Abstract
OBJECTIVES:
Familial steroid-sensitive idiopathic nephrotic syndrome is rare, and only approximately 3% of patients have affected siblings.
METHODS:
Herein, we report seven cases of patients with steroid-sensitive idiopathic nephrotic syndrome from three Chinese families. Mutational screening of the Nphs2 gene was performed in all the patients.
RESULTS:
All seven of the familial steroid-sensitive idiopathic nephrotic syndrome cases in our sample exhibited minimal change disease, and one case also presented with mesangial proliferative glomerulonephritis, according to the renal pathology. No significant was associations were found between Nphs2 gene mutations and the onset of proteinuria and nephrotic syndrome in these familial cases.
CONCLUSIONS:
The presence of minimal change disease is important, but it is not an unusual finding in patients with familial steroid-sensitive idiopathic nephrotic syndrome, which appears to be clinically benign and genetically distinct from other types of nephrosis.
Keywords: Steroid-Sensitive Idiopathic Nephrotic Syndrome, Familial, Nphs2 Gene, Pathology
INTRODUCTION
Nephrotic syndrome is defined by the presence of proteinuria, hypoalbuminemia, edema, and hyperlipidemia. This syndrome is one of the most common diagnoses in pediatric nephrology, and it is emerging as a leading cause of uremia. The most common variant of nephrotic syndrome in children is idiopathic nephrotic syndrome (INS).
Podocin, which is encoded by the Nphs2 gene, is an important molecule expressed in glomerular podocytes and the slit diaphragm. Since the identification of Nphs2 (1), several groups have demonstrated that mutations in the Nphs2 gene represent a frequent cause of steroid-resistant nephrotic syndrome (SRNS) and are found in 20–30% of sporadic cases of SRNS (2).
Most children with sporadic INS respond to steroid treatment, in which case the disease has a favorable outcome and does not progress to end-stage renal disease. Few reports of familial cases of steroid-sensitive idiopathic nephrotic syndrome (SSINS) have been published (3–7). Herein, we report seven cases of SSINS from three families in China.
CASE PRESENTATION
Family 1
The first case was an 8-year-old girl who presented on March 3, 2004 with edema, massive proteinuria, and hypoalbuminemia that had persisted for seven days. The level of protein in a 24-hr urine sample was 3.42 g, and the blood albumin concentration was 14.5 g/L. Primary nephrotic syndrome was diagnosed, and treatment with 1.5 mg/kg/day of prednisone was initiated. Over time, the patient developed frequently relapsing nephrotic syndrome, and a renal biopsy performed in 2006 revealed minimal change disease (MCD). Subsequently, pulse cyclophosphamide (CTX) therapy was applied for four months at a total dose of 145 mg/kg CTX. The patient achieved remission and has since maintained this state.
The second case was a 5-year-old boy (the brother of the first patient) who was admitted for edema and proteinuria on April 10, 2008. The level of protein in a 24-hr urine sample was 2.65 g, and the blood albumin concentration was 15.1 g/L. This patient was treated with 2 mg/kg/day prednisolone, and he achieved remission after 12 days. He had five episodes of relapse during the following six months, and a renal biopsy revealed MCD. The patient received 25 mg/kg/day mycophenolate mofetil (MMF) and has since remained in remission.
Direct DNA sequencing was performed after polymerase chain reaction (PCR) amplification of the 29 exons of the Nphs1 gene and the 8 exons of the Nphs2 gene in both patients. No homozygous or compound heterozygous mutations in either gene were found in either patient, except for 1 known single-nucleotide polymorphism (SNP; V763V) in Nphs1 in the second case. These patients were not born to consanguineous parents.
Family 2 (8)
The third case was a 13-year-old boy with steroid-sensitive nephrotic syndrome. The patient underwent a biopsy, which revealed MCD. He achieved remission after prednisone therapy and has had no relapses in the 9.5 years since the biopsy.
The fourth case was a 15-year-old boy (the brother of the third patient) who had four relapses at 17, 18, 20, and 22 years of age. After the fourth relapse, the patient underwent a renal biopsy, which identified MCD. After two weeks of prednisone therapy, the proteinuria disappeared, and the patient has maintained normal renal function for the past 3.6 years.
The fifth patient was a 35-year-old man (the brother of the third and fourth patients) who developed nephrotic syndrome. Despite a 2-year history of hypertension, he had not undergone urine testing. Immunofluorescence analysis of a renal biopsy revealed IgG and IgA deposits, and an electron microscopic analysis revealed segmental foot process effacement and mild electron-dense deposits in the mesangial area (Figure 2). The pathology diagnose was mesangial proliferative glomerulonephritis (MsPGN). The patient received prednisone treatment and achieved remission.
Figure 2.

Slight mesangial proliferation and matrix expansion in glomeruli from patient 5.
Denaturing high-performance liquid chromatography (DHPLC) was performed following the PCR amplification of all eight exons of the Nphs2 gene in patients 3, 4, and 5 (described above). No homozygous or compound heterozygous mutations in the Nphs2 gene were found in any of these three patients. There was no parental consanguinity in this family.
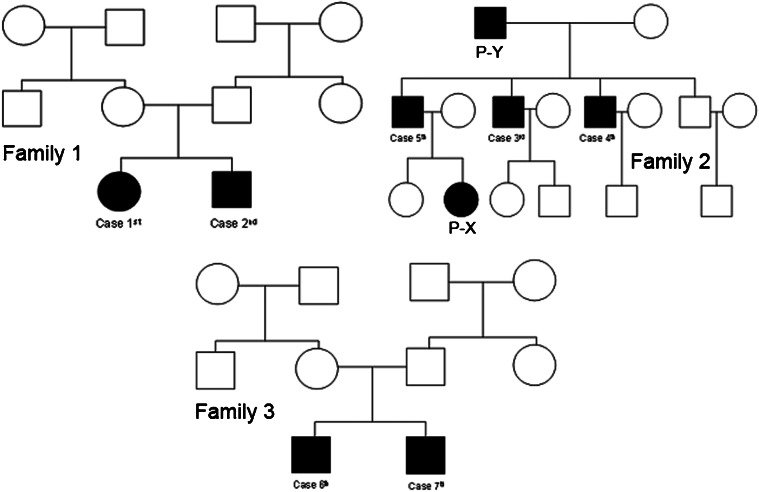
Patient X (P-X) and patient Y (P-Y) (Figure 1) also exhibited moderate proteinuria (a result of ++) based on a urine dipstick test, but no further investigations were performed. No quantitative evaluation of the proteinuria was undertaken in either patient.
Figure 1.
The pedigrees of families 1–3, which include individuals with steroid-sensitive idiopathic nephrotic syndrome.
Family 3
The sixth case was a 2.8-year-old boy who was admitted with palpebral edema. A quantitative analysis revealed that his 24-hr urine protein level was 1.82 g. The serum albumin concentration was 12.7 g/L, the serum cholesterol was 12.89 mmol/L, and the serum creatinine level was 26.7 μmol/L. The patient received 2 mg/kg/day prednisone and achieved remission 6 days after treatment. One month later, he experienced a single episode of relapse involving an upper respiratory infection and exhibited proteinuria, which resolved within 10 days. This patient has remained in remission for 3 years.
The seventh case was a 3-year-old boy who was the dizygotic twin of patient 6. The onset of his proteinuria and edema occurred 2 months later than that of patient 6. The 24-hr urine protein level was 2.16 g, the serum albumin concentration was 12.4 g/L, and the cholesterol level was 11.77 mmol/L. The patient was treated with prednisone at a dose of 1.8 mg/kg/day, and remission was observed 15 days after the steroid therapy was initiated. His urinary test results have remained normal for the past 2.8 years.
Patients 6 and 7 were born to nonconsanguineous parents. Neither boy underwent a renal biopsy. The mutational screening of the Nphs2 gene using PCR and direct sequencing revealed no significant mutations in either dizygotic twin.
Secondary causes of nephrotic syndrome, including systemic lupus erythematosus, malignancy, hepatitis B infection, and Henoch-Schonlein purpura, were excluded in all the patients. Hematuria, hypertension, and hypocomplementemia were absent in all the cases. The clinical parameters and treatment responses to prednisone/immunosuppressants of patients 1–7 are summarized in Table 1). This study was approved by the ethics committee of The Children's Hospital of Zhejiang University School of Medicine.
Table 1.
Seven cases of steroid-sensitive nephrotic syndrome from three families.
| Family | 1 | 2(8) | 3 | ||||
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Sex (M/F) | F | M | M | M | M | M | M |
| Age at onset (years) | 8 | 5 | 13 | 15 | 35 | 2.8 | 2.9 |
| Interval of onset (months) | ? | 49 | ? | 36 | 96 | ? | 2 |
| Hematuria | No | No | No | No | No | No | No |
| Hypertension | No | No | No | No | Yes | No | No |
| Renal function | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| Immunosuppressant | Pre+CTX | Pre+MMF | Pre | Pre | Pre | Pre | Pre |
| Days to remission | 13 | 12 | Unknown | 7 | Unknown | 10 | 15 |
| Renal pathology | MCD | MCD | MCD | MCD | MsPGN | No | No |
| Number of relapses | 3 | 5 | 0 | 4 | 0 | 1 | 0 |
Pre: prednisone, CTX: cyclophosphamide, MMF: mycophenolate mofetil, MCD: minimal change disease, MsPGN: mesangial proliferative glomerulonephritis, F: female, M: male.
DISCUSSION
Familial SSINS is a rare condition, and only approximately 3% of patients have affected siblings (9). Nonetheless, the occasional occurrence of familial SSINS points to a genetic predisposition for this syndrome (2,10). No genes involved in familial SSINS have yet been identified. Kari et al. (5) investigated the major histocompatibility complex class I and II loci in three Bengali families with nine children affected by steroid-sensitive nephrotic syndrome. However, no correlations between a predisposition for this disease and the human leukocyte antigen (HLA)-encoding genes were found. Fuchshuber (4) reported a cohort of 32 cases of SSINS from 15 families who experienced disease onset during childhood. The results of linkage studies and mutational analyses indicated that familial SSINS was not associated with the Nphs2 gene. This gene has been linked to focal segmental glomerulosclerosis (FSGS) in certain cases of autosomal recessive SRNS based on the pathology and rapid progression to end stage renal disease (ESRD). In the present study, a mutational analysis of the Nphs2 gene (11) was conducted for all seven patients from three families, and no homozygous or compound heterozygous mutations were found. One known polymorphism (V736V) in the Nphs1 gene, which does not involve an amino acid substitution, was found in patient 2. We have also reported finding this polymorphism in Chinese children with sporadic steroid-resistant nephrotic syndrome (11). For that reason, we assumed that V736V was not a pathogenic mutation in this patient with familial SSINS.
Motoyama et al. (3) summarized the histological findings from 41 familial SSINS patients, all of whom were found to have MCD. In the present study, two cases from Family 1 and 2 cases from Family 2 presented with MCD, whereas one case from Family 2 presented with MsPGN. To our knowledge, this is the first reported case of familial SSINS with MsPGN pathology, and it is unclear whether there was an association between the age at onset (35 years vs. 2.5–15 years) and the pathology (MsPGN vs. MCD) in these cases. For patient 5 (who presented with MsPGN), we excluded minimal change nephrotic syndrome with mesangial hypercellularity (no IgA deposits were observed using immunofluorescence and electron microscopy) and IgA nephropathy associated with minimal change nephrotic syndrome (the immunofluorescence analysis revealed predominantly IgA deposits in the mesangial area). Renal biopsies are not typically conducted in familial SSINS patients because the course of the disease is favorable in children. In a study reported by Fuchshuber (4), only 12 of 32 familial SSINS patients underwent renal biopsies. It is possible that MCD is not a unique pathological finding in patients with familial SSINS; further research is needed.
Environmental factors (e.g., preceding infection, infectious agents, or medications) may trigger the onset of nephrotic syndrome in several members of the same family over a short period of time. In Family 3, the onset of proteinuria and edema was separated by only two months in patients 6 and 7. Environmental factors may have played a role in the pathogenesis of proteinuria in this family (12). However, prior to the onset of proteinuria, neither patient had any history of viral, mycoplasma, or bacterial infections; allergies to milk or dust; asthma; neoplasms; or the use of medications associated with proteinuria, such as NSAIDs or antimicrobials. Therefore, we favor the hypothesis that there is a genetic predisposition to SSINS in this family.
In conclusion, familial SSINS is rare, but it is occasionally encountered in the clinic. No genes related to this condition have yet been identified, and further research is necessary.
ACKNOWLEDGMENTS
This project was supported by the National Natural Science Foundation of China (Grant Nos. 81270792, 81070561, and 81170664), the Research Fund for the Doctoral Program of Higher Education of China (20120101110018), the Zhejiang Provincial Healthy Science Foundation of China (WKJ2010-2-014, 2012KYA119), the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents, and the Zhejiang Provincial Natural Science Foundation of China (LY12H050037). We thank all the patients for participating in this study.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Ruf RG, Lichtenberger A, Karle SM, Haas JP, Anacleto FE, Schultheiss M, et al. Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol. 2004;15(3):722–32. doi: 10.1097/01.asn.0000113552.59155.72. [DOI] [PubMed] [Google Scholar]
- 2.Chang JW, Lin CY. Long-term outcome of heavy proteinuria in patients under 2 years of age. Pediatr Nephrol. 2003;18(10):1044–8. doi: 10.1007/s00467-003-1238-6. [DOI] [PubMed] [Google Scholar]
- 3.Motoyama O, Sugawara H, Hatano M, Fujisawa T, Iitaka K. Steroid-sensitive nephrotic syndrome in two families. Clin Exp Nephrol. 2009;13(2):170–3. doi: 10.1007/s10157-008-0117-7. [DOI] [PubMed] [Google Scholar]
- 4.Fuchshuber A, Gribouval O, Ronner V, Kroiss S, Karle S, Brandis M, et al. Clinical and genetic evaluation of familial steroid-responsive nephrotic syndrome in childhood. J Am Soc Nephrol. 2001;12(2):374–8. doi: 10.1681/ASN.V122374. [DOI] [PubMed] [Google Scholar]
- 5.Kari JA, Sinnott P, Khan H, Trompeter RS, Snodgrass GJ. Familial steroid-responsive nephrotic syndrome and HLA antigens in Bengali children. Pediatr Nephrol. 2001;16(4):346–9. doi: 10.1007/s004670000549. [DOI] [PubMed] [Google Scholar]
- 6.Bensman A, Vasmant D, Mougenot B, Baudon JJ, Muller JY. Steroid-responsive nephrotic syndrome in infants: 2 familial case reports. Arch Fr Pediatr. 1982;39(6):381–3. [PubMed] [Google Scholar]
- 7.Ruf RG, Fuchshuber A, Karle SM, Lemainque A, Huck K, Wienker T, et al. Identification of the first gene locus (SSNS1) for steroid-sensitive nephrotic syndrome on chromosome 2p. J Am Soc Nephrol. 2003;14(7):1897–00. doi: 10.1097/01.asn.0000070070.03811.02. [DOI] [PubMed] [Google Scholar]
- 8.Chen YQ, Zhang H, Lv JC, Zhou Y, Wang HY. A familial steroid-responsive nephrotic syndrome report. Chin J Nephrol. 2003;19:378–80. [Google Scholar]
- 9.Moncrieff MW, White RH, Glasgow EF, Winterborn MH, Cameron JS, Ogg CS. The familial nephrotic syndrome . II. A clinicopathological study. Clin Nephrol. 1973;1(4):220–9. [PubMed] [Google Scholar]
- 10.Wang DY, Mao JH, Zhang Y, Gu WZ, Zhao SA, Chen YF, et al. Kimura disease: a case report and review of the Chinese literature. Nephron Clin Pract. 2009;111(1):c55–61. doi: 10.1159/000178980. [DOI] [PubMed] [Google Scholar]
- 11.Mao J, Zhang Y, Du L, Dai Y, Gu W, Liu A, et al. NPHS1 and NPHS2 gene mutations in Chinese children with sporadic nephrotic syndrome. Pediatr Res. 2007;61(1):117–22. doi: 10.1203/01.pdr.0000250041.19306.3d. [DOI] [PubMed] [Google Scholar]
- 12.Bockenhauer D, Debiec H, Sebire N, Barratt M, Warwicker P, Ronco P, et al. Familial Membranous Nephropathy: An X-Linked Genetic Susceptibility. Nephron Clin Pract. 2008;108(1):c10–c15. doi: 10.1159/000112466. [DOI] [PubMed] [Google Scholar]