Abstract
OBJECTIVE:
The aim of this study is to verify the expression of proteins that are controlled by miR-let7c, 100 and 218 using immunohistochemistry in tissue microarray representative of localized and metastasized the lymph nodes and bone prostate cancer.
METHODS:
To verify the expression of proteins that are controlled by miR-let7c (C-MYC, BUB1, RAS) 100 (SMARCA5, RB) and 218 (LAMB3) and cell proliferation (Ki-67) we used immunohistochemistry and computerized image system ImageJ MacBiophotonics in three tissue microarrays representative of localized prostate cancer and lymph node and bone metastases. miRNA expression was evaluated by qRT-PCR using 60 paraffin blocks to construct the tissue microarray representative of localized disease.
RESULTS:
RAS expression was increased in localized prostate cancer and bone metastases compared to the lymph nodes (p = 0.017). RB showed an increase in expression from localized prostate cancer to lymph node and bone metastasis (p = 0.036). LAMB3 was highly expressed in localized and lymph node metastases (p<0.001). Cell proliferation evaluated by Ki-67 showed an increase from localized prostate cancer to metastases (p<0.001). We did not found any relationship between C-MYC (p = 0.253), BUB1 (p = 0.649) and SMARCA5 (p = 0.315) protein expression with prognosis or tumor behavior.
CONCLUSION:
We found that the expression of RAS, RB, LAMB3 and Ki-67 changed in the different stages of prostate cancer. Furthermore, we confirmed the overexpression of the miRNAs let7c, 100 and 218 in localized prostate cancer but failed to show the control of protein expression by the putative controller miRNAs using immunohistochemistry.
Keywords: Prostate Cancer; Prognosis; Tumor Markers; Micro RNA; Immunohistochemistry; RAS; C-MYC; BUB1; SMARCA5; LAMB3; Ki-67, RB
INTRODUCTION
Prostate cancer (PC) is a common disease with a multifactorial and complex etiology. PC is the most common male malignancy and the second leading cause of death among men in many countries, including Brazil. In the United States, 241,740 new cases and 28,170 deaths related to PC are estimated for the year 2012 (1).
The widespread use of prostate-specific antigen (PSA) has increased prostate cancer detection rates at earlier stages. However, up to 20% of clinically localized cases recur during a 10-year follow-up period after local radical treatment. In addition, current clinical and pathological parameters fail to determine an accurate prognosis in many cases (2). Prior identification of patients with poor prognoses is of paramount importance in clinical practice, especially for determining whether additional treatments are necessary. Currently, there are no clinical parameters or molecular markers that can accurately assess the aggressive behavior and metastatic potential of PC. Nevertheless, both tumor invasion and progression to metastasis, which are the signatures of malignancy, are related to cell proliferation and chromosomal stability.
We have recently described alterations in the expression profile of micro RNAs (miRNAs) during the progression from localized to metastatic PC characterized by the loss of expression of miR-let7c, miR-100 and miR-218 (3). miRNAs are a class of noncoding RNAs responsible for the control of one third of the human genes involved in many carcinogenic processes. C-MYC, RAS and BUB1 are the target genes for miR-let7c (4,5) while SMARCA5 and Retinoblastoma (RB) are the target genes for miR-100 (http://atlasgeneticsoncology.org/Genes/MIR100ID51447ch11q24.html) and Laminin 5 β3 (LAMB3) is the target for miR-218 (6).
C-MYC is a multifunctional, nuclear phosphoprotein that plays a role in cell cycle progression, apoptosis and cellular transformation; this protein also functions as a transcription factor that regulates the transcription of specific target genes (7). The RAS protein functions as a binary molecular switch that controls intracellular signaling networks involved in cytoskeletal integrity, cell proliferation, differentiation, adhesion, apoptosis, and migration (8). Bub-1 is a kinase protein involved in spindle checkpoint by phosphorylating a member of the mitotic checkpoint complex (9). SMARCA5 mediates DNA accessibility by sliding the histone octamer, which is important for gene expression, DNA replication, DNA repair, and the maintenance of the chromatin structure (10). The RB protein is a key negative regulator of the cell cycle and an important tumor suppressor gene (11). Finally, LAMB3 and its ligand α6β4-integrin have a role in cell migration, and their expression has been related to cell transformation in SCID mice (12).
The aim of this study was to verify the expression of proteins that are controlled by miR-let7c, 100 and 218 using immunohistochemistry (IHC) in tissue microarrays (TMA) representative of both localized PC and PC that had metastasized to the lymph nodes and bone.
METHODS
Patients
For the representative TMA of localized PC, we retrospectively evaluated 954 patients with clinically localized PC who underwent radical prostatectomy with a curative intention between January 1994 and April 2000; from this population, we randomly selected the patients of our study. All the surgeries were performed by the same surgeon (MS). The cohort consisted of 45 cases with biochemical recurrence after surgery and 67 cases without recurrence. The mean age was 63.6 years, the mean PSA was 10.8 ng/mL and the mean Gleason score was 7.2. The patients were followed for a median of 79 months. Ninety-seven percent of patients were Caucasian, and 2.7% were Asian. Tumor recurrence was defined as a PSA level above 0.4 ng/ml. The tumor-node-metastasis (TNM) staging designations were assigned according to the TNM 2010 classification. For the construction of the second TMA, 19 cases of lymph node metastases were selected from 1,619 patients who underwent radical prostatectomy between March 1997 and July 2006. The mean age was 66 years, the mean PSA was 11.9 ng/mL, and the mean Gleason score was 8.8. The slides containing the metastasis and the primary tumor for each patient were selected by considering the area that best represented the whole tumor. One area from the metastasis and two areas from the primary tumor were selected and marked with permanent ink; these areas correspond to those included in the TMA. The third TMA consisted of twenty-eight patients with a mean age of 67.3 years, a mean PSA of 553 ng/mL and a mean Gleason score was 8.3. This cohort of patients had PC bone metastases (femur = 16, iliac = 2, vertebra = 6, scapula = 2, humerus = 1, and pubis = 1) and had undergone surgery for the treatment of secondary bone events at the Orthopedic Institute of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP). This study conformed to the provisions of the Declaration of Helsinki and was submitted to and approved by the Ethical Board of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo under protocol 1074/04.
Immunohistochemical study
The TMA were constructed on Superfrost slides. The samples underwent a heat antigen retrieval process using citrate buffer (1 mM, pH 6.0). The slides were incubated overnight at 4°C with the monoclonal antibodies specified in Table 1). The LSAB system was used for immunostaining (Dako Cytomation, CA). Color was developed by a reaction with a 3,3′diaminobenzidine substrate-chromogen solution followed by counterstaining with Harris hematoxylin. The slides were dehydrated, coverslipped and observed under a light microscope. Cytokeratin 18 was used to test the preservation of the antigens, and it was strongly positive in all cases.
Table 1.
Antibodies used in TMAs representatives of localized and metastatic prostate cancer to lymph nodes and bones.
| Antibody | Clone | Brand |
| RAS | F132 | Santa Cruz |
| C-MYC | 0.N.222 | Santa Cruz |
| BUB1 | Polyclonal | Abcam |
| Ki-67 | Polyclonal | Millipore |
| LAMB3 | 4C7 | Dako |
| RB | IF8 | Chemicon |
| SMARCA5 | H-300 | Santa Cruz |
Computerized analysis of the immunohistochemical reactions
The expression of each marker was evaluated by one pathologist (KRML). The images were captured by an optical microscope with a 40 × objective (Nikon Eclipse E200) coupled to a camera (Nikon DsFi1) using the software NIS-Elements D 3.1. The luminescence images were quantified densitometrically using the ImageJ MacBiophotonics (National Institutes of Health, U.S.) software package “plug-ins” developed by McMaster University (http://www.macbiophotonics.ca/ImageJ). The antibodies that bound to nuclear proteins (C-MYC, SMARCA5) were evaluated by the percentage of stained nuclei, and the antibodies that bound to cytoplasmic proteins (Bub 1, RAS and Lamb3) were evaluated as an arbitrary unit correspondent to the area occupied by stained tumor cells; Ki-67 was used to establish the proliferative index.
MiRNA expression
To compare the miRNA expression with the expression of the associated target proteins, we evaluated the miRNA expression by qRT-PCR using 60 paraffin blocks that were used for the construction of the TMA representative of localized disease as previously described (4). Briefly, small RNA fractions were isolated and enriched using a mirVana miRNA isolation kit (Ambion, Austin, TX), and the cDNA was obtained using a TaqMan® miRNA Reverse Transcription kit (Applied Biosystems, Foster City, CA). Next, 10 ng of miRNA was reverse-transcribed using sequence-specific stem-loop primers to the has-miR-let7c, hsa-miR-100 and hsa-miR-218 genes. The reaction was performed in 9600 Emulation mode with the following parameters: 30 min at 16°C, 5 min at 42°C, 5 min at 85°C and 4°C until analysis. Quantitative RT-PCR was carried out using the ABI 7500 Fast Real-Time PCR System and a Taqman Universal PCR Master Mix (Applied Biosystems, Foster City, CA). The expression of the individual miRNAs mentioned above was analyzed using miRNA sequence-specific primers. These miRNAs were selected for verification based on their predicated target genes listed in the Sanger miRBase database (http://microrna.sanger.ac.uk/sequences) (13). miRNA expression levels were assessed by relative quantification, and the fold expression changes were determined by the 2−ΔΔCT method (14). All RT-PCRs were performed in duplicate, and the small nucleolar RNA RNU43 was used as the endogenous control.
Statistical analysis
Statistical analyses were performed with SPSS 19.0 for Windows, and all reported p-values are two sided. Comparisons of the miRNA and protein expression levels were performed using Student's t and Mann-Whitney tests for homogenous and heterogeneous variables, respectively. For comparison among three or more variables, we used an ANOVA test or the Kruskal-Wallis test for homogenous and heterogeneous variables, respectively. A Chi-squared test was used to compare nominal scale values. A p-value≤0.05 was considered statistically significant.
RESULTS
Statistical analysis showed that RAS expression was significantly increased in localized PC (124.93) compared to the lymph nodes (17.64) and that there was an increase in expression when the PC metastasized to the bones (104.04) (p = 0.017). RB showed an increase in expression from localized PC (12.5%) to lymph node and bone metastases (25.7% and 20.2%, respectively; p = 0.036). LAMB3 was highly expressed in localized PC (127.3) and lymph node metastases (132.5), but the expression was low in the bone metastases (9.2) (p<0.001). Cell proliferation using Ki-67 increased from localized PC (11.2%) to the lymph node metastasis (22.3%) and bone metastasis (42.5%) (p<0.001). C-MYC, Smarca-5 and Bub-1 expression did not show differences during PC progression (p = 0.253, p = 0.315 and p = 0.649, respectively) (Table 2 and Figure 1).
Table 2.
Immunohistochemical analyses of proteins that are target of miR-let7c, miR-100 and miR-218. The nuclear staining was evaluated as percentage of positive nuclei whereas cytoplasmic expression was evaluated as an arbitrary unit correspondent to the area occupied by tumor cells.
| Antibody | Localized prostate cancer | Lymph node metastasis | Bone metastasis | p-value |
| RAS**) | 124.9 | 17.6 | 104.0 | 0.017 |
| RB*) | 12.5% | 25.7% | 20.2% | 0.036 |
| LAMB3**) | 127.3 | 132.5 | 9.2 | <0.001 |
| Ki-67*) | 11.2% | 22.3% | 42.5% | <0.001 |
| C-MYC*) | 40.2% | 57.3% | 51.7% | 0.253 |
| BUB1**) | 164.65 | 144.2 | 152.6 | 0.649 |
| SMARCAR5*) | 68.0% | 48.4% | 80.5% | 0.315 |
percentage of stained nuclei.
arbitrary unit correspondent to the area occupied by tumor cells.
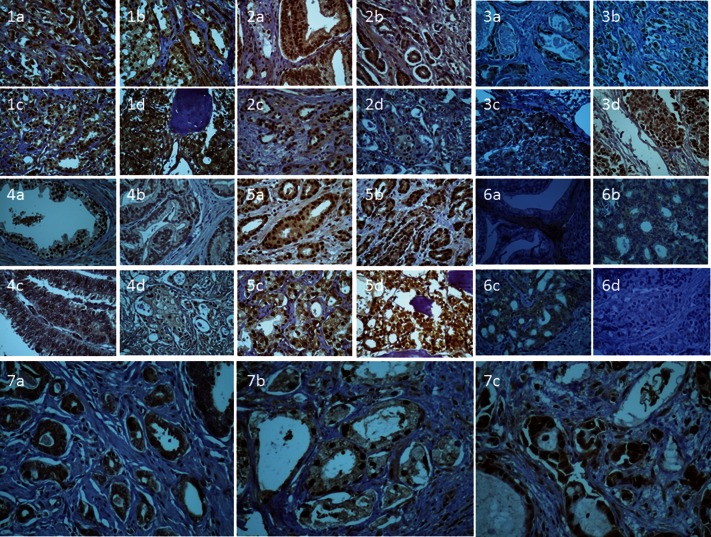
Figure 1.
1) Cytoplasmic and nuclear immunoexpression of BUB1 in localized prostate cancer (A and B), in lymph node metastases (C), and in bone metastases (D). 2) Nuclear immunoexpression of c-C-MYC in localized prostate cancer (A and B), in lymph node metastases (C), and in bone metastases (D). 3) Nuclear (A) and cytoplasmic (B) immunoexpression of RAS in localized prostate cancer, in lymph node metastases (C), and in bone metastases (D). 4) Nuclear immunoexpression of RB in normal prostate glands (A), in localized prostate cancer (B), in lymph node metastases (C), and in bone metastases (D). 5) Nuclear immunoexpression of SMARCA5 in localized prostate cancer (A and B), in lymph node metastases (C), and in bone metastases (D). 6) Immunoexpression of LAMB3 in the basal membrane (A) and cytoplasmic staining in localized prostate cancer (B), in lymph node metastases (C), and in bone metastases (D). 7) Nuclear Ki-67 immunoexpression in localized prostate cancer (A), in the lymph node (B), and in bone metastasis (C).
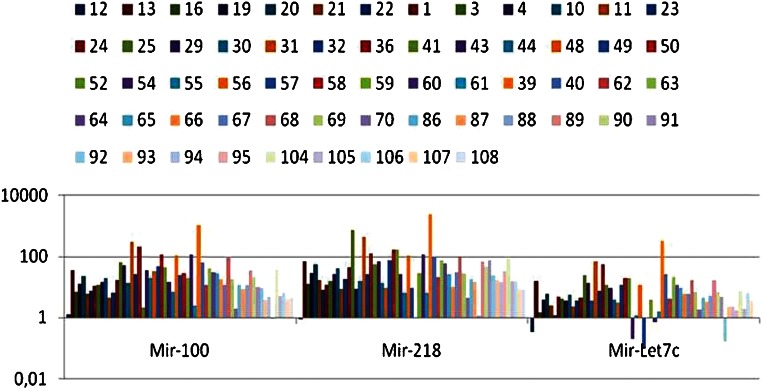
We confirmed the overexpression of miR-let7c, 100 and 218 by qRT-PCR. The results are expressed in Figure 2. MiR-100 and 218 were overexpressed in 98.4% of the cases, and miR-let7c was overexpressed in 91.8% of the cases. The IHC expression levels of the proteins were compared to the mean expression of miRNAs, and no statistical relationship was detected for the results that are shown in Table 3).
Figure 2.
Expression of miR-let7c, miR-100 and miR-218 in 60 cases of localized prostate cancer as part of a TMA to evaluate the protein immunoexpression of their supposed target genes.
Table 3.
Correlation between the IHC expression of proteins and the expression of their respective controllers miRNAs analyzed by qRT-PCR.
| miRNA | Protein | Mean | p-value |
| Let7c | C-MYC | Positive – 17.09 | 0.489 |
| Negative – 9.01 | |||
| BUB1 | Positive – 8.19 | 0.421 | |
| Negative – 17.57 | |||
| RAS | Positive – 10.09 | 0.412 | |
| Negative – 19.4 | |||
| 100 | SMARCA5 | ≤99% – 19.96 | 0.213 |
| = 100% – 66.14 | |||
| RB | ≤9 – 68.04 | 0.356 | |
| >9 – 33.15 | |||
| 218 | LAMB3 | Positive – 136.55 | 0.140 |
| Negative – 45.90 |
DISCUSSION
Carcinoma of the prostate can vary greatly in biological behavior, ranging from slowly progressive to highly aggressive metastasizing tumors. The introduction of PSA into clinical practice allowed PC to be diagnosed in its early stages, increasing the chances of cure with appropriate treatment. However, 20 to 30% of patients still suffer recurrence after surgery and commonly receive a salvage therapy, such as radiotherapy; however, the disease-free survival rate decreases considerably at this time (2). Therefore, understanding which factors contribute to the prognosis of PC is of paramount importance.
The establishment of biomarkers that reflect crucial biological functions in oncogenesis, such as cell cycling, proliferation and chromosomal instability, would enable better prognostication. We hypothesized that changes in RAS, C-MYC, BUB1, RB, SMARCA5, LAMB3 and Ki-67 expression would be related to these events.
In this study, we found that the expression of RAS, RB, LAMB3 and Ki-67 changed in different stages of PC. There was an increase in proliferation during tumor progression reflected by the higher percentage of Ki-67 expression in bone metastases, followed by lymph node metastases and finally the primary tumors. Ki-67 is a nuclear antigen present throughout the whole cell cycle (G1, S, G2 and M phases) except during the rest phase (GO phase) or in the early G1 phase (15). Ki-67 nuclear staining has been related to biological aggressiveness and prognosis in several cancers, and a greater proliferative index indicates more aggressive behavior of the neoplasia (16). Ki-67 is a reliable marker of cellular proliferation that correlates well with the uptake of bromodeoxyuridine and thymidine labeling (17). Statistically significant differences in the mean Ki-67 indices of benign prostatic hyperplasia and prostate cancer have been uniformly reported (18,19). Previous studies of Ki-67 staining in prostate cancer have shown a variable relationship with tumor stage and grade; however, significant associations have been found between Ki-67 expression and the time to progression, metastatic status and tumor volume (20–22).
We were unable to show a correlation between the expression of proteins related to the control of cell proliferation and Ki-67 expression. RAS was highly expressed in localized PC; however, a significant decrease in the expression was observed in the lymph node metastases that was regained in the bone metastases. The family of RAS oncogenes has been extensively studied for their involvement in the multistep process of carcinogenesis (23). Overexpression of RAS oncogenes has also been detected in several human cancers, including breast, colon, bladder and lung, and has been associated with the development of the disease (24). RAS activation is implicated in human carcinogenesis mainly by inhibiting apoptosis and promoting cellular proliferation. However, there is considerable evidence that the activated RAS oncogene can also have the opposite action by promoting apoptosis and inhibiting cellular proliferation. Furthermore, this oncogene may have an oncosuppressive role under many circumstances. Therefore, we argue that RAS is important for local tumor growth, but it is not essential for tumor progression, as it is secondary during invasion and metastases processes (25).
RB responds in part to the presence of mitogenic signals and controls the cell cycle mainly during the transition from G0 to S by binding and inactivating transcription factors. Comparing the RB expression between normal prostate tissue and PC, we could see an enormous loss of staining; however, the PC progression from local tissue to the metastases was accompanied by an increase in RB expression. We believe that this increase is a reactive process associated with the increase in cell proliferation. Ambrosch et al. (26) have also shown a loss in the expression of RB in head and neck carcinomas, but this loss was independent of tumor progression. Maddison et al. (27) showed in an in vivo model that the loss of RB induces cell proliferation in the prostate without a transformation; this finding reinforces the fact that RB is important for the control of cell proliferation but may not be related to tumor progression and metastasization.
LAMB3 was maintained in localized and lymph node metastases but was almost completely absent in the bone metastases. Hao et al. (28) have previously shown the same phenomenon in prostate cancer. LAMB3 is a glycoprotein abundant in the basal membrane with important biological activities involved in cell adhesion, migration, angiogenesis and metastasization (29). In the prostate, LAMB3 is expressed in normal and tumoral tissues but as different isoforms. LM332 is predominant in normal prostate tissue (30), and its expression levels decrease during tumor progression. The authors believe that the loss of this specific isoform promotes a crucial change in the extra-cellular matrix that turns it into a weaker environment conducive to metastatic dissemination.
In conclusion we found that the expression of RAS, RB, LAMB3 and Ki-67 changed in the different stages of prostate cancer. Furthermore, we confirm that miRNAs let7c, 100 and 218 are overexpressed in localized PC but failed to show the control of protein expression by the supposed controller miRNAs. We believe that the regulation of these miRNAs is subtle and not detectable by IHC. Protein expression control is not a binary system, and no evidence of a loss of protein expression related to miRNA expression was detected even with computerized image analysis. Therefore, western blot analysis may be more suitable for the evaluation of the control of proteins by miRNAs.
ACKNOWLEDGMENTS
This study was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) under protocol number 2009/52158-1.
Footnotes
No potential conflict of interest was reported.
REFERENCE
- 1.Siegel R, Desantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Whitmore WJ., Jr Natural history and staging of prostate cancer. Urol Clin North Am. 1984;11(2):205–20. [PubMed] [Google Scholar]
- 3.Leite KR, Sousa-Canavez JM, Reis ST, Tomiyama AH, Camara-Lopes LH, Sañudo A, et al. Change in expression of miR-let7c, miR-100, and miR-218 from high grade localized prostate cancer to metastasis. 2011;29(3):265–9. doi: 10.1016/j.urolonc.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120(5):635–47. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, et al. MicroRNA let-7a downregulates C-MYC and reverts C-MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67(20):9762–70. doi: 10.1158/0008-5472.CAN-07-2462. [DOI] [PubMed] [Google Scholar]
- 6.Martinez I, Gardiner AS, Board KF, Monzon FA, Edwards RP, Khan SA. Khan Human papillomavirus type 16 reduces the expression of microRNA-218 in cervical carcinoma cells. Oncogene. 2008;27(18):2575–82. doi: 10.1038/sj.onc.1210919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackwood EM, Eisenman RN. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with C-MYC. Science. 1991;251(4998):1211–7. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- 8.Stopka T, Skoultchi AI. The ISWI ATPase Snf2h is required for early mouse development. PNAS. 2003;100(24):14097–102. doi: 10.1073/pnas.2336105100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.BaRBacid M: RAS genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 10.Adams PD, Kaelin WG., Jr Negative control elements of the cell cycle in human tumors. Curr Opin Cell Biol. 1998;10(6):791–7. doi: 10.1016/s0955-0674(98)80123-3. [DOI] [PubMed] [Google Scholar]
- 11.Bullwinkel J, Baron-Lühr B, Lüdemann A, Wohlenberg C, Gerdes J, Scholzen T. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferatingcells. J Cell Physiol. 2006;206(3):624–35. doi: 10.1002/jcp.20494. [DOI] [PubMed] [Google Scholar]
- 12.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99(24):15524–9. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams BD, Furneaux H, White BA. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-alpha (ERe) and represses ERe messenger RNA and protein expression in breast cancer cell lines. Mol Endocrinol. 2007;21(5):1132–47. doi: 10.1210/me.2007-0022. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2deltadeltaCT method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133(4):1710–5. [PubMed] [Google Scholar]
- 16.Brown DC, Gatter KC. Monoclonal antibody Ki-67: its use in histopathology. Histopathology. 1990;17(6):489–503. doi: 10.1111/j.1365-2559.1990.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 17.Cher ML, Chew K, Rosenau W, Carroll PR. Cellular proliferation in prostatic adenocarcinoma as assessed by bromodeoxyuridine uptake and Ki-67 and PCNA expression. Prostate. 1995;26(2):87–93. doi: 10.1002/pros.2990260205. [DOI] [PubMed] [Google Scholar]
- 18.Gallee MPW, Visser-de Jong E, ten Kate FWJ, Schroeder FH, van der Kwast TH. Monoclonal antibody Ki-67 DEFINED Growth fraction in benign prostatic hyperplasia and prostatic cancer. J Urol. 1989;142(5):1342–6. doi: 10.1016/s0022-5347(17)39094-8. [DOI] [PubMed] [Google Scholar]
- 19.Raymond WA, Leong ASY, Bolt JW, Milios J, Jose JS. Growth fractions in human prostatic carcinoma determined by the Ki-67 immunostaining. J Pathol. 1988;156(2):161–7. doi: 10.1002/path.1711560211. [DOI] [PubMed] [Google Scholar]
- 20.McLoughlin J, Foster CS, Price P, Williams G, Abel PD. Evaluation of Ki-67 monoclonal antibody as prognostic indicator for prostatic carcinoma. Br J Urol. 1993;72(1):92–7. doi: 10.1111/j.1464-410x.1993.tb06466.x. [DOI] [PubMed] [Google Scholar]
- 21.Sadi MV, Barrack ER. Determination of growth fraction in advanced prostate cancer by Ki-67 immunostaining and its relationship to the time to tumor progression after hormonal therapy. Cancer. 1991;67(12):3065–71. doi: 10.1002/1097-0142(19910615)67:12<3065::aid-cncr2820671222>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Van Weerden WM, Moerings EP, van Kreuningen A, de Jong FH, van Steenbrugge GJ, Schröder FH. Ki-67 expression and BrdUrd incorporation as markers of proliferative activity in human prostate tumour models. Cell Prolif. 1993;26(1):67–75. doi: 10.1111/j.1365-2184.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 23.BaRBacid M: RAS genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 24.Zachos G, Spandidos DA. Expression of RASproto-oncogenes: regulation and implications in the development of human tumors. Crit Rev Oncol Hematol. 1997;26(2):65–75. doi: 10.1016/s1040-8428(97)00013-9. [DOI] [PubMed] [Google Scholar]
- 25.Lim KH, Counter CM. Reduction in the requirement of oncogenic Ras signaling to activation of PI3K/AKT pathway during tumor maintenance. Cancer Cell. 2005;8(5):381–92. doi: 10.1016/j.ccr.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Ambrosch P, Schlott T, Hilmes D, Ruschenburg I. p16 alterations and retinoblastoma protein expression in squamous cell carcinoma and neighboring dysplasia from the upper aerodigestive tract. Virchows Arch. 2001;438(4):343–9. doi: 10.1007/s004280000368. [DOI] [PubMed] [Google Scholar]
- 27.Maddison LA, Sutherland BW, Barrios RJ, Greenberg NM. Conditional deletion of RB causes early stage prostate cancer. Cancer Res. 2004;64(17):6018–25. doi: 10.1158/0008-5472.CAN-03-2509. [DOI] [PubMed] [Google Scholar]
- 28.Hao J, Yang Y, McDaniel KM, Dalkin BL, Cress AE, Nagle RB. Differential expression of laminin 5 (alpha 3 beta 3 gamma 2) by human malignant and normal prostate. Am J Pathol. 1996;149(4):1341–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol. 2003;200(4):423–8. doi: 10.1002/path.1437. [DOI] [PubMed] [Google Scholar]
- 30.Nagle RB. Role of the extracellular matrix in prostate carcinogenesis. J Cell Biochem. 2004;91(1):36–40. doi: 10.1002/jcb.10692. [DOI] [PubMed] [Google Scholar]