Abstract
Infectious pneumonias exact an unacceptable mortality burden worldwide. Efforts to protect populations from pneumonia have historically focused on antibiotic development and vaccine-enhanced adaptive immunity. However, we have recently reported that the lungs’ innate defenses can be therapeutically induced by inhalation of a bacterial lysate that protects mice against otherwise lethal pneumonia. Here, we tested in mice the hypothesis that Toll-like receptors (TLRs) are required for this antimicrobial phenomenon, and found that resistance could not be induced in the absence of the TLR signaling adaptor protein MyD88. We then attempted to recapitulate the protection afforded by the bacterial lysate by stimulating the lung epithelium with aerosolized synthetic TLR ligands. While most single or combination treatments yielded no protection, simultaneous treatment with ligands for TLR2/6 and TLR9 conferred robust, synergistic protection against virulent Gram-positive and Gram-negative pathogens. Protection was associated with rapid pathogen killing in the lungs, and pathogen killing could be induced from lung epithelial cells in isolation. Taken together, these data demonstrate the requirement for TLRs in inducible resistance against pneumonia, reveal a remarkable, unanticipated synergistic interaction of TLR2/6 and TLR9, reinforce the emerging evidence supporting the antimicrobial capacity of the lung epithelium, and may provide the basis for a novel clinical therapeutic that can protect patients against pneumonia during periods of peak vulnerability.
INTRODUCTION
Despite decades of antibiotic development and hygiene programs, pneumonia continues to affect hundreds of millions of people annually and remains the leading cause of infectious mortality worldwide (1–4). In the course of normal ventilation, the sterile lower respiratory tract is recurrently exposed to inhaled pathogens, often resulting in serious infections (5–7). Although the lungs have traditionally been regarded as passive gas exchange barriers, it is now apparent that they have robust intrinsic defense mechanisms that prevent the incidence of lower respiratory tract infections from being much higher (6–13).
Epithelial surfaces that are constantly in contact with microbes, such as the colonic mucosa, constitutively generate antimicrobial effectors to modulate their local microbiome (14). As lung epithelial cells are only intermittently exposed to pathogens, and because chronic immune activation would negatively impact gas exchange, their baseline innate immune activity is relatively low. However, when exposed to pathogens, the lung epithelium rapidly responds by enhancing barrier function, recruiting leukocytes, and expressing antimicrobial products (13).
Viewing this epithelial plasticity as an opportunity for intervention, we recently demonstrated that the lungs’ antimicrobial defenses are therapeutically inducible. The inhalational pretreatment of mice with an aerosolized lysate of nontypeable Haemophilus influenzae (NTHi) protected against otherwise lethal pneumonia from a variety of pathogens (15–18). Supporting an innate immune mechanism underlying this inducible resistance was the observation of protection against pathogens non-cognate with the stimulus, protecting against all tested bacterial, fungal and viral organisms (15, 17, 18). Furthermore, induction of resistance occurred too rapidly for an adaptive immune response (onset within 2 h of treatment, maximal by 24 h) and did not rely upon innate immune leukocytes (neutrophils, macrophages, or mast cells) (15, 17).
In contrast to the highly refined epitope-sensing of T- and B-cell-expressed adaptive immune receptors, innate immune signaling depends upon recognition of conserved pathogen associated molecular patterns (PAMPs) by host pattern recognition receptors (PRRs). Toll-like receptors (TLRs) remain the best characterized of the PRRs (19, 20). They are highly conserved transmembrane proteins, consisting of an ectodomain with multiple leucine-rich repeats for pattern recognition, a membrane-spanning α-helix, and a Toll/interleukin-1 receptor (TIR) domain for intracellular signaling. At least 13 mammalian TLRs have been identified, each specifically localizing to either the plasma membrane or endosomal membranes, and each detecting unique complements of PAMPs (20–22). Upon PAMP recognition, signal transduction occurs via TLR-specific recruitment of cytosolic TIR adaptor protein combinations. The TIR adaptor protein MyD88 is required for signaling from most TLRs. The MyD88-independent signaling events observed from TLR3 and TLR4 require the TIR adaptor TRIF (also known as TICAM-1), with or without participation of TRAM (23). The TLR-specific TIR adaptor signaling cascades activate receptor-specific transcription factors, such as NF-κB, activating protein-1 (AP-1) and interferon regulatory factors (IRFs), leading to expression of inflammatory and antimicrobial genes (21, 24, 25).
To test our hypothesis that lysate-induced resistance is at least partially dependent upon TLR signaling (15, 17), mice deficient in TIR adaptors were challenged with virulent pathogens with or without NTHi lysate pre-treatment. We present here our observation that lysate-inducible resistance is MyD88-dependent, but not TRIF-dependent. Moreover, we report the robust and unanticipated synergistic protection afforded by simultaneous stimulation of two MyD88-dependent TLRs. These findings may provide the basis for the clinical use of synthetic TLR ligand combinations to prevent respiratory infections in high risk human populations.
MATERIALS AND METHODS
Animals and Reagents
All general reagents were obtained from Sigma (St Louis, MO), except as indicated. All mice were handled in accordance with the policies of the Institutional Animal Care and Use Committee of the University of Texas M. D. Anderson Cancer Center. Wild type five to eight week-old female Swiss-Webster mice (Charles River, Wilmington, MA) were used for most protection and cell count experiments. As indicated, five to eight week-old female Myd88−/− mice provided by Shizuo Akira (26), Trif−/− mice (The Jackson Laboratory, Bar Harbor, ME), Il1r−/− (Jackson) and Tlr2−/− mice (Jackson), all backcrossed >10 generations to C57BL/6J, were used in comparison to wild type mice C57BL/6J (Jackson).
Aerosolized treatments
Frozen stock of non-typeable Haemophilus influenzae (NTHi) was grown on chocolate agar (Remel, Lenexa, KS), expanded in brain-heart infusion broth (Acumedia, Baltimore, MD) supplemented with 3.5 µg/ml NAD, and disrupted with an EmulsiFlex C5 (Avestin, Mannheim, Germany), as described (15, 17, 27). The protein concentration was adjusted to 2.5 mg/ml in saline by bicinchoninic assay (Pierce, Rockford, IL), and the lysate was frozen in 10 ml aliquots at −80°C. For treatment, a thawed aliquot was placed in an AeroMist CA-209 nebulizer (CIS-US) driven by 10 l/min air supplemented with 5% CO2 (to promote deep breathing) for 20 min. The nebulizer was connected by polyethylene tubing (30 cm × 22 mm) to a 10 liter polyethylene exposure chamber, with an identical efflux tube with a low resistance microbial filter (BB50T, Pall, East Hills, NY) at its end vented to a biosafety hood.
Pam3CSK4, Pam2CSK4, Poly (I:C), MPLA, synthetic flagellin, imiquimod, and ODN 2395 were purchased from InvivoGen (San Diego, CA). To treat the animals, all synthetic TLR agonists except flagellin were reconstituted in endotoxin-free water, suspended in 8 ml sterile PBS at indicated concentrations, and aerosolized to the animals for 20 min using the same technique as used for NTHi lysate treatment. Flagellin was reconstituted in endotoxin free PBS and administered intranasally to mice anesthetized with isofluorane.
In vivo infectious challenges
As previously described (15–17), mice were inhalationally challenged with bacterial inocula targeted to LD80 – LD100. P. aeruginosa strain PA103 was obtained from ATCC and stored as frozen stock (1 × 108 CFU/ml) in 20% glycerol in LB medium (Bio 101 Systems). One ml of stock was incubated for 16 h in 100 ml LB medium at 37°C in 5% CO2, then diluted in 1 l of fresh broth and grown at 37°C for 6–7 h to OD600 of 0.3, yielding 1–4 × 1010 CFU/ml. S. pneumoniae serotype 4 was stored as frozen stock (1 × 109 CFU) in 20% glycerol in Todd-Hewett broth (Becton Dickinson). One ml of thawed stock was incubated for 16 h in 150 ml Todd-Hewitt broth at 37°C in 5% CO2, then diluted in 1.5 l of fresh broth and grown in logarithmic phase for 6–7 h to an OD600 of 0.3, yielding 2–6 × 1011 CFU/ml. The bacterial suspensions were centrifuged, washed, resuspended in 10 ml PBS and aerosolized over a period of 60 min using a system identical to that used for the treatments. Bacterial concentrations were determined by plating serial dilutions onto tryptic soy agar plates (Becton Dickinson).
Quantification of lung pathogen burden
As previously described (15–17), immediately after infection with bacterial pathogens, mice were anesthetized and their lungs were harvested and homogenized in 1 ml of PBS utilizing a 2 ml tissue grinder (Kontes, Vineland, NJ). Serial dilutions of the homogenates were plated on tryptic soy agar (TSA) plates, incubated at 37°C for 16 h, and bacterial colonies were counted.
Bronchoalveolar lavage fluid analysis
As previously described (15–17), bronchoalveolar lavage (BAL) fluid was obtained by instilling and collecting two aliquots of 1 ml each of PBS through a luer stub adapter cannula (Becton Dickinson) inserted through rings of the exposed trachea at indicated time points. Total leukocyte count was determined with a hemacytometer (Hauser Scientific, Horsham, PA), and differential count by cytocentrifugation of 300 µl of BAL fluid at 2,000 rpm for 5 min, followed by Wright-Giemsa staining.
In vitro killing assay
As previously described (15, 17), MLE-15 cells and A549 cells were cultured on 6-well plates in RPMI-1640 supplemented with 10% heat-inactivated FCS and 1% penicillin/streptomycin (Invitrogen). When grown to ~80% confluence, cells were washed with PBS, supplied with fresh antibiotic-free media with 10% heat-inactivated FCS, and treated with 20 µl PBS or a 20 µl volume of ODN 2395 (20 µg/ml), Pam2CSK4 (10 µg/ml) or both in RPMI-1640 containing 10% heat-inactivated FCS. After 4 h, 1000 spores of Bacillus anthracis Sterne strain or 2000 CFU P. aeruginosa strain PA103 were then added to all wells. 4 h after infection, 20 µl of the supernatant from each well was aspirated, serially diluted, plated on a TSA agar plate, incubated for 16 h at 37 °C, and CFUs were counted.
To test killing by primary epithelial cells, mouse tracheal epithelial cells (mTEC) were isolated as previously described (28). In brief, tracheas from wild type Swiss Webster mice were isolated and digested overnight at 4°C in Ham’s F-12 media (Mediatech) containing 25 units penicillin-streptomycin (Invitrogen) and 0.15% pronase (Roche). Disaggregated cells were incubated in Ham’s F12/DMEM (Invitrogen) containing 4 mM glutamine (Invitrogen), 10 units penicillin-streptomycin, 0.25µg/ml amphotericin B (Fisher Scientific), and 50 µg/ml gentamicin (Fisher Scientific) at 37°C in 5% CO2 for 5 h. After incubation, floating cells were collected and 3.8 × 105 cells/well were plated on collagen I/III (Sigma Aldrich) coated 24 well tissue culture plates in Ham’s F12/DMEM containing 5% heated inactivated FCS, 4mM glutamine, 10 units penicillin-streptomycin, 0.25 µg/ml amphotericin B, 50 µg/ml gentamicin, 10 µg/ml insulin (Sigma Aldrich), 30 µg/ml bocine pituitary extract (Lonza), 5 µg/ml human transferrin (Fisher Scientific), 0.1 µg/ml cholera toxin (Sigma Aldrich), 25 ng/ml epidermal growth factor (Fisher Scientific) and 10−8 M retinoic acid (Sigma Aldrich). After incubating at 37°C in 5% CO2 for 3–4 days, confluent cells were washed and treated with 20 µl PBS or a 20 µl volume of ODN 2395 (20 µg /ml) and Pam2CSK4 (10 µg /ml) in antibiotic-fee mTEC culture media. Four hours later, the wells were infected with 150 CFU luminescent S. pneumoniae (a gift from Dr. Jon McCullers, St. Jude Children’s Research Hospital). Culture luminescence was measured after infection by a Biotek Synergy 2 plate reader. Culture luminescence correlated with serial dilution CFUs with an R2>0.998. Similar experiments were performed with primary alveolar macrophages harvested from wild type Swiss Webster. Alveolar macrophages were collected by BAL, as described above. The cells were resuspended in RPMI-1640 with 10% heat inactivated FCS supplemented with penicillin and streptomycin, then plated on 24 well plates. Once adherent to the wells, the wells were washed and antibiotic-free RPMI-1640 was added. Treatment and infection then proceeded as described for the mTECs.
Microarray gene expression analysis
MLE-15 cells were treated with PBS, ODN 2395 (20 µg/ml), Pam2CSK4 (10 µg/ml) or both ligands for 4 h. Total RNA from MLE-15 cells was isolated using RNeasy Mini kit (QIAGEN). cRNA was then synthesized and amplified using Ilumina TotalPrep RNA amplification kit (Ambion), labeled and hybridized onto Sentrix Mouse-6 Expression BeadChips, then scanned by a BeadStation 500 (Illumina). Consistent with minimum information about a microarray experiment (MIAME) standards, all primary microarray data were deposited at the NCBI Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/, accession number GSE18725).
Immunofluorescence microscopy
A549 cells were cultured on Lab-Tek II chamber slides (Nunc, Rochester, NY) in RPMI-1640 supplemented with 10% heat-inactivated FCS and 1% penicillin/streptomycin (Invitrogen) for 48 h, then treated with treated with a 20 µl volume of Texas Red-labeled ODN 2395 (20 µg/ml, Invivogen), fluorescein isothiocyanate (FITC)-labeled Pam2CSK4 (10 µg/ml, Invivogen) or both in RPMI-1640 containing 10% heat-inactivated FCS. After 2 h, the media was suctioned, the chambers were detached, and the cells were washed three times with iced PBS. The cells were then fixed with 4% paraformaldehyde, quenched with glycine, washed three times with PBS, nuclear counterstained with 4′,6-diamidino-2-phenylindole (DAPI; 0.1 µg/ml), and examined with fluorescence microscopy (Olympus BX-60 microscope, Melville, NY) using appropriate optics (Texas Red: excitation = 540 nm; emission = 620 nm; FITC: excitation = 495 nm, emission = 520 nm; DAPI: excitation = 360 nm; emission = 460 nm). Images were collected sequentially with a computer-regulated Spot RT Camera (Diagnostic Instruments, Sterling Heights, MI) and assembled in Photoshop CS3 (Adobe, San Jose, CA). Overlapping red and green fluorescence appeared yellow.
Statistical analysis
Statistical analysis was performed using SPSS v19 (SAS Institute). Student’s t-test was used to compare the lung bacterial burdens between groups. Percentage of mice surviving pathogen challenges was compared using Fisher’s exact test, and the log-rank test was used to compare the survival distribution estimated by the Kaplan-Meier method. One-way ANOVA with Dunnett’s post hoc test was used to compare the BAL fluid differential counts between the treated and untreated animals.
Gene expression data were background corrected using the robust microarray averaging (RMA) method, then transformed by taking the base-two logarithm, and quantile normalized. Analysis of the microarray output was performed using a gene-by-gene class comparison ANOVA to identify treatment-induced changes. To identify genes with synergistic effects of the TLR ligand treatments, we fit a model of Y = β0 + β1 × ODN + β2 × Pam2 + β3 × ODN × Pam2, where Y is the expression value of a gene and the β terms are the coefficients for main effects of ODN (β1), Pam2 (β2) and the interaction between ODN and Pam2 (β3). Then, to test whether the interaction term is significant, we compared this linear model with one lacking an interaction term. To identify genes with additive effects, we fit the ANOVA model without interaction term, and test whether both main effects (ODN and Pam2) are significantly non-zero. To adjust for multiple testing, we used a beta-uniform mixture (BUM) model to estimate the false discovery rate (FDR). Tukey honestly significant difference (HSD) test is used to compare the differences between each pair of means with appropriate adjustment for multiple testing on the level of individual gene.
RESULTS
MyD88, but not TRIF, is required for the induction of resistance to pneumonia by an aerosolized bacterial lysate
We have previously shown that stimulation of the lung epithelium by an aerosolized bacterial lysate induces a high level of resistance to a broad array of microbial pathogens (15–18). To test whether TLR signaling is required for lysate-induced protection, mice deficient in TIR adaptors were inhalationally challenged with P. aeruginosa. Wild type and TRIF-deficient (Trif−/−) mice were fully protected against lethal P. aeruginosa challenges by pretreatment with the aerosolized bacterial lysate, whereas resistance could not be induced in mice deficient in MyD88 (Myd88−/−; Figure 1A and B, left panels). Consistent with our prior observations (15–17), protection closely correlated with the induction of rapid pathogen killing in the lungs (Figure 1A and B, right panels). The IL-1 receptor also signals through MyD88 (26, 29), but responds to host cytokine signaling, rather than responding directly to microbial products. Pathogen killing was fully preserved in IL-1 receptor deficient mice (Il1r−/−; Figure 1C) stimulated by the aerosolized bacterial lysate. This finding indicates that not all receptors signaling through MyD88 are required for lysate-induced protection, and suggests that direct microbial signaling through TLRs is more important than indirect signaling through host cytokines for inducible epithelial resistance.
Figure 1. MyD88, but not TRIF, signaling is required for bacterial lysate-induced resistance to pneumonia.
(A) Myd88−/− and wild type mice were inhalationally challenged with P. aeruginosa with or without pretreatment 24 h earlier with an aerosolized lysate of nontypeable H. influenzae (NTHi). Left, survival (N = 10 mice/group, *p<0.0001). Right, bacterial lung burden immediately after infection (right, N = 3 mice/group, **p<0.004, †p=0.39 vs. wild type control). (B) P. aeruginosa challenge of Trif−/− mice with or without pretreatment with the bacterial lysate. Left, survival (N = 10 mice/group, *p<0.0001). Right, bacterial lung burden immediately after infection (N = 3 mice/group, *p<0.0001). (C) Il1r−/− and wild type mice were treated with aerosolized PBS or the lysate 24 h before challenge with P. aeruginosa. Shown is the bacterial burden of lung homogenates immediately after infection. (N = 3 mice/group, *p=0.001 vs. wild type + PBS, **p=0.01 vs. Il1r−/− + PBS, †p=0.66 vs. wild type + PBS, ‡p=0.89 vs. wild type + NTHi)
Individual TLR agonists fail to induce a high level of resistance to pneumonia
In view of the requirement for MyD88 signaling, we tested whether any individual synthetic TLR agonists could induce resistance similar to that afforded by the aerosolized bacterial lysate. As TLR1 and TLR6 are expressed as heterodimers with TLR2, and as TLR7 and TLR8 both recognize imiquimod, mouse TLRs 1 through 9 could all be stimulated with the following seven synthetic ligands: Pam3CSK4 (TLR2/1 agonist), Pam2CSK4 (TLR2/6 agonist), Poly(I:C) (TLR3 agonist), synthetic lipid A (MPLA, TLR4 agonist), synthetic flagellin (TLR5 agonist), imiquimod (TLR7 and TLR8 agonist), or ODN2395 (TLR9 agonist).
The appropriate airway doses of these agonists is not known, so a strategy was formulated to insure that an adequate dose was delivered to the lungs to avoid a type II (β) error. Each of the synthetic TLR agonists we used has a reported concentration at which maximal cytokine secretion is stimulated from dendritic cells ([DCmax]) (23, 30–35). Based on our calculations of effective airway delivery of aerosolized compounds (16, 36), we detemined the nebulizer fluid concentrations required to achieve [DCmax] at the airway epithelial surface. Although aerosolized lysate-induced resistance does not depend upon leukocyte influx, we have previously reported that the protective phenomenon is tightly correlated with the timing and magnitude of induced lung neutrophilia (15). Therefore, to identify TLR agonist doses sufficient for testing, we began at the reported [DCmax] for each ligand and increased the starting concentrations logarithmically until leukocyte infiltration was achieved, as a biomarker of ligand activity in the lungs.
As shown in Figure 2, in PBS treated mice, the number of neutrophils in BAL fluid is low (0.1 × 103 ± 0.2 cells/ml). Only Pam2CSK4 demonstrated a significant increase in neutrophils at [DCmax], though all but poly(I:C) showed significant increases in neutrophil levels at concentrations one to two logs above [DCmax]. Alternately, poly(I:C) induced significant influx of macrophages in BAL fluid 24 h after treatment. Flagellin and imiquimod each had a ligand concentration above which there was a reduction in neutrophil infiltration. Pam2CSK4 induced a level of neutrophilia nearly 5-fold higher than Pam3CSK4 and 15-fold higher than any other ligand.
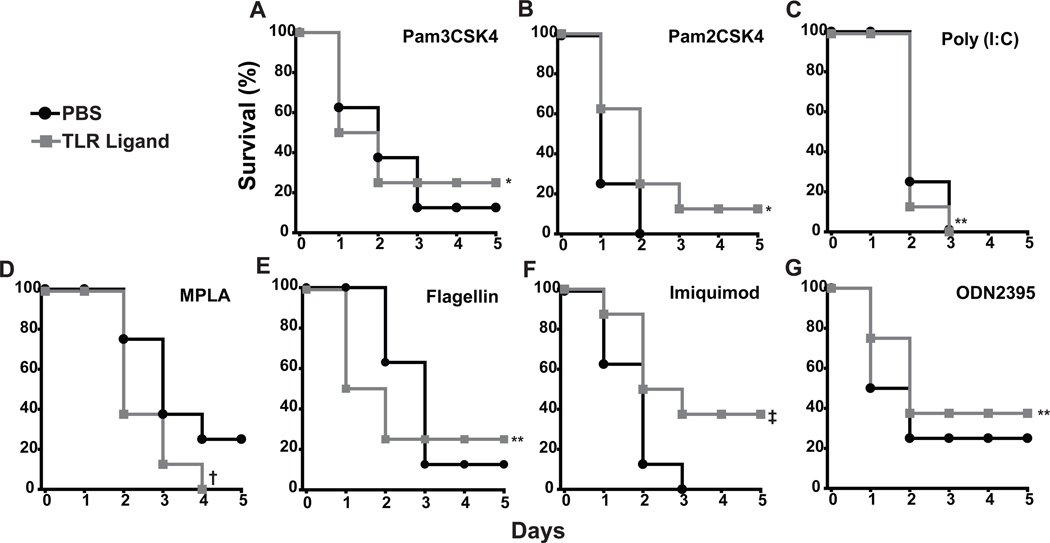
Figure 2. Leukocyte counts in bronchoalveolar lavage fluid after treatment with single synthetic TLR ligands.
Mice were submitted to BAL 24 h after treatment with PBS or one of the following TLR ligands: Pam3CSK4 (TLR2/1 agonist, 1 µg/ml, 3µg/ml,10µg/ml), Pam2CSK4 (TLR2/6 agonist, 1 µg/ml, 3 µg/ml, 10 µg/ml), Poly(I:C) (TLR3 agonist, 1 µg/ml, 10 µg/ml, 100 µg/ml), synthetic lipid A (MPLA, TLR4 agonist, 1 µg/ml, 10 µg/ml, 100 µg/ml), Flagellin (TLR5 agonist, 40 ng, 400 ng, 4000 ng), imiquimod (TLR7 and TLR8, 100 µg/ml, 300 µg/ml, 1000 µg/ml), or ODN2395 (TLR9 agonist, 2 µg/ml, 20 µg/ml). All ligands delivered as 8 ml suspensions nebulized over 20 minutes, except Flagellin which was delivered intranasally in 40 µl. Shown are neutrophil (black bars) and macrophage (gray bars) counts in BAL fluid
The concentration chosen for each ligand was the lowest dose to induce a 10-fold increase in neutrophils/ml or to induce doubling of the macrophages (none did both). While some of the ligands induced robust cellular infiltration, none of the synthetic agonists provided significant protection against lethal P. aeruginosa pneumonia (Figure 3). There was a modest trend towards protection with Pam2CSK4 and imiquimod though these did not reach statistical significance for individual experiments or in the mean of multiple experiments. MPLA-treated mice showed a non-significant trend towards increased mortality after pathogen challenge.
Figure 3. Aerosolized treatment with individual synthetic TLR ligands does not induce a high level of resistance against pneumonia.
Wild type mice were challenged with P. aeruginosa after treatment with PBS or the following synthetic TLR ligands 24 prior: (A) TLR2/1 agonist Pam3CSK4 100 µg/ml nebulized for 20 min, (B) TLR2/6 agonist Pam2CSK4 10 µg/ml nebulized for 20 min, (C) TLR3 agonist poly (I:C) 100 µg/ml nebulized for 20 min, (D) TLR4 agonist MPLA 100 µg/ml nebulized for 20 min, (E) TLR5 agonist flagellin 400 ng intranasal, (F) TLR7 and TLR8 agonist imiquimod 1 mg/ml nebulized for 20 min, or (G) TLR9 agonist ODN 2395 20 µg/ml nebulized for 20 min. Survival curves are representative examples of at least three distinct experiments for treated and untreated mice (N = 8 mice/group, *p=0.5, **p=1.0, †p=0.47, ‡p=0.2)
A combination of TLR2/6 and TLR9 agonists induces a high level of resistance against pneumonia
Although we did not observed significant protection following treatment with single synthetic TLR agonists, we and others have postulated that simultaneous stimulation of multiple PRRs is required to induce a high level of resistance (13, 15, 17). To determine whether combinations of TLR agonists could induce resistance, we tested the 21 non-redundant pairwise permutations of the seven synthetic ligands.
Remarkably, the first tested combination, simultaneous treatment with Pam2CSK4 and ODN2395 (Pam2-ODN), resulted in survival of 100% of mice from an otherwise lethal challenge with Gram-negative P. aeruginosa (Figure 4A, left), and survival of 80% from a lethal challenge with Gram-positive S. pneumoniae (Figure 4B, left). Doubling the concentration of both ligands in the aerosol treatment resulted in 90% survival of the challenge with S. pneumoniae (Figure 4B). Protection of mice from lethal infectious challenges was associated with synergistic killing of the pathogens within the lungs (Figure 4A and 4B, right), and doubling the concentration of the ligands was associated with a trend towards greater pathogen killing. Synergistic interactions between Pam2CSK4 and ODN2395 were also observed in leukocyte recruitment to the lungs at 4 and 24 h (Figure 4C). Together, these results indicate that ligands for TLR2/6 and TLR9 induce synergistic activation of antimicrobial effector responses, including those for pathogen killing and leukocyte recruitment, which result in a synergistic level of protection against pneumonia. Similar to the kinetics of the aerosolized bacterial lysate-induced resistance, protection was present by 4 h after treatment (data not shown). This protection persisted for up to 8 days after a single inhaled treatment (Figure 4D). Moderate protection was also observed when the treatment was delivered up to 24 h after infection (Figure 4E). Later post-infection time points cannot be assessed in our model as too many untreated/sham treated mice meet euthanasia criteria prior to receiving planned treatments (15). Similar to our findings with lysate-induced protection (15, 16), Pam2-ODN-enhanced survival rates also correlate with containment of the infection within the lungs. Table 1 shows that Pam2-ODN treatment induces pathogen killing at the time of infection, promotes ongoing pathogen clearance, and prevents hematogenous dissemination of infection relative to PBS-treatment. Thus, we observe evidence of direct intrapulmonary pathogen killing and enhanced containment of pathogens within the lungs.
Figure 4. TLR2/6 and TLR9 agonists cooperate to induce resistance against bacterial pneumonia.
(A) Left, survival of mice challenged with P. aeruginosa 24 h after treatment with PBS, Pam2CSK4 10 µg/ml, ODN 2395 20 µg/ml, the combination, or the combination at double dose (N = 6 mice/group, ‡p=0.008 vs. PBS). Right, Bacterial burden of lung homogenates immediately after infection with P. aeruginosa (N = 3 mice/group, #p=0.045 vs. PBS, ##p=0.030 vs. PBS). (B) Left, survival of mice challenged with S. pneumoniae 24 h after treatment with PBS, Pam2CSK4 10 µg/ml, ODN 2395 20 µg/ml, the combination, or the combination at double dose (N = 10 mice/group, †p<0.001 vs. PBS, ‡p<0.0001 vs. PBS treated). Right, bacterial burden of lung homogenates immediately after S. pneumoniae infection 2×1010 (N = 3 mice/group, †p<0.001, ‡p<0.0001). (C) BAL cell counts from mice 4 or 24 h after treatment with PBS, Pam2CSK4 10 µg/ml, ODN 2395 20 µg/ml, or the combination of Pam2CSK4 and ODN2395 (N = 3 mice/group, *p=0.02 vs. PBS, **p<0.0001 vs. PBS, †p=0.04 vs. Pam2 alone). (D) Survival of mice challenged with P. aeruginosa after treatment with Pam2-ODN 8 d, 6 d, or 4 d prior to infection. (N = 8 mice/group; * p=0.01 vs PBS, ** p=0.001 vs. PBS). (E) Survival of P. aeruginosa challenge in mice receiving Pam2-ODN treatment 24 h before infection, at the time of infection, or after infection (PBS group N = 9 mice, other groups N = 8 mice; *p=0.01, **p=0.05)
Table 1.
Temporal dissemination of bacteria following inhalational challenge with P. aeruginosa.
| Organ | Treatment | 0 hpi | 24 hpi | 48 hpi |
|---|---|---|---|---|
| Lung | PBS | 16000±2121 | 39000±30405 | ••• |
| Pam2-ODN | 1500±1322* | 1100±1678 | 23±22 | |
| Blood | PBS | 0 | 5±2 | ••• |
| Pam2-ODN | 0 | 0** | 0 | |
| Spleen | PBS | 0.5±0.1 | 60.25±23 | ••• |
| Pam2-ODN | 0 | 0** | 0.3±0.5 | |
| Liver | PBS | 0 | 365±262 | ••• |
| Pam2-ODN | 0 | 0** | 0.6±1.2 | |
N=3 mice per group at each time point, except the PBS group 24 h after infection in which one mouse did not survive to the pre-designated time point.
All mice in the PBS group died prior to 48 h timepoint.
p<0.001 vs. PBS;
p=0.01 vs. PBS; hpi, hours post-infection.
Not all TLR agonist combinations protect against infection
As shown in representative examples of other TLR combinations with Pam2CSK4 (Figure 5A–C) and with ODN2395 (Figure 5D–F), few other treatments induced protection that was statistically superior to PBS treatment. These results indicate that not all TLR agonist combinations confer the same immune stimulation as Pam2-ODN, even when both ligands signal via MyD88, suggesting that a degree of specificity must account for the immune activation.
Figure 5. Not all TLR agonist combinations protect against pneumonia.
Wild type mice were challenged with P. aeruginosa following treatment with PBS or the following TLR agonist combinations 24 h prior: (A) Pam2CSK4 and poly (I:C), (B) Pam2CSK4 and flagellin, (C) Pam2CSK4 and imiquimod, (D) ODN2395 and poly (I:C), (E) ODN2395 and flagellin, (F) ODN2395 and Pam3CSK4. (N = 8 mice/group, *p=0.20, **p=0.01, †p=1.0, ‡p=0.5)
TLR2 is required to promote protective Pam2CSK4 and ODN2395 synergy, but not required for induced resistance by the bacterial lysate
The detection of synergistic effects of TLR ligands Pam2CSK4 and ODN2395 with well-defined receptor specificities provides presumptive evidence of the participation of TLR2/6 and TLR9. We sought further evidence using knockout mice and additional ligands.
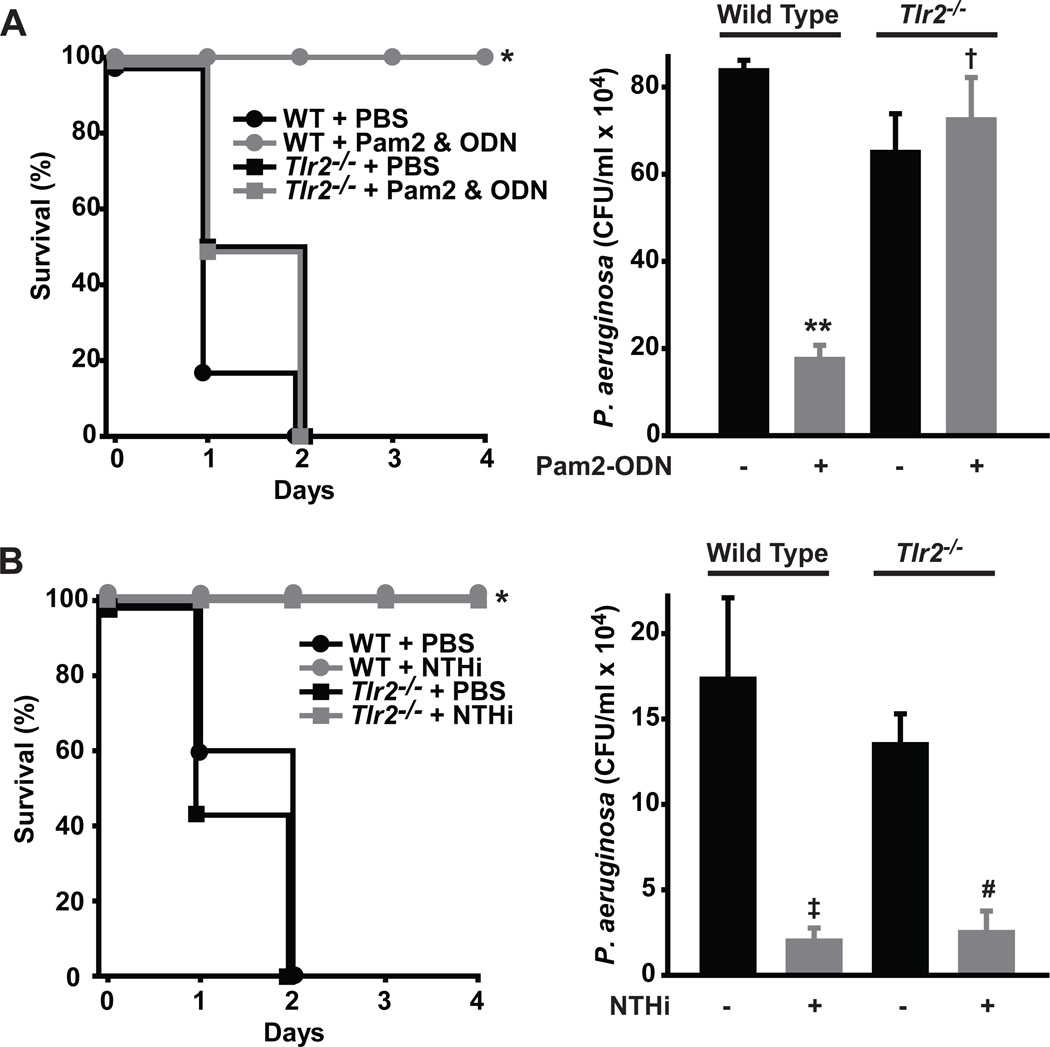
First, we compared the survival of wild type and TLR2-deficient mice pretreated with Pam2-ODN or PBS prior to challenge with P. aeruginosa. While the wild type mice were fully protected by Pam2-ODN, there was no survival in the sham-treated wild type group or either Tlr2−/− group (Figure 6A, left panel), confirming the requirement for TLR2 in Pam2-ODN-induced protection. Consistent with our prior findings, the loss of protection in the Tlr2−/− mice correlated tightly with the loss of Pam2-ODN-induced intrapulmonary pathogen killing (Figure 6A, right panel).
Figure 6. TLR2 is sufficient to promote protective Pam2CSK4 and ODN2395 synergy, but not required for induced resistance.
(A) Left, survival of Tlr2−/− and wild type mice challenged with P. aeruginosa with or without ODN2395 and Pam2CSK4 treatment 24 h prior (N = 8 mice/group, *p<0.0002). Right, Bacterial burden of lung homogenates immediately after infection with P. aeruginosa (N = 4 mice/group, **p<0.0001 vs. wild type + PBS, †p=0.59 vs. Tlr2−/− + PBS) (B) Left, survival of TLR2−/− and wild type mice challenged with P. aeruginosa with or without treatment 24 h prior with an aerosolized lysate of nontypeable H. influenzae (NTHi) (N = 10 mice/group, *p<0.0002). Right, Bacterial burden of lung homogenates immediately after infection with P. aeruginosa (N = 3 mice/group, ‡p=0.03 vs. wild type + PBS, #p=0.002 vs. Tlr2−/− + PBS)
Since Pam2CSK4 and Pam3CSK4 discriminate between TLR2/6 and TLR2/1, and Pam2CSK4 but not Pam3CSK4 interacts synergistically with ODN2395, this suggested a requirement for TLR2/6 heterodimers in inducing lung epithelial resistance. However, we also challenged Tlr2−/− and wild type mice after treatment with the aerosolized bacterial lysate and found neither loss of protection (Figure 6B, left panel) nor a defect in lysate-induced bacterial killing (Figure 6B, right panel). Taken together, we found that TLR2/6 is sufficient to synergistically interact with TLR9, but not required for all induced resistance.
Class C CpG ODNs, but not Class A or B CpG ODNs, interact synergistically with Pam2CSK4 to induce resistance to bacterial pneumonia
We next sought to confirm that TLR9 is required for the synergistic interaction of Pam2-ODN. Tlr9−/− mice were not available to us, so we further tested TLR9 involvement using a scrambled ODN known to not bind TLR9. Whereas pretreatment with Pam2-ODN resulted in 90% survival of P. aeruginosa-challenged mice, none survived when pretreated with Pam2CSK4 and the control ODN (Figure 7A), indicating that TLR9 binding by the ODN is required for the synergistic protection.
Figure 7. TLR9-binding Class C, but not Class A or B, CpG ODNs interact synergistically with Pam2CSK4 to induce resistance to bacterial pneumonia.
(A) Survival of wild type mice treated with Pam2CSK4 and ODN2395 or Pam2CSK4 and a scrambled control ODN 24 h prior to P. aeruginosa challenge (N = 10 mice/group, *p<0.0001). (B) Survival of wild type mice challenged with P. aeruginosa 24 h after treatment with PBS or Pam2CSK4 combined with a Class A CpG ODN (ODN 1585 or ODN2216), a Class B CpG ODN (ODN 2006-G5) or a Class C CpG ODNs (M362 or ODN2395, dashed lines) (N = 10 mice/group, *p=0.01 vs. PBS, **p=0.0001 vs. PBS; †p=0.3 vs. Pam2 + ODN2395)
To further explore the specificity of the Pam2-ODN interaction, we treated wild type mice with Pam2CSK4 and different classes of TLR9-binding CpG ODNs prior to challenge with P. aeruginosa. The combination of a Class A ODN (ODN 1585 or ODN 2216) or a Class B ODN (ODN 2006-G5) with Pam2SCK4 conferred no protection, whereas equimolar combinations of Pam2CSK4 with a Class C ODN (ODN M362 or ODN 2395) promoted significant resistance against otherwise lethal pneumonia (Figure 7B). These results indicate that, not only do TLR2/6 and TLR9 ligands synergize, but there are specific TLR9 ligands that interact more favorably than others.
Pam2CSK4 and ODN2395 induce bacterial killing by epithelial cells in vitro
We have previously reported the induction of bacterial killing by lung epithelial cells in vitro when stimulated with the bacterial lysate (16, 17). Since Pam2-ODN recapitulates the immunostimulatory effect of the bacterial lysate in vivo, we tested whether the combination could induce pathogen killing by isolated lung epithelial cells in vitro, as well. Pretreatment of murine MLE-15 respiratory epithelial cells for 4 h with Pam2-ODN significantly reduced bacterial CFUs in cell culture media after inoculation with the Gram-positive organism B. anthracis (Figure 8A). Similarly, treatment of human A549 cells with Pam2-ODN resulted in significant reductions in the Gram-negative organism P. aeruginosa CFUs 4 h after infection (Figure 8B). Demonstrating that the observed pathogen killing occurs through the stimulation of epithelial cells, rather than through direct antibiotic effects of Pam2-ODN, bacteria grew to equal numbers in wells containing no cells, whether the wells were treated with Pam2-ODN or PBS (Figure 8C and D). Demonstrating that this was not an artifact of immortalized cells, we found that S. pneumoniae was killed by Pam2-ODN-treated primary mTECs (Figure 7C). However, freshly isolated alveolar macrophages could not be induced to kill S. pneumoniae by Pam2-ODN in vitro (Figure 8F).
Figure 8. TLR2/6 and TLR9 agonists cooperate to induce bacterial killing by murine and human respiratory epithelial cells in vitro.
(A) MLE-15 cells were treated with Pam2CSK4 (10 µg/ml) and/or ODN2395 (20 µg/ml) for 4 h prior to infection with B. anthracis (1000 spores). Shown are bacterial CFU 4 h after infection (*p=0.05 vs. PBS, **p=0.016 vs. PBS, #p>0.05 vs. either single agonist). (B) A549 cells were treated with ODN2395 and Pam2CSK4 for 4 h prior to infection with P. aeruginosa (2700 CFU). Shown are bacterial CFU 4 h after infection (*p=0.01 vs. PBS, **p=0.003 vs. PBS, ***p=0.001 vs. PBS, #p=>0.05 vs. either single agonist). (C) A549 culture media (without cells) was treated with ODN2395 and Pam2CSK4, infected with B. anthracis (1000 spores), and cultured after 4 h (†p=1.0). (D) MLE culture media (without cells) was treated with ODN2395 and Pam2CSK4, infected with P. aeruginosa (4000 CFU), and cultured after 4 h (‡p=0.58 (E) Primary mouse tracheal epithelial cells were grown at air-liquid interface, then treated with PBS or Pam2CSK4 (10 µg/ml) and ODN2395 (20 µg/ml) for 4 h prior to infection with S. pneumoniae (150 CFU). Shown are bacterial CFUs 19 h after infection (*p=0.03 vs. PBS). (F) Freshly isolated mouse alveolar epithelial cells were treated with Pam2CSK4 (10 µg/ml) and ODN2395 (20 µg/ml) for 4 h prior to infection with S. pneumoniae (150 CFU). Shown are bacterial CFUs 7 h after infection (†p=1.0 vs. PBS). (G) Microarray analysis was performed on MLE-15 cells treated with PBS, Pam2CSK4 (10 µg/ml), ODN2395 (20 µg/ml), or both for 2 h. Shown are heatmaps reporting relative enrichment by TLR ligand treatments for differentially expressed antimicrobial effectors (left), TLR signaling elements (center) and differentially expressed cytokine signaling (right), N=6 replicates/condition
Thus, the antimicrobial effect of Pam2-ODN is induced in both murine and human epithelial cells and results in killing of both Gram-positive and Gram-negative bacterial pathogens. These data mimic the bacterial killing seen in vivo following Pam2-ODN treatment. Serial increases in Pam2-ODN dosing up to 32-fold higher than indicated here did not significantly increase pathogen killing (data not shown).
To further explore the mechanisms by which the epithelial cells are induced to kill pathogens, we performed microarray analysis of MLE-15 cells after treatment with PBS (sham), ODN2395, Pam2CSK4 or both. Compared to PBS treated samples, 1,129 genes were differentially expressed 2 h after ODN2395 and/or Pam2CSK4 treatment with a FDR <0.05. Of these DEGs, 338 demonstrated additive or synergistic effects of the two ligands. To emphasize treatment-specific gene expression differences, Figure 8G shows fold-change log ratios of the three ligand treatments (Pam2, ODN or both) for selected DEGs. These include antimicrobial effectors, TLR signaling genes and inflammatory mediators that are differentially expressed following Pam2-ODN treatment (compared to PBS). To reveal detail, the scale is truncated at log ratio +/− 1, so differences >2.7-fold are shown as saturated blue or yellow. There is high concordance between these induced changes and the gene expression enrichment demonstrated 2 h after treatment of MLE-15 cells with the NTHi lysate. In fact, more than half of the Pam2-ODN-induced effector genes were upregulated by treatment with the lysate, and almost all of the enriched cytokines were induced by the lysate (17). These results not only further substantiate that the lung epithelia have the necessary machinery to generate antimicrobial responses, but demonstrates that combined treatment with TLR agonists is sufficient to induce effective responses.
Pam2CSK4 and ODN2395 co-localize intracellularly in vitro
The mechanism by which Pam2CSK4 and ODN2395 interact to induce synergy remains unresolved. As TLR2/6 is reported to localize to the plasma membrane and TLR9 is reported to localize to endosomes (37, 38), we did not anticipate physical interaction of their cognate ligands. However, in light of recent reports that TLR4 may require internalization in order to signal (39), we investigated whether the two ligands were internalized by epithelial cells. A549 cells were treated with FITC-labeled Pam2CSK4 and Texas Red-labeled ODN2395 at the same concentrations used in the pathogen killing experiments for 2 h, then submitted to fluorescence microscopy. As shown in Figure 9, both Pam2CSK4 (green) and ODN2395 (red) were internalized by the epithelial cells. Further, they co-localize (yellow) in the cytoplasmic compartment, presumably within endosomes. While this does not prove that the receptors interact in that compartment, it supports that possibility. To assess whether they physically interact will require additional testing.
Figure 9. TLR2/6 and TLR9 agonists co-localize when applied in vitro.
FITC-labeled ODN2395 (20 µg/ml, green) and Texas Red-labeled Pam2CSK4 (10 µg/ml, red) were added to A549 cells in monolayer for 2 h, the cells were then washed, stained with DAPI (blue), and submitted to fluorescence microscopy. Overlapping red and green fluorescence is shown yellow. Microscopy experiments performed four times
DISCUSSION
We describe here an unanticipated, synergistic combination of TLR agonists that broadly protects mice against bacterial pneumonia, recapitulating the survival advantage previously reported after inhalational of a crude bacterial lysate. The combination of Pam2CSK4 and ODN2395 promoted intrapulmonary pathogen killing and survival of infectious challenges to an extent that far surpassed most other tested TLR ligand doublets, suggesting that the mechanisms underlying these observations are more specific than anticipated when we hypothesized that the bacterial lysate induced resistance by simultaneous stimulation of multiple PRRs. Similar to our earlier reports of inducible resistance, Pam2-ODN-induced protection appears to involve elaboration of antimicrobial effectors from the lung epithelium and containment of infection within the lungs.
We demonstrate here the requirement for TLR signaling in lysate-induced resistance in the discovery of MyD88-dependence. That the deficiency of a single gene could result in such a profound loss of function was remarkable given the numerous PRRs potentially engaged by the lystate. Given the importance of MyD88 in bacterial defense (24, 29), the susceptibility of MyD88 deficient mice to infection was not surprising. However the Myd88−/− mice did not die more rapidly than the PBS-treated wild type mice, nor did they exhibit higher lung bacterial CFUs after challenge than PBS-treated wild type mice, suggesting that the central defect was an inability to induce resistance rather than a diminution of baseline resistance, although both likely contribute to some extent. Further, while the MyD88 studies refined our focus from PRRs in general to TLRs, the lack of a defect in the TRIF-deficient mice revealed that only a subset of TLRs were required for induced resistance.
Single TLR ligands have previously been described to induce cytokine responses from leukocytes and to confer survival benefits in some models of infection (40–43), but their ability to therapeutically induce lung mucosal defenses is incompletely characterized and likely inferior to stimulation of multiple PRRs (6, 15, 44–46). To more precisely define the receptors involved in inducible resistance than is possible with the crude lysate, we investigated the use of synthetic TLR ligands. Despite our aggressive ligand dosing strategy, we did not observe high level protection from any single TLR ligand against P. aeruginosa infections. Because we and others (15, 40, 43) have observed protection with similar doses of single TLR ligands in other models, we believe the lack of protection reflects the extreme lethality of our cytotoxic Pseudomonas model, underscoring the robustness of Pam2-ODN-induced resistance.
To investigate whether simultaneous TLR stimulation could induce antibacterial resistance, we combined TLR ligands into doublets and failed to induce a high level of protection with most combinations, but achieved protection that was similar to that induced by the lysate when treating mice with the synergistic combination of TLR2/6 and TLR9 agonists.
In recent years, a number of TLR ligands have been investigated therapeutically for infectious and non-infectious conditions, with a handful presently in clinical use (46–48). However, to our knowledge, this is the first description of the therapeutic combination of TLR2/6 and TLR9 agonists and the first TLR treatment reported to confer broad protection against pneumonia. While we did not anticipate such exceptional performance of this specific doublet, retrospective review of the literature provides some clues that this protective effect is consistent with a more broadly observed biologic phenomenon.
TLR2 and TLR9 cooperate in the detection and control of several pathogens, including Mycobacterium tuberculosis and Trypanosoma cruzi (49, 50), herpes simplex virus (51, 52), Helicobacter pylori (53, 54) and possibly S. pneumoniae (55), by unresolved mechanisms. Sato and colleagues claimed that the dual recognition of herpes simplex viruses required serial detection by TLR2 then TLR9 (51), though Sørensen and colleagues subsequently found no evidence for sequential signaling (52). Certainly, signal amplification of one TLR by another in series could theoretically result in such an effect. However, a potentially more appealing explanation than serial signaling is synergistic coincident detection of dual TLR-dependent signals. Such convergence could occur either in the process of intracellular secondary signaling or by extracellular interactions of elaborated antimicrobial products. Our gene expression data indicate that at least some of the synergy arises from intracellular signaling convergence, as the two ligands synergistically enrich TLR signaling pathways. However, extracellular synergy has also been shown in humans with sub-microbicidal concentrations of lysozyme and lactoferrin enhancing bacterial killing by LL-37 and β-defensin 2 (9, 56); combinations of lactoferrin, SLPI, and lysozyme showing synergistic killing; and antimicrobial proteins interacting with reactive oxygen species (13, 57). As we have found orthologs of several of these effector molecules to be inducible in mouse epithelial cells by the lysate (17, 18) and by Pam2-ODN (Figure 8), but have infrequently seen synergistic unregulation of individual effectors by combination treatment, such an extracellular interaction seems likely to contribute, as well.
Potentially lending some insight into the mechanism of synergistic TLR interaction is our finding that Class C CpG ODNs combine with Pam2CSK4 to yield greater protection than is observed when Class A or Class B ODNs are delivered with Pam2CSK4. Synthetic CpG ODN classes can be defined both functionally and structurally (58–61). Class A ODNs have phosphodiester backbones with palindromic sequences and characteristically induce interferon-α and -γ secretion from leukocytes. Class B ODNs have phosphorothioate backbones with 6-mer linear sequences that induce B cell proliferation and interleukins-6 and -10 responses. Class C ODNs have characteristics of both A and B classes (60, 62). These class-specific cytokine responses appear to arise from differential endosomal compartmentalization and signaling, with Class A ODNs predominantly triggering IRF-7-mediated signaling from early endosomes, while Class B ODNs primarily induce NF-κB activation from late endosomes (60). As such, we hypothesize that Class C ODN-induced concurrent signaling by both IRF-7 and NF-κB pathways may be required for the robust Pam2-ODN effect, perhaps resulting in elaboration of distinct sets of effector molecules that positively interact. This hypothesis is particularly intriguing given our observation of apparent co-localization of the ligands in endosomes, and the recent demonstrations of endolysosomal IRF-7 signaling induction by a TLR2/6 agonist (63) and of endosomal trafficking of TLR9 (64).
Given the worldwide mortality burden of pneumonia, therapeutics to broadly induce lung mucosal immunity could be of tremendous potential benefit, particularly for susceptible populations during self-limited periods of peak vulnerability, such as cancer patients undergoing myeloablative therapies, healthy people during an emerging epidemics, or individuals at risk for exposure to weaponized bioterror agents. Building on our discovery that MyD88 is required for inducible resistance, our finding of robust protection synergistically induced by synthetic TLR ligands may provide the basis for a clinical intervention to protect broadly against pneumonia.
ACKNOWLEDGEMENTS
Drs. Evans, Tuvim and Dickey are also authors on a related U.S. patent application entitled “Stimulation of innate resistance of the lungs to infection with synthetic ligands,” and Drs. Tuvim and Dickey are authors on related patent US60/833,857 entitled “Compositions and methods for stimulation of lung innate immunity.” Drs. Evans, Tuvim and Dickey own stock in Pulmotect, Inc., which holds the commercial options on these patent disclosures.
Dr. Evans is supported by grants KL2-RR02419 from the National Center for Research Resources, National Institutes of Health and U01-AI82226 from the National Institute of Allergy and Infectious Disease, National Institutes of Health. Bioinformatics resources for this work and a Physician-Scientist Award to Dr. Evans were supported by Cancer Center Support Grant P30-CA016672 to the University of Texas M. D. Anderson Cancer Center from the National Cancer Institute, National Institutes of Health
Abbreviations used in this paper
- NTHi
nontypeable Haemophilus influenzae
- PAMP
pathogen associated molecular pattern
- PRR
pattern recognition receptor
- TIR
Toll/interleukin-1 receptor
- [DCmax]
ligand concentration resulting in maximal dendritic cell cytokine expression
- IRF
interferon regulatory factor
Footnotes
The other authors have no potential conflicts to declare.
REFERENCES
- 1.Mizgerd JP. Lung infection--a public health priority. PLoS Med. 2006;3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. The World Health Report 2004 -- Changing History. Geneva: World Health Organization; 2004. [Google Scholar]
- 4.WHO. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bulletin of the World Health Organization. 2004;82:891–970. [PMC free article] [PubMed] [Google Scholar]
- 5.Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8:142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 6.Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc. 2005;2:403–411. doi: 10.1513/pats.200508-090JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med. 2008;358:716–727. doi: 10.1056/NEJMra074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23:327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett JA, Fischer AJ, McCray PB., Jr Innate immune functions of the airway epithelium. Contrib Microbiol. 2008;15:147–163. doi: 10.1159/000136349. [DOI] [PubMed] [Google Scholar]
- 10.Hiemstra PS. The role of epithelial beta-defensins and cathelicidins in host defense of the lung. Exp Lung Res. 2007;33:537–542. doi: 10.1080/01902140701756687. [DOI] [PubMed] [Google Scholar]
- 11.Hippenstiel S, Opitz B, Schmeck B, Suttorp N. Lung epithelium as a sentinel and effector system in pneumonia--molecular mechanisms of pathogen recognition and signal transduction. Respir Res. 2006;7:97. doi: 10.1186/1465-9921-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schutte BC, McCray PB., Jr [beta]-defensins in lung host defense. Annu Rev Physiol. 2002;64:709–748. doi: 10.1146/annurev.physiol.64.081501.134340. [DOI] [PubMed] [Google Scholar]
- 13.Evans SE, Xu Y, Tuvim MJ, Dickey BF. Inducible innate resistance of lung epithelium to infection. Annu Rev Physiol. 2010;72:413–435. doi: 10.1146/annurev-physiol-021909-135909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clement CG, Evans SE, Evans CM, Hawke D, Kobayashi R, Reynolds PR, Moghaddam SJ, Scott BL, Melicoff E, Adachi R, Dickey BF, Tuvim MJ. Stimulation of lung innate immunity protects against lethal pneumococcal pneumonia in mice. Am J Respir Crit Care Med. 2008;177:1322–1330. doi: 10.1164/rccm.200607-1038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clement CG, Tuvim MJ, Evans CM, Tuvin DM, Dickey BF, Evans SE. Allergic lung inflammation alters neither susceptibility to Streptococcus pneumoniae infection nor inducibility of innate resistance in mice. Respir Res. 2009;10:70. doi: 10.1186/1465-9921-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans SE, Scott BL, Clement CG, Pawlik J, Bowden MG, Hook M, Kontoyiannis DP, Lewis RE, LaSala PR, Peterson JW, Tuvim MJ, Dickey BF. Stimulation of lung innate immunity protects mice broadly against bacterial and fungal pneumonia. Am J Respir Cell Molec Biol. 2010;42:40–50. doi: 10.1165/rcmb.2008-0260OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuvim MJ, Evans SE, Clement CG, Dickey BF, Gilbert BE. Augmented lung inflammation protects against influenza A pneumonia. PLoS ONE. 2009;4:e4176. doi: 10.1371/journal.pone.0004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–363. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 21.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Shi Z, Cai Z, Wen S, Chen C, Gendron C, Sanchez A, Patterson K, Fu S, Yang J, Wildman D, Finnell RH, Zhang D. Transcriptional regulation of the novel Toll-like receptor Tlr13. J Biol Chem. 2009;284:20540–20547. doi: 10.1074/jbc.M109.022541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 24.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 25.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 27.Moghaddam SJ, Clement CG, De la Garza MM, Zou X, Travis EL, Young HW, Evans CM, Tuvim MJ, Dickey BF. Haemophilus influenzae lysate induces aspects of the chronic obstructive pulmonary disease phenotype. Am J Respir Cell Mol Biol. 2008;38:629–638. doi: 10.1165/rcmb.2007-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1315–L1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- 29.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 30.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 31.Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Ulmer AJ. Lipopeptide structure determines TLR2 dependent cell activation level. FEBS J. 2005;272:6354–6364. doi: 10.1111/j.1742-4658.2005.05029.x. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 33.Krug A, Rothenfusser S, Hornung V, Jahrsdorfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, Hartmann G. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Chuang TH, Redecke V, She L, Pitha PM, Carson DA, Raz E, Cottam HB. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci U S A. 2003;100:6646–6651. doi: 10.1073/pnas.0631696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin M, Michalek SM, Katz J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect Immun. 2003;71:2498–2507. doi: 10.1128/IAI.71.5.2498-2507.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, Stripp BR, Dickey BF. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dostert C, Meylan E, Tschopp J. Intracellular pattern-recognition receptors. Adv Drug Deliv Rev. 2008;60:830–840. doi: 10.1016/j.addr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cluff CW, Baldridge JR, Stover AG, Evans JT, Johnson DA, Lacy MJ, Clawson VG, Yorgensen VM, Johnson CL, Livesay MT, Hershberg RM, Persing DH. Synthetic toll-like receptor 4 agonists stimulate innate resistance to infectious challenge. Infect Immun. 2005;73:3044–3052. doi: 10.1128/IAI.73.5.3044-3052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JS, Frevert CW, Matute-Bello G, Wurfel MM, Wong VA, Lin SM, Ruzinski J, Mongovin S, Goodman RB, Martin TR. TLR-4 pathway mediates the inflammatory response but not bacterial elimination in E. coli pneumonia. Am J Physiol Lung Cell Mol Physiol. 2005;289:L731–L738. doi: 10.1152/ajplung.00196.2005. [DOI] [PubMed] [Google Scholar]
- 42.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 43.Yu FS, Cornicelli MD, Kovach MA, Newstead MW, Zeng X, Kumar A, Gao N, Yoon SG, Gallo RL, Standiford TJ. Flagellin stimulates protective lung mucosal immunity: role of cathelicidin-related antimicrobial peptide. J Immunol. 2010;185:1142–1149. doi: 10.4049/jimmunol.1000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng JC, Moore TA, Newstead MW, Zeng X, Krieg AM, Standiford TJ. CpG oligodeoxynucleotides stimulate protective innate immunity against pulmonary Klebsiella infection. J Immunol. 2004;173:5148–5155. doi: 10.4049/jimmunol.173.8.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reppe K, Tschernig T, Luhrmann A, van Laak V, Grote K, Zemlin MV, Gutbier B, Muller HC, Kursar M, Schutte H, Rosseau S, Pabst R, Suttorp NW, Witzenrath M. Immunostimulation with Macrophage-Activating Lipopeptide-2 Increased Survival in Murine Pneumonia. Am J Respir Cell Mol Biol. 2008 doi: 10.1165/rcmb.2008-0071OC. [DOI] [PubMed] [Google Scholar]
- 46.Standiford TJ, Deng JC. Immunomodulation for the prevention and treatment of lung infections. Semin Respir Crit Care Med. 2004;25:95–108. doi: 10.1055/s-2004-822309. [DOI] [PubMed] [Google Scholar]
- 47.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 48.Ulevitch RJ. Therapeutics targeting the innate immune system. Nat Rev Immunol. 2004;4:512–520. doi: 10.1038/nri1396. [DOI] [PubMed] [Google Scholar]
- 49.Bafica A, Santiago HC, Goldszmid R, Ropert C, Gazzinelli RT, Sher A. Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J Immunol. 2006;177:3515–3519. doi: 10.4049/jimmunol.177.6.3515. [DOI] [PubMed] [Google Scholar]
- 50.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci U S A. 2006;103:17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorensen LN, Reinert LS, Malmgaard L, Bartholdy C, Thomsen AR, Paludan SR. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J Immunol. 2008;181:8604–8612. doi: 10.4049/jimmunol.181.12.8604. [DOI] [PubMed] [Google Scholar]
- 53.Chang YJ, Wu MS, Lin JT, Chen CC. Helicobacter pylori-Induced invasion and angiogenesis of gastric cells is mediated by cyclooxygenase-2 induction through TLR2/TLR9 and promoter regulation. J Immunol. 2005;175:8242–8252. doi: 10.4049/jimmunol.175.12.8242. [DOI] [PubMed] [Google Scholar]
- 54.Chang YJ, Wu MS, Lin JT, Sheu BS, Muta T, Inoue H, Chen CC. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol Pharmacol. 2004;66:1465–1477. doi: 10.1124/mol.104.005199. [DOI] [PubMed] [Google Scholar]
- 55.Lee KS, Scanga CA, Bachelder EM, Chen Q, Snapper CM. TLR2 synergizes with both TLR4 and TLR9 for induction of the MyD88-dependent splenic cytokine and chemokine response to Streptococcus pneumoniae. Cell Immunol. 2007;245:103–110. doi: 10.1016/j.cellimm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh PK, Tack BF, McCray PB, Jr, Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 58.Jurk M, Kritzler A, Debelak H, Vollmer J, Krieg AM, Uhlmann E. Structure-activity relationship studies on the immune stimulatory effects of base-modified CpG toll-like receptor 9 agonists. ChemMedChem. 2006;1:1007–1014. doi: 10.1002/cmdc.200600064. [DOI] [PubMed] [Google Scholar]
- 59.Vollmer J, Jurk M, Samulowitz U, Lipford G, Forsbach A, Wullner M, Tluk S, Hartmann H, Kritzler A, Muller C, Schetter C, Krieg AM. CpG oligodeoxynucleotides stimulate IFN-gamma-inducible protein-10 production in human B cells. J Endotoxin Res. 2004;10:431–438. doi: 10.1179/096805104225006534. [DOI] [PubMed] [Google Scholar]
- 60.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 61.Vollmer J, Weeratna R, Payette P, Jurk M, Schetter C, Laucht M, Wader T, Tluk S, Liu M, Davis HL, Krieg AM. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol. 2004;34:251–262. doi: 10.1002/eji.200324032. [DOI] [PubMed] [Google Scholar]
- 62.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 63.Dietrich N, Lienenklaus S, Weiss S, Gekara NO. Murine toll-like receptor 2 activation induces type I interferon responses from endolysosomal compartments. PLoS One. 2010;5:e10250. doi: 10.1371/journal.pone.0010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]