Abstract
The relationship between Toxocara infection and epilepsy was previously demonstrated by several case-control studies and case reports. These previous studies were often based on the enzyme-linked immunosorbent assay (ELISA) using Toxocara excretory-secretory antigens, which are not specific due to cross-reactivity with other parasitic infections such as ascariasis, trichuriasis, and anisakiasis. An immunoblot analysis is highly specific and can detect low levels of Toxocara antibodies. Therefore, this assay may be useful in the identification of toxocariasis in epileptic patients. We examined patients who had epilepsy and healthy subjects for seropositivity for Toxocara infection by ELISA and Western blotting. Out of 85 epileptic patients, 10 (11.8%) and 3 (3.5%) persons exhibited Toxocara immunoglobulin G (IgG) antibodies responses by ELISA and by both techniques, respectively. Moreover, in the healthy group (n = 85), 3 (3.5%) persons were positive by ELISA, but none was detected by Western blotting. This study indicates that Toxocara infection is a risk factor for epilepsy in Iran. These findings strongly suggest the need to perform Western blotting immunodiagnosis, as well as the ELISA using Toxocara excretory-secretory antigens, to improve diagnosis of human toxocariasis in patients with epilepsy.
1. Introduction
Human toxocariasis is a zoonotic parasitic disease caused by migration of the nematode worms Toxocara canis (dog) or Toxocara cati (cat) larvae to the organs and tissues of animals and birds. Human infection occurs when Toxocara eggs containing infective larvae are accidentally ingested or through consumption of raw or undercooked meat and giblets [1]. The larvae hatch in small intestine and migration through somatic organs, preferably the liver, brain, and eyes, and cause at least three syndromes: visceral larva migrans (VLM), ocular larva migrans (OLM), and covert toxocariasis [2].
The tests are available for the immunodiagnosis including enzyme-linked immunosorbent assay (ELISA) and Western blotting, both using Toxocara canis excretory-secretory (TES) antigens [3]. ELISA using TES antigens was the first method to be employed, irrespective of the clinical form of toxocariasis [4–6]. However, Magnaval et al. (1991) reported the high sensitivity and specificity of WB for immunodiagnosis of toxocariasis [7]. In addition, previous reports using both techniques indicated that Western blotting is a better method than ELISA to investigate patients suffering from visceral larva migrans or ocular larva migrans [8].
The epidemiological studies have noted the presence of Toxocara spp. infection in stray cats and dogs in various parts of Iran [9–11]. In Khorramabad, Iran, 22.2% of the public parks studied showed Toxocara eggs [12].
Toxocara is one of the common helminthic parasites reported that affect the human central nervous system (CNS). Several epidemiological and clinical studies have reported a correlation between Toxocara canis infection and epilepsy. Recently some authors have investigated this association in different geographic locations through case-control studies using serological tests [13–18].
To the best of our knowledge, there is no precise report from the anti-Toxocara antibodies in epileptic patients in the region. Therefore, this study afforded an opportunity to investigate the antibody response to Toxocara infection in epileptic patients by the performance of TES-ELISA and Western blotting.
2. Materials and Methods
2.1. Study Population
This study was conducted on 85 consecutive patients with idiopathic epilepsy, who were evaluated regarding their medical history, clinical characteristic, cranial imaging such as computed tomography (CT) scans, or magnetic resonance imaging (MRI) at the Neurology Division of Shohadaye-Ashayer Hospital from March to December 2011. Controls subjects were 85 volunteers from health care workers and the relatives of patients without history of neurological disorders. Epidemiological data determining the socioeconomic status of the participants groups were collected by a paper-and-pencil questionnaire. Neurological examination for each individual was performed, and the physical findings were recorded.
2.2. Serological Tests
Serum samples of all participations were collected and stored at −20°C until used. Anti-Toxocara antibodies (IgG) were detected by a commercial Enzyme-Linked Immunosorbent Assay (ELISA) kit (IBL, International Gmbh, Hamburg, Germany) following the manufacture's guidance. Briefly, diluted sera samples (1 : 100) were added to wells; after 30 min incubation at 37°C, horseradish peroxidase-conjugated goat anti-human IgG was added at a 1 : 1000 dilution (30 min at 37°C), followed by the tetramethylbenzidine (TMB) substrate. Absorbance readings were made at 450 nm; a cut-off absorbance value was defined as the mean absorbance reading for three negative control sera plus two standard deviations. Antibody levels were expressed as reactivity indices, which were calculated as the cut-off value; positive samples had reactivity indices more than 0.553.
Immunodiagnosis by the Western blotting technique relied also upon a commercial kit (LDBIO Diagnostic, Lyon, France). In brief, the strips incubated for 2 h at room temperature, with sera at a dilution of 1/100. Before being washed at least three times in PBS containing 0.1% Tween 20, the strips were further incubated for 2 h at room temperature with the second antibody, anti-human immunoglobulin G peroxidase conjugate (dilution 1/1,000). After washing as mentioned previously, the substrate diaminobenzidine was added and the reaction stopped with several washes in distilled water. The results were considered as positive, when the samples react to two or more low-molecular-weight bands (LMWB; 24–35 kDa).
2.3. Statistical Analysis
We conducted statistical analysis using SPSS version 15.0 of windows 2003. Chi-squire test and Fisher's exact test were used for categorical data. A P value that is less than 0.05 was considered statistically significant.
2.4. Ethical Considerations
The study was approved by ethical committee of the Lorestan University of Medical Sciences, and informed consent was obtained from the participants prior to data collection.
3. Results
3.1. Characteristics of the Collected Pairs
Patients and control groups comprised 85 cryptogenic epileptic patients and 85 healthy persons, respectively. Of the patient group, 55 participants were males and 30 were females. The participants' ages ranged from 8 to 62 years old. Of the control group, 57 participants were males and 28 were females. These participants' ages in control group ranged from 7 to 59 years old. The epidemiologic and demographical factors in patients and healthy groups are shown in Table 1.
Table 1.
The epidemiological and demographical factors in patients and healthy participants.
| Factors | Patients, n (%) | Healthy, n (%) | Total | P value |
|---|---|---|---|---|
| Age group (years) | ||||
| <20 | 42 (49.5) | 38 (44.7) | 80 | >0.05 |
| 20–39 | 28 (32.9) | 34 (40.0) | 62 | |
| 40–59 | 12 (14.1) | 13 (15.3) | 25 | |
| ≥60 | 3 (3.5) | 0 (0.0) | 3 | |
| Sex | ||||
| Female | 30 (5.3) | 28 (32.9) | 58 | >0.05 |
| Male | 55 (64.7) | 57 (67.1) | 112 | |
| Residence | ||||
| Rural | 67 (72.8) | 70 (82.4) | 137 | >0.05 |
| urban | 18 (21.2) |
15 (17.6) |
33 |
|
| Metier | ||||
| Student | 37 (45.1) | 22 (26.2) | 59 | <0.05 |
| Worker | 26 (31.7) | 38 (45.2) | 64 | |
| Other |
22 (23.2) |
25 (28.6) |
47 |
|
| Schooling level | ||||
| No school | 13 (15.3) | 21 (24.7) | 34 | >0.05 |
| Some high | 15 (17.7) | 6 (7.1) | 21 | |
| High school | 40 (47.1) | 32 (37.6) | 72 | |
| Some college graduate school |
27 (31.8) |
26 (30.6) |
53 |
|
|
| ||||
| Total | 85 (100) | 85 (100) | ||
Neuroradiologic findings (CT scan or cranial MRI examination) were normal in 78.8% (n = 67) of the patients, whilst 21.8% (n = 18) had abnormal electrical activity in all of the cerebral cortices simultaneously. On the basis of the 1981 ILAE classification, 70 patients (82.4%) had partial seizure, out of whom 53 had secondary generalization and 17 were without secondary generalization.
3.2. Enzyme-Linked Immunosorbent Assay Testing
The population seroprevalence of Toxocara antibodies was significantly higher in the epileptic patients compared with the healthy group (Figure 1). Based on the ELISA results, 10 (11.8%) persons with epilepsy presented antibodies against Toxocara (Table 2). Among the seropositive patients, six (60%) were males and four (40%) females. These seropositive patient's ages ranged from 14 to 29 years old (mean: 19.8 ± 11.42). Of 85 healthy individuals, 3 (3.5%) were positive for Toxocara by initial ELISA screening. Among the seropositive persons in control group, 2 (66.7%) were males and one (33.3%) was female. According to the questionnaire response, patients were categorized according to their age groups and also living environment. The difference between presence of Toxocara infection and age groups of the patients was not found statistically significant (P > 0.05). The characteristics and distributions of the epileptic patients are shown in Table 3.
Figure 1.
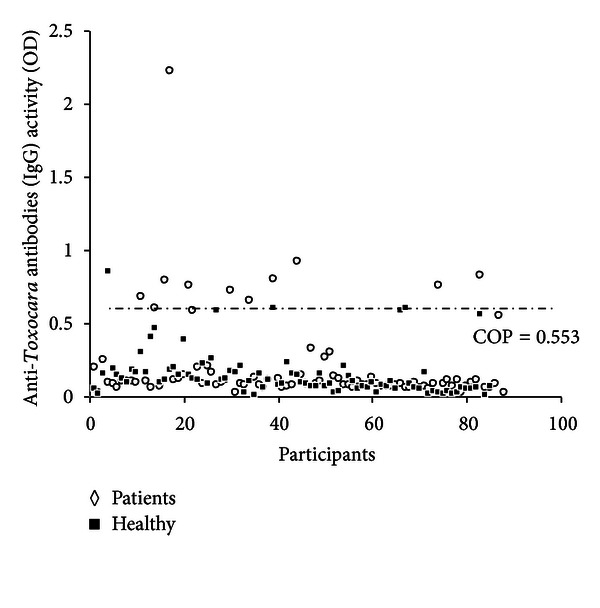
Dispersion of the anti-Toxocara IgG in serum samples of epileptic patients and healthy persons. Numbers in X-axis represent each sample (COP: cut-off point).
Table 2.
Seroprevalence of antibodies to Toxocara canis in the epilepsy patients and healthy persons by ELISA.
| Epilepsy patients (%) | Healthy (%) | Total (n) | |
|---|---|---|---|
| Seropositive | 10 (11.8) | 3 (3.5) | 13 |
| Seronegative | 75 (88.2) | 82 (96.5) | 157 |
Table 3.
Seroprevalence of Toxocara seropositive and seronegative in the epilepsy cases considering residency and age group.
| Seropositive, n (%) |
Seronegative, n (%) |
Total |
Statistic |
|
|---|---|---|---|---|
| Residence | ||||
| Rural | 7 (70.0) | 60 (80.0) | 67 | Not significant |
| Urban | 3 (30.0) | 15 (20.0) | 18 | |
| Age group (years) | ||||
| <20 | 6 (60.0) | 36 (48.0) | 42 | Not significant |
| 20–39 | 3 (30.0) | 25 (33.3) | 28 | |
| 40–59 | 1 (10.1) | 11 (14.7) | 12 | |
| ≥60 | 0 (0.0) | 3 (4.0) | 3 |
3.3. Western Blotting
Serum samples from patients and control groups that were positive for anti-Toxocara antibodies by ELISA were investigated using confirmatory Western blotting analysis. The positive Western blotting strips indicating the presence of specific anti-Toxocara IgG in the samples showed at least two or more bands of lower molecular weight between 24 and 35 kDa (Figure 2). Anti-Toxocara antibodies were detected in 3 (3%) ELISA positive patients, whereas all ELISA positive persons in control group were negative by Western blotting. Of 18 patients with abnormal neuroradiologic findings, 2 persons were seropositive by ELISA and immunoblot.
Figure 2.

Western blotting results for sera sample of the study population. Lane 1: reference ladder; lanes 2–4: sera testing positive by ELISA; Lane 5: Toxocara-negative control.
4. Discussion
Epilepsy is considered an important health problem in the developing countries [19]. A possible association between toxocariasis and epilepsy has been hypothesized, and Toxocara infection has been suggested as cofactor for epilepsy [13].
Serological studies conducted in various parts of the world demonstrate a variation in Toxocara seroprevalence ranging from 1.8 to 58.3 percent [20]. There are only a few studies reporting toxocariasis prevalence in Iran. A previous study has shown that the seroprevalence was 25.6% in school children [21]. We have previously reported that Toxocara eggs prevalence was 22.2% in public parks in Khorramabad, an urban region in Iran [12].
According to the literature research, neurological disorders in humans due to the presence of Toxocara canis larvae in the central nervous system might not be an uncommon event [22]. Despite the higher seroprevalence of Toxocara canis antibodies found in people with epilepsy versus controls in previous studies, there has been doubt about whether this implicated causality [13].
In this study, frequency of Toxocara infection in epileptic patients was 11.8%, scientifically higher than the healthy group (3.5%). Therefore, due to the reported prevalence of toxocariasis in the general population in Iran, the relatively low frequency of Toxocara infection in our control group required cautions interpretation. Of all the epilepsy patients seropositive for Toxocara, seven cases (70%) were males. However, this was not higher than the overall proportion of the male epilepsy patients of 64.7 percent. Toxocariasis is seen more frequently among children or young adults probably due to more frequent contact with infective eggs or by the ingestion of encapsulated larvae contained in the raw tissues of paratenic hosts, such as cows, sheep, or chicken. In the present study, we did not observe any significant difference between either of the seropositive and seronegative epileptic patients in terms of rural and urban populations and also various age groups. The seropositivity was not affected by age, although there were significantly more seropositive epilepsy patients who were students.
The diagnosis of human toxocariasis depends on serological test (ELISA) by using excretory-secretory antigens from Toxocara larvae, because it is very difficult to detect infective Toxocara larva in biopsy specimens. The antigens used in TES-ELISA are a complex mixture of glycoproteins, and cross-reactivity has been demonstrated with other helminthic infections (ascariasis, enterobiasis, trichuriasis, anisakiasis, taeniasis, echinococcosis, fascioliasis, and schistosomiasis). Therefore, ELISA and Western blotting are the most commonly used methods to determine anti-Toxocara antibodies [23–26]. Western blotting is the most sensitive and specific of the two assays available for immunodiagnosis of human toxocariasis. The high specificity of the test is based on the distinction between clusters of higher and lower molecular weight fractions. A previous study has showed that higher molecular weight bands are not specific and are suggestive of cross-reactions with other helminths, while lower molecular weight bands demonstrated a high level of specificity [7]. In this study, the immunoblot method that was applied to diagnosis of toxocariasis was more sensitive and specific than detecting by TES-ELISA. Therefore, our results confirm previous findings that the ELISA can be used for screening of patients depending on the specific antigen used and the Toxocara species [27].
5. Conclusion
Toxocariasis is a prevalent and treatable disease; the findings confirm that it could play an important role in the incidence of epilepsy in endemic areas of developing countries, and Toxocara infection could increase the risk of epilepsy that results from central nervous system disorders. On the other hand, results of our study demonstrate that a Western blotting based on TES performed a high specificity and is an appropriate confirmatory test for Toxocara monitoring in epilepsy patients. Serological monitoring in patients should follow a two-step testing scheme, that is, screening for Toxocara antibodies by ELISA followed by confirmation of seropositive reactions by means of Western blotting.
Conflict of Interests
The authors report no conflict of interests in this work.
Acknowledgments
The authors are grateful to the people and staff of Shohadaye-Ashayer Hospital, especially the Division of Neurology, for their collaboration and everlasting support. They thank Dr. Mohammad Hassan Kayedi, who is the Chief of Office of Vice Chancellor for Research, Lorestan University of Medical Sciences, and Dr. Saeedeh Shahali for their cooperation.
References
- 1.Azizi S, Oryan A, Sadjjadi SM, Zibaei M. Histopathologic changes and larval recovery of Toxocara cati in experimentally infected chickens. Parasitology Research. 2007;102(1):47–52. doi: 10.1007/s00436-007-0722-5. [DOI] [PubMed] [Google Scholar]
- 2.Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clinical Microbiology Reviews. 2003;16(2):265–272. doi: 10.1128/CMR.16.2.265-272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zibaei M, Sadjjadi SM, Ishiyama S, Sarkari B, Uga S. Production of monoclonal antibody against Toxocara cati second-stage larvae and its application for the detection of circulating antigens. Hybridoma. 2010;29(3):217–220. doi: 10.1089/hyb.2009.0108. [DOI] [PubMed] [Google Scholar]
- 4.Glickman LT, Magnaval JF, Domanski LM. Visceral larva migrans in French adults: a new disease syndrome? American Journal of Epidemiology. 1987;125(6):1019–1034. doi: 10.1093/oxfordjournals.aje.a114618. [DOI] [PubMed] [Google Scholar]
- 5.Jacquier P, Gottstein B, Stingelin Y, Eckert J. Immunodiagnosis of toxocarosis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. Journal of Clinical Microbiology. 1991;29(9):1831–1835. doi: 10.1128/jcm.29.9.1831-1835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollard ZF, Jarret WH, Hagler WS. ELISA for diagnosis of ocular toxocariasis. Ophthalmology. 1979;86(5):743–749. doi: 10.1016/s0161-6420(79)35465-3. [DOI] [PubMed] [Google Scholar]
- 7.Magnaval JF, Fabre R, Maurieres P, Charlet JP, De Larrard B. Application of the Western blotting procedure for the immunodiagnosis of human toxocariasis. Parasitology Research. 1991;77(8):697–702. doi: 10.1007/BF00928685. [DOI] [PubMed] [Google Scholar]
- 8.Logar J, Soba B, Kraut A, Stirn-Kranjc B. Seroprevalence of Toxocara antibodies among patients suspected of ocular toxocariasis in Slovenia. The Korean journal of parasitology. 2004;42(3):137–140. doi: 10.3347/kjp.2004.42.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirzayans S, Eslami A, Anwar M, Sajar M. Gastrointestinal parasite of dogs in Iran. Tropical Animal Health and Production. 1972;4(3):35–60. doi: 10.1007/BF02357096. [DOI] [PubMed] [Google Scholar]
- 10.Eslami A, Mohebali M. Parasitisme des chencs de bergers et implication en sante public en Iran. Bulletin de la Société de Pathologie Exotique. 1988;81(3):94–96. [PubMed] [Google Scholar]
- 11.Zibaei M, Sadjjadi SM, Sarkari B. Prevalence of Toxocara cati and other intestinal helminths in stray cats in Shiraz, Iran. Tropical biomedicine. 2007;24(2):39–43. [PubMed] [Google Scholar]
- 12.Zibaei M, Abdollahpour F, Birjandi M, Firoozeh F. Soil contamination with Toxocara spp. eggs in the public parks from three areas of Khorram Abad, Iran. Nepal Medical College Journal. 2010;12(2):63–65. [PubMed] [Google Scholar]
- 13.Arpino C, Gattinara GC, Piergili D, Curatolo P. Toxocara infection and epilepsy in children: a case-control study. Epilepsia. 1990;31(1):33–36. doi: 10.1111/j.1528-1157.1990.tb05356.x. [DOI] [PubMed] [Google Scholar]
- 14.Nicoletti A, Bartoloni A, Reggio A, et al. Epilepsy, cysticercosis, and toxocariasis: a population-based case-control study in rural Bolivia. Neurology. 2002;58(8):1256–1261. doi: 10.1212/wnl.58.8.1256. [DOI] [PubMed] [Google Scholar]
- 15.Nicoletti A, Sofia V, Mantella A, et al. Epilepsy and toxocariasis: a case-control study in Italy. Epilepsia. 2008;49(4):594–599. doi: 10.1111/j.1528-1167.2007.01432.x. [DOI] [PubMed] [Google Scholar]
- 16.Stommel EW, Seguin R, Thadani VM, et al. Cryptogenic epilepsy: an infectious etiology? Epilepsia. 2001;42(3):436–438. doi: 10.1046/j.1528-1157.2001.25500.x. [DOI] [PubMed] [Google Scholar]
- 17.Akyol A, Bicerol B, Ertug S, Ertabaklar H, Kiylioglu N. Epilepsy and seropositivity rates of Toxocara canis and Toxoplasma gondii . Seizure. 2007;16(3):233–237. doi: 10.1016/j.seizure.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Winkler AS, Blocher J, Auer H, Gotwald T, Matuja W, Schmutzhard E. Anticysticercal and antiToxocaral antibodies in people with epilepsy in rural Tanzania. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102(10):1032–1038. doi: 10.1016/j.trstmh.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Zibaei M, Zamani Z, Chahichi-Esfahani A, Anbari K, Nazer MR. Toxoplasma infection and epilepsy: a case-control study in Iran. Neurology Asia. 2011;16(4):299–302. [Google Scholar]
- 20.Kaplan M, Kalkan A, Kuk S, Demirdag K, Ozden M, Kilic SS. Toxocara seroprevalence in schizophrenic patients in Turkey. Yonsei Medical Journal. 2008;49(2):224–229. doi: 10.3349/ymj.2008.49.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadjjadi SM, Khosravi M, Mehrabani D, Oryan A. Seroprevalence of Toxocara infection in school children in Shiraz, Southern Iran. Journal of Tropical Pediatrics. 2000;46(6):327–330. doi: 10.1093/tropej/46.6.327. [DOI] [PubMed] [Google Scholar]
- 22.Magnaval JF, Galindo V, Glickman LT, Clanet M. Human Toxocara infection of the central nervous system and neurological disorders: a case-control study. Parasitology. 1997;115(5):537–543. doi: 10.1017/s0031182097001558. [DOI] [PubMed] [Google Scholar]
- 23.Gillespie SH, Bidwell B, Voller A, Robertson BD, Maizels RM. Diagnosis of human toxocariasis by antigen capture enzyme linked immunosorbent assay. Journal of Clinical Pathology. 1993;46(6):551–554. doi: 10.1136/jcp.46.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akao N, Chu AE, Tsukidate S, Fujita K. A rapid and sensitive screening kit for the detection of anti-Toxocara larval ES antibodies. Parasitology International. 1997;46(3):189–195. [Google Scholar]
- 25.Yamasaki H, Taib R, Watanabe YI, et al. Molecular characterization of a cDNA encoding an excretory-secretory antigen from Toxocara canis second stage larvae and its application to the immunodiagnosis of human toxocariasis. Parasitology International. 1998;47(3):171–181. [Google Scholar]
- 26.Park HY, Lee SU, Huh S, Kong Y, Magnaval JF. A seroepidemiological survey for toxocariasis in apparently healthy residents in Gangwon-do, Korea. Korean Journal of Parasitology. 2002;40(3):113–117. doi: 10.3347/kjp.2002.40.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith H, Holland C, Taylor M, Magnaval JF, Schantz P, Maizels R. How common is human toxocariasis? Towards standardizing our knowledge. Trends in Parasitology. 2009;25(4):182–188. doi: 10.1016/j.pt.2009.01.006. [DOI] [PubMed] [Google Scholar]


