS+IBC).
| Variable | Group 1 (pure DCIS) | Group 2(DCIS+IBC) | p-value | OR (95% CI) | |
| n | 90 | 146 | |||
| Patient's age | (mean±SD) | 58.45 | 58.53 | NS | – |
| Nuclear grade | 1–2 | 63 (70%) | 93/144 (64.6%) | 0.47*) | 1.28 |
| 3 | 27 (30%) | 51/144 (35.4%) | (0.73–2.25) | ||
| ER/PR | Positive | 76 (84.4%) | 104 (71.2%) | 0.03*) | 0.45 |
| Negative | 14 (15.6%) | 42 (28.8%) | (0.23–0.89) | ||
| HER2 | Negative (0/1+) | 69 (76.7%) | 119 (81.5%) | 0.46*) | 1.34 |
| Positive (3+) | 21 (23.3%) | 27 (18.5%) | (0.70–2.55) | ||
| Molecular profile | Luminal (A+B) | 65 (72.2%) | 98 (67.1%) | 0.01*) | – |
| TP | 11 (12.2%) | 6 (4.1%) | |||
| HER2 | 10 (11.1%) | 21 (14.4%) | |||
| TN | 4 (4.4%) | 21 (14.4%) | |||
| CK 5/6 | Positive | 26/85 (30.6%) | 15/133 (11.3%) | 0.0007*) | 0.29 |
| Negative | 59/85 (69.4%) | 118/133 (88.7%) | (0.14–0.58) | ||
| EGFR | Positive | 9/85 (10.6%) | 16/116 (13.8%) | 0.64*) | 1.35 |
| Negative | 76/85 (89.4%) | 100/116 (86.2%) | (0.57–3.22) |
Chi-square; IBC: invasive breast cancer; ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor 2; CK 5/6: cytokeratin 5/6; EGFR: epidermal growth factor receptor (human epidermal growth factor 1); TP: triple-positive (ER/PR/HER2-positive); TN: triple-negative (ER/PR/HER2-negative).
Table 2.
Results of a multivariate analysis of the pathological characteristics associated with invasion in ductal carcinoma in situ (DCIS).
| DCIS | Variable | OR | 95% CI | Coefficient | SE | p-value |
| Total | ER/PR-negative | 4.67 | 1.87–11.70 | 1.54 | 0.47 | 0.0010 |
| HER2-positive | 0.39 | 0.17–0.93 | −0.93 | 0.44 | 0.0333 | |
| CK 5/6-positive | 0.22 | 0.10–0.48 | −1.5 | 0.39 | 0.0001 | |
| Low-grade | CK 5/6-positive | 0.22 | 0.09–057 | −1.5 | 0.49 | 0.0019 |
| High-grade | Triple-positive | 0.09 | 0.02–0.51 | −2.37 | 0.87 | 0.006 |
| CK 5/6-positive | 0.28 | 0.09–0.93 | −1.24 | 0.59 | 0.04 |
ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor 2; CK 5/6: cytokeratin 5/6; OR: odds ratio; SE: standard error.
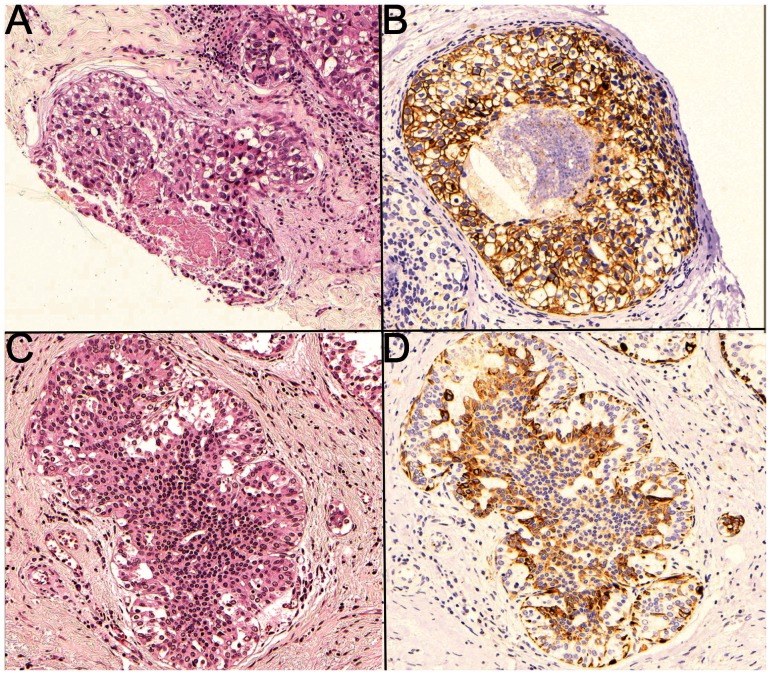
The pathological characteristics of high-grade DCIS are described in Table 3). The presence of invasion was associated with negative ER, PR and HER2 expression. The TN phenotype was more common in group 2 (35.3% vs. 14.8%), but the most striking difference in molecular profiles was the lower frequency of triple-positive cases observed in group 2 (3.9% vs. 33.3%) (p = 0.003). Although CK 5/6 expression (Figure 1) was lower in group 2 than in group 1 (17% vs. 34.6%) (p = 0.09), this difference did not reach statistical significance.
Table 3.
Pathological characteristics of the high-grade ductal carcinoma in situ (DCIS) cases (78 cases) according to the presence or absence of associated invasive carcinoma (invasive breast carcinoma).
| Variable | Group 1 (pure DCIS) | Group 2 (DCIS+IBC) | Total | p-value | OR (95% CI) | |
| n | 27 | 51 | 78 | |||
| ER/PR | Positive | 14 (51.8%) | 15 (29.4%) | 29 | 0.05 | 0.38 (0.14–1.01) |
| Negative | 13 (48.2%) | 36 (70.6%) | 49 | |||
| HER2 | Positive (3+) | 18 (66.7%) | 20 (39.2%) | 38 | 0.02 | 0.32 (0.12–0.86) |
| Negative (0/1+) | 9 (33.3%) | 31 (60.8%) | 40 | |||
| Molecular profile | Luminal (A+B) | 5 (27.8%) | 13 (25.5%) | 18 | 0.003 | – |
| Triple-positive | 9 (33.3%) | 2 (3.9%) | 11 | |||
| HER2 | 9 (33.3%) | 18 (35.3%) | 27 | |||
| Triple-negative | 4 (14.8%) | 18 (35.3%) | 22 | |||
| CK 5/6 | Positive | 9/26 (34.6%) | 8/47 (17%) | 17 | 0.09 | 0.38 (0.13–1.17) |
| Negative | 17/26 (65.4%) | 39/47 (83%) | 56 | |||
| EGFR | Positive | 7 (25.9%) | 13/40 (32.5%) | 20 | 0.56 | 1.37 (0.46–4.07) |
| Negative | 20 (74.1%) | 27/40 (67.5%) | 47 |
ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor 2; CK 5/6: cytokeratin 5/6; EGFR: epidermal growth factor receptor (human epidermal growth factor 1).
Figure 1.
High-grade ductal carcinoma in situ (DCIS) (A) showing positive membranous-cytoplasmic immunostaining for basal cytokeratin (CK 5/6) (B); and low-grade DCIS (C) showing positive membranous-cytoplasmic immunostaining for CK 5/6 (D) (original magnification 100X).
The characteristics of low-grade DCIS are presented in Table 4. In this subgroup, CK 5/6 expression was the only characteristic that differed between the cases with and without invasion (8.1% and 28.8%, respectively, p = 0.002) (Figure 1).
Table 4.
| Variable | Group 1 (pure DCIS) | Group 2 (DCIS+IBC) | p-value | OR (95% CI) | |
| n | 63 | 93 | |||
| Age (average) | 58.45 | 58.53 | - | ||
| ER/PR | Positive | 62 (98.4%) | 87 (93.5%) | 0.18 | 0.23 (0.02–1.99) |
| Negative | 1 (1.6%) | 6 (6.5%) | |||
| HER2 | Positive (3+) | 3 (4.8%) | 7 (7.5%) | 0.49 | 1.63 (0.40–6.5) |
| Negative (0/1+) | 60 (95.2%) | 86 (92.5%) | |||
| Molecular profile | Luminal (A+B) | 60 (95.2%) | 83 (89.2%) | 0.44 | – |
| Triple-positive | 2 (3.1%) | 4 (4.3%) | |||
| HER2 | 1(1.6%) | 3 (3.2%) | |||
| Triple-negative | 0 | 3 (3.2%) | |||
| CK 5/6 | Positive | 17/59 (28.8%) | 7/86 (8.1%) | 0.002 | 0.21 (0.08–0.57) |
| Negative | 42/59 (71.2%) | 79/86 (91.9%) | |||
| EGFR | Positive | 2/58 (3.4%) | 3/76 (3.9%) | 0.88 | 1.15 (0.18–7.12) |
| Negative | 56/58 (96.5%) | 73/76 (96%) |
ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor 2; CK 5/6: cytokeratin 5/6; EGFR: epidermal growth factor receptor (human epidermal growth factor 1).
DISCUSSION
Basal CKs are intermediate filaments present in the myoepithelial and basal epithelial cells of the mammary gland (14) and are recognized by the monoclonal antibody CK 5/6. Basal CKs have been described in many types of cancer, including breast tumors (15); along with other CKs, they have been studied in the breast since the 1980s, either to characterize normal histology or to evaluate the prognosis of cancer (16–19). However, interest in basal CKs increased after a study by Perou et al. (20) identified a “basal-like” subgroup of breast carcinomas characterized by a more aggressive phenotype and by the expression of genes that are normally active in the basal/myoepithelial cells of the breast (14). Since then, numerous studies have described surrogate immunohistochemical markers for characterizing molecular subgroups, including a marker recognized by the antibody CK 5/6 (9,21–23). Nielsen et al. defined the basal-like subtype as a combination of the TN phenotype and positive CK 5/6 and/or EGFR expression. The use of this gene expression signature as the gold standard for identifying the basal-like subtype was associated with 76% sensitivity and 100% specificity (22). The same criteria have been extended to the identification of a basal-like profile in DCIS cases (12,24). Livasy et al. (12) identified the basal-like phenotype in 19 of 245 (8%) patients with pure DCIS; furthermore, all of their TN cases exhibited CK 5/6 and/or EGFR expression. Among our cases, not all of the TN lesions were basal-like. Among the 236 DCIS samples that we examined, 25 (10.6%) cases were classified as the TN subtype, and only 18 of these (72% of the TN cases and 7.6% of all the DCIS cases) also exhibited the basal-like phenotype. The remaining 7 TN DCIS cases were also negative for both CK 5/6 and EGFR. Although Livasy et al. (12) reported no basal-like phenotypes among their low-grade DCIS cases, we noted three cases with TN profiles in the low-grade group, all of which were associated with invasive carcinoma and two of which exhibited a basal-like phenotype. Bryan et al. (24) observed the TN phenotype in 4 of 66 (6%) cases of high-grade DCIS, all of which exhibited a basal-like phenotype. In that study, the expression of basal CK was also observed among in a subset of the non-TN lesions. CK 5/6 was expressed in 16 of their 66 (24%) high-grade DCIS cases. Besides, we observed the TN phenotype in 22 of our 78 (28.2%) high-grade DCIS cases. Among these cases, 17 exhibited the basal-like phenotype (CK 5/6- and/or EGFR-positive), and most of them (15 cases) were CK 5/6-negative but EGFR-positive. Besides, CK 5/6 expression was identified in 17 of our 78 (21.8%) high-grade DCIS cases, most of which were non-TN. Different from the conclusion of Bryan et al., our results suggest that CK 5/6 expression in DCIS cases is not correlated with basal-like invasive carcinoma. In fact, the most striking feature of the neoplastic epithelial DCIS cells examined here was their expression of basal CK 5/6, which indicates a low probability of invasive disease, in both the low-grade and high-grade groups. In addition, our results support a negative association between CK 5/6 expression and the TN phenotype; the latter was associated with more invasive cases and higher-grade tumors. Interestingly, Steinman et al. (25) observed a discordance between the CK marker expression and ER/PR/EGFR status in cases of high-grade DCIS and DCIS associated with invasive carcinoma (25). The authors considered the hypotheses that pure DCIS lesions correspond to earlier lesions than DCIS/IC that lack some of the genetic changes necessary for progression to IBC or that some pure DCIS cases are genetically programmed to not progress to IBC. Among our cases, both invasive and in situ components of the same lesion presented the same morphological and immunohistochemical profile (data not shown). Based on this finding, it is possible that basal CKs (identified by CK 5/6 staining) play different roles in in situ neoplasms and invasive carcinomas, particularly those of the basal-like TN subtype.
Zhou et al. (26) compared the risk of local recurrence/invasive cancer between basal-like DCIS cases and DCIS cases belonging to the other molecular subgroups (26). Although basal-like DCIS was associated with a worse prognosis, this difference was not significant. In the same study, the TN phenotype and nuclear grade showed weak power for stratifying the cases according to prognosis. However, the authors did not explore the influence of basal CK expression, which might partly explain their results.
Our findings suggest a possible utility of basal CK 5/6 for selecting patients with low-grade DCIS who can be spared radiation treatment without increasing the risk of invasive disease. Radiation therapy following lumpectomy significantly reduces the risk of local recurrence; however, there are ongoing efforts to identify the subset of patients with low-risk DCIS who may safely forgo radiation. Such a finding would alter the paradigm of the necessity of radiation treatment in all cases (27–31).
Another source of debate concerns the appropriate indications for sentinel lymph node biopsy in patients with pure DCIS (32–34). Most studies agree that sentinel lymph node biopsy should be considered only in cases of DCIS with a risk of invasive disease (32). The challenge is to identify this subset of patients after a biopsy results in a DCIS diagnosis. The features generally considered to indicate lymph node biopsy are extensive DCIS, areas of suspected microinvasion, irregular mass lesions and planned mastectomy (34,35). Immunohistochemical markers have been correlated with the risk of recurrence, but there has been minimal research analyzing these markers as indicators of the risk of invasion. In this regard, our results yield some interesting data. We could speculate that the low-grade DCIS patients who would benefit the most from conservative approaches might be those with positive CK 5/6 expression, regardless of their molecular profile, because this group did not demonstrate evidence of an influence of hormonal status or HER2 expression. In contrast, high-grade DCIS cases with a triple-positive phenotype (ER/PR/HER2) and positive CK 5/6 expression might correspond to less aggressive lesions. Our study had some limitations due to its retrospective design. Additional investigations to confirm and extend our findings would be valuable.
Another interesting issue is the role of HER2 overexpression in DCIS. In this study, we observed that the proportions of both HER2 positivity and HER2-enriched profiles were similar between the pure DCIS and DCIS/IC cases. However, triple-positive profile was predominant in the high-grade pure DCIS group compared with the high-grade DCIS/IBC group (33.3% vs. 3.9%, p = 0.003). This difference remained after a multivariate analysis, with an OR of 0.09 (CI 0.02-0.51) for predicting invasion. A comparison of our results with the published data would be difficult because HER2 overexpression is mainly found in high-grade DCIS cases. In addition, HER2 overexpression is generally analyzed independently of ER/PR co-expression, except when the focus is determining its predictive value for therapeutic response (36). However, we found support for our results in a study by Rakovitch et al. (37), who identified a combination of HER2 overexpression and Ki-67 expression as an independent profile predictor of noninvasive recurrence following breast-conserving therapy. This result is interesting because it does not deny the more aggressive nature and greater risk of recurrence associated with HER2-positive DCIS, but it shows that recurrence is more common in in situ disease. Han et al. (38) reported high rates (40% and 38%) of local recurrence among DCIS cases with either co-expression of ER/PR or HER2 enrichment, respectively. However, although those authors observed a trend toward higher rates of invasive recurrence in HER-positive cases, they did not analyze cases of invasive and in situ recurrence separately (38). In contrast, Harada et al. (39) identified the ER/PR/HER2-positive phenotype as exhibiting the highest risk of progression to invasive disease. The influence of the co-expression of ER/PR in HER2-positive neoplasms is far from clear.
At this point, the challenge is to investigate which basal CKs, such as those recognized by CK 5/6, can be combined with other biomarkers to define a low-risk DCIS subgroup. Larger studies involving systematic evaluations of pathological and molecular parameters, longer follow-ups, adjustments for treatment effects and validation are needed to improve risk stratification and treatment planning for women with DCIS.
According to our results, the immunoexpression of basal CK 5/6 in both high-grade and low-grade DCIS lesions indicates a lower risk of invasive carcinoma.
ACKNOWLEDGMENTS
This study was supported by FAPESP (São Paulo Research Foundation), process number 2011/14741-7.
Table 4.
Pathological characteristics of the low-grade ductal carcinoma in situ (DCIS) cases (156 cases) according to the presence or absence of associated invasive carcinoma (invasive breast carcinoma).
| Variable | Group 1 (pure DCIS) | Group 2 (DCIS+IBC) | p-value | OR (95% CI) | |
| n | 63 | 93 | |||
| Age (average) | 58.45 | 58.53 | - | ||
| ER/PR | Positive | 62 (98.4%) | 87 (93.5%) | 0.18 | 0.23 (0.02–1.99) |
| Negative | 1 (1.6%) | 6 (6.5%) | |||
| HER2 | Positive (3+) | 3 (4.8%) | 7 (7.5%) | 0.49 | 1.63 (0.40–6.5) |
| Negative (0/1+) | 60 (95.2%) | 86 (92.5%) | |||
| Molecular profile | Luminal (A+B) | 60 (95.2%) | 83 (89.2%) | 0.44 | – |
| Triple-positive | 2 (3.1%) | 4 (4.3%) | |||
| HER2 | 1(1.6%) | 3 (3.2%) | |||
| Triple-negative | 0 | 3 (3.2%) | |||
| CK 5/6 | Positive | 17/59 (28.8%) | 7/86 (8.1%) | 0.002 | 0.21 (0.08–0.57) |
| Negative | 42/59 (71.2%) | 79/86 (91.9%) | |||
| EGFR | Positive | 2/58 (3.4%) | 3/76 (3.9%) | 0.88 | 1.15 (0.18–7.12) |
| Negative | 56/58 (96.5%) | 73/76 (96%) |
ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor 2; CK 5/6: cytokeratin 5/6; EGFR: epidermal growth factor receptor (human epidermal growth factor 1).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Tavassoli F, Devilee P. Wordl Health Organization of Tumors: Pathology and genetics of tumors of the breast and female genital organs. 2003 IARC WHO Classification of Tumours (Book 4) [Google Scholar]
- 2.Ernster VL, Ballard-Barbash R, Barlow WE, Zheng Y, Weaver DL, Cutter G, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94(20):1546–54. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
- 3.Ottesen GL, Christensen IJ, Larsen JK, Larsen J, Baldetorp B, Linden T, et al. Carcinoma in situ of the breast: correlation of histopathology to immunohistochemical markers and DNA ploidy. Breast Cancer Res Treat. 2000;60(3):219–26. doi: 10.1023/a:1006453420088. [DOI] [PubMed] [Google Scholar]
- 4.Allred DC, Wu Y, Mao S, Nagtegaal ID, Lee S, Perou CM, et al. Ductal carcinoma in situ and the emergence of diversity during breast cancer evolution. Clin Cancer Res. 2008;14(2):370–8. doi: 10.1158/1078-0432.CCR-07-1127. [DOI] [PubMed] [Google Scholar]
- 5.Sgroi DC. Preinvasive breast cancer. Annu Rev Pathol. 2010;5:193–221. doi: 10.1146/annurev.pathol.4.110807.092306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudloff U, Brogi E, Reiner AS, Goldberg JI, Brockway JP, Wynveen CA, et al. The influence of margin width and volume of disease near margin on benefit of radiation therapy for women with DCIS treated with breast-conserving therapy. Ann Surg. 2010;251(4):583–91. doi: 10.1097/SLA.0b013e3181b5931e. [DOI] [PubMed] [Google Scholar]
- 7.Bijker N, van Tienhoven G. Local and systemic outcomes in DCIS based on tumor and patient characteristics: the radiation oncologist's perspective. J Natl Cancer Inst Monogr. 2010;2010(41):178–80. doi: 10.1093/jncimonographs/lgq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yen TW, Hunt KK, Ross MI, Mirza NQ, Babiera GV, Meric-Bernstam F, et al. Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: a guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg. 2005;200(4):516–26. doi: 10.1016/j.jamcollsurg.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19(2):264–71. doi: 10.1038/modpathol.3800528. [DOI] [PubMed] [Google Scholar]
- 10.Thike AA, Iqbal J, Cheok PY, Chong AP, Tse GM, Tan B, et al. Triple negative breast cancer: outcome correlation with immunohistochemical detection of basal markers. Am J Surg Pathol. 2010;34(7):956–64. doi: 10.1097/PAS.0b013e3181e02f45. [DOI] [PubMed] [Google Scholar]
- 11.Liu ZB, Wu J, Ping B, Feng LQ, Di GH, Lu JS, et al. Basal cytokeratin expression in relation to immunohistochemical and clinical characterization in breast cancer patients with triple negative phenotype. Tumori. 2009;95(1):53–62. doi: 10.1177/030089160909500110. [DOI] [PubMed] [Google Scholar]
- 12.Livasy CA, Perou CM, Karaca G, Cowan DW, Maia D, Jackson S, et al. Identification of a basal-like subtype of breast ductal carcinoma in situ. Hum Pathol. 2007;38(2):197–204. doi: 10.1016/j.humpath.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 14.Gusterson BA, Ross DT, Heath VJ, Stein T. Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res. 2005;7(4):143–8. doi: 10.1186/bcr1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu PG, Weiss LM. Expression of cytokeratin 5/6 in epithelial neoplasms: an immunohistochemical study of 509 cases. Mod Pathol. 2002;15(1):6–10. doi: 10.1038/modpathol.3880483. [DOI] [PubMed] [Google Scholar]
- 16.Moll R, Krepler R, Franke WW. Complex cytokeratin polypeptide patterns observed in certain human carcinomas. Differentiation. 1983;23(3):256–69. doi: 10.1111/j.1432-0436.1982.tb01291.x. [DOI] [PubMed] [Google Scholar]
- 17.Wetzels RH, Holland R, van Haelst UJ, Lane EB, Leigh IM, Ramaekers FC. Detection of basement membrane components and basal cell keratin 14 in noninvasive and invasive carcinomas of the breast. Am J Pathol. 1989;134(3):571–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Dairkee SH, Mayall BH, Smith HS, Hackett AJ. Monoclonal marker that predicts early recurrence of breast cancer. Lancet. 1987;1(8531):514. doi: 10.1016/s0140-6736(87)92129-5. [DOI] [PubMed] [Google Scholar]
- 19.Malzahn K, Mitze M, Thoenes M, Moll R. Biological and prognostic significance of stratified epithelial cytokeratins in infiltrating ductal breast carcinomas. Virchows Arch. 1998;433(2):119–29. doi: 10.1007/s004280050226. [DOI] [PubMed] [Google Scholar]
- 20.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 21.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736–50. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10(16):5367–74. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 23.Bhargava R, Striebel J, Beriwal S, Flickinger JC, Onisko A, Ahrendt G, et al. Prevalence, morphologic features and proliferation indices of breast carcinoma molecular classes using immunohistochemical surrogate markers. Int J Clin Exp Pathol. 2009;2(5):444–55. [PMC free article] [PubMed] [Google Scholar]
- 24.Bryan BB, Schnitt SJ, Collins LC. Ductal carcinoma in situ with basal-like phenotype: a possible precursor to invasive basal-like breast cancer. Mod Pathol. 2006;19(5):617–21. doi: 10.1038/modpathol.3800570. [DOI] [PubMed] [Google Scholar]
- 25.Steinman S, Wang J, Bourne P, Yang Q, Tang P. Expression of cytokeratin markers, ER-alpha, PR, HER-2/neu, and EGFR in pure ductal carcinoma in situ (DCIS) and DCIS with co-existing invasive ductal carcinoma (IDC) of the breast. Ann Clin Lab Sci. 2007;37(2):127–34. [PubMed] [Google Scholar]
- 26.Zhou W, Jirström K, Johansson C, Amini RM, Blomqvist C, Agbaje O, et al. Long-term survival of women with basal-like ductal carcinoma in situ of the breast: a population-based cohort study. BMC Cancer. 2010;10:653. doi: 10.1186/1471-2407-10-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solin LJ. The impact of adding radiation treatment after breast conservation surgery for ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010(41):187–92. doi: 10.1093/jncimonographs/lgq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lari SA, Kuerer HM. Biological Markers in DCIS and Risk of Breast Recurrence: A Systematic Review. J Cancer. 2011;2:232–61. doi: 10.7150/jca.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang SY, Shamliyan T, Virnig BA, Kane R. Tumor characteristics as predictors of local recurrence after treatment of ductal carcinoma in situ: a meta-analysis. Breast Cancer Res Treat. 2011;127(1):1–14. doi: 10.1007/s10549-011-1387-4. [DOI] [PubMed] [Google Scholar]
- 30.Allred DC. Ductal carcinoma in situ: terminology, classification, and natural history. J Natl Cancer Inst Monogr. 2010;2010(41):134–8. doi: 10.1093/jncimonographs/lgq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnitt SJ. Local outcomes in ductal carcinoma in situ based on patient and tumor characteristics. J Natl Cancer Inst Monogr. 2010;2010(41):158–61. doi: 10.1093/jncimonographs/lgq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veronesi P, Intra M, Vento AR, Naninato P, Caldarella P, Paganelli G, et al. Sentinel lymph node biopsy for localised ductal carcinoma in situ. Breast. 2005;14(6):520–2. doi: 10.1016/j.breast.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Son BK, Bong JG, Park SH, Jeong YJ. Ductal carcinoma in situ and sentinel lymph node biopsy. J Breast Cancer. 2011;14(4):301–7. doi: 10.4048/jbc.2011.14.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith BL. Clinical applications of breast pathology: management of in situ breast carcinomas and sentinel node biopsy issues. Mod Pathol. 2010;23(Suppl 2):S33–5. doi: 10.1038/modpathol.2010.53. [DOI] [PubMed] [Google Scholar]
- 35.Collado MV, Ruiz-Tovar J, García-Villanueva A, Rojo R, Latorre L, Rioja ME, et al. Sentinel lymph node biopsy in selected cases of ductal carcinoma in situ. Clin Transl Oncol. 2010;12(7):499–502. doi: 10.1007/s12094-010-0543-3. [DOI] [PubMed] [Google Scholar]
- 36.Collins LC, Schnitt SJ. HER2 protein overexpression in estrogen receptor-positive ductal carcinoma in situ of the breast: frequency and implications for tamoxifen therapy. Mod Pathol. 2005;18(5):615–20. doi: 10.1038/modpathol.3800360. [DOI] [PubMed] [Google Scholar]
- 37.Rakovitch E, Nofech-Mozes S, Hanna W, Narod S, Thiruchelvam D, Saskin R, et al. HER2/neu and Ki-67 expression predict non-invasive recurrence following breast-conserving therapy for ductal carcinoma in situ. Br J Cancer. 2012;106(6):1160–5. doi: 10.1038/bjc.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han K, Nofech-Mozes S, Narod S, Hanna W, Vesprini D, Saskin R, et al. Expression of HER2neu in ductal carcinoma in situ is associated with local recurrence. Clin Oncol (R Coll Radiol) 2012;24(3):183–9. doi: 10.1016/j.clon.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Harada S, Mick R, Roses RE, Graves H, Niu H, Sharma A, et al. The significance of HER-2/neu receptor positivity and immunophenotype in ductal carcinoma in situ with early invasive disease. J Surg Oncol. 2011;104(5):458–65. doi: 10.1002/jso.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]