Synopsis
The development of the integumentary system is a series of events, which start in utero and continue throughout life. Although at birth, skin in full-term infants is anatomically mature, functional maturity develops during the first year of life. Pediatric skin transitions again with the onset of puberty. At each stage, there are changes in transepidermal water loss, skin hydration, and skin acidity that define the specific period of development.
Keywords: Fetal, Pediatric, Skin Development, Transepidermal Water Loss, Skin Hydration, Skin Acidity
Introduction
Fetal and pediatric skin are commonly believed to have advantageous properties for wound repair, with the ability of fetal skin to undergo scarless wound repair and the inherent youthful appearance of pediatric skin[1]. However, infant and pediatric skin are also considered to be more sensitive and prone to injury than adults with the cosmetic industry marketing an extensive line of ‘delicate’ products exclusively for the care and hygiene of infants and pediatrics[2].
Skin Development
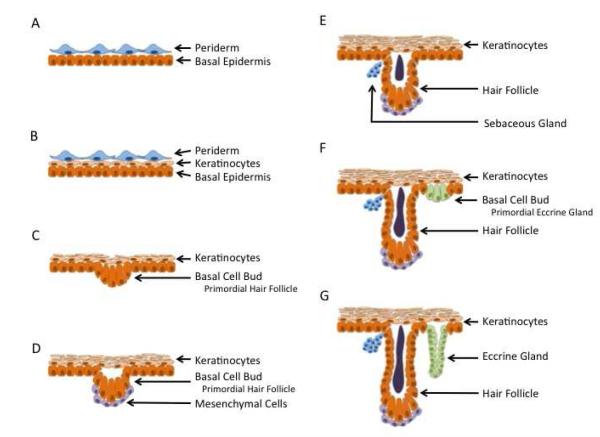
The development of the dermal/epidermal layers is a continuous process starting early in pregnancy with discrete patterns related to gestation age, developing from the cranial to caudal pole[3]. (Table 1) At four weeks gestation, fetal skin can be visualized as two distinct layers with a basal cell layer covered by an outer layer, termed the periderm (Figure 1). The periderm is uniquely found in humans without an analog in animal models, such as mice or rats [4]. Keratinization begins at nine weeks gestation, and at thirteen weeks gestation, stratification into different layers becomes apparent [3]. Hair follicles begin to form as epidermal buds along the basal layer at fourteen weeks gestation. In the subsequent two weeks, these epidermal buds are associated with a local proliferation of mesenchymal cells associated with the epidermal bud as hair follicles rapidly develop[3]. This is followed by the continued elongation of the hair follicles. The development of eccrine sweat glands begins as epidermal buds in the basal layer at twenty weeks gestation and continue to develop over the next ten weeks, first by elongating and then by coiling [3]. At 24 weeks gestation, fetal skin continues to heal without scar, as keratinization and stratification into mature morphologic layers continues [4]. After 24 weeks gestation, there is a “transitional period”, where skin heals without characteristic scar deposition, but fails to reconstitute the dermal appendages. This transitional period has been found in multiple animal models of fetal wound healing. The scarless wound healing phenotype is lost by third trimester. At 34 weeks gestation, mature keratinocytes characterized by flattened, keratinized morphology is observed in conjunction with adult type dermo-epidermal undulations [5, 6].
Table 1.
Clinical Review of the Literature: Summary Table of Various Reviews
| Author | Groups Compared | Findings |
|---|---|---|
| Stamatas GN, Nikolovski J, Mack MC, et al (2011)[15] |
Full term infants (birth to 3 years old) vs Adults |
|
| Fluhr JW, Darlenski R, Lachmann N, et al (2012)[26] |
Newborns (1-15 days) vs Infants (5-6 weeks) vs Infants (6 months) vs Infants (1-2 years) vs Pediatrics (4-5 years) vs Adults (20-35 years) |
Skin Function
|
| Giusti F, Martella A, Bertoni L and Seidenari S (2001)[2] |
Infants (8-24 months) vs Adults (25-35 years) |
Skin Function:
|
| Firooz A, Sadr B, Babakoohi S, et al (2012)[24] |
Pediatrics (10-20 years) vs Adults (20-30 years, 30- 40 years, 40-50 years) |
Skin Function
|
| Man MQ, Xin SJ, Song SP, et al (2009)[25] |
Prepuberty (0-12 years) vs Young group (13-35 years) vs Middle age (36-50 years) vs Old group (51-70 years) |
Skin Function:
|
Figure 1.
Fetal development of skin. (A) At 4 weeks gestation, skin is composed of two layers, the periderm and basal epidermis. (B) At 9 week gestation, keratinization becomes apparent. (C) At 14 weeks gestation, stratification of the epidermal layer is apparent along with budding of the basal layer as the primordial hair follicle develops. (D) At 16 weeks gestation, mesenchymal cells may be seen associated with the epidermal bud. (E) At 18 weeks, sebaceous glands become apparent, along with hair follicle elongation. (F) At 23 weeks, the hair follicle continues to elongate while the basal layer bud to form primordial eccrine glands. (G) At 30 week gestation, the eccrine glands continue to elongate and coil.
In later gestation, the fetal dermis is primarily thickened by a increase in collagen content[4]. This dermis has higher levels of type III collagen, chondroitin sulfate, proteoglycans and hyaluronan compared to adult dermis[4]. Fetal dermis is also notable in its absence of dermal elastin[7, 8].
Also during the last trimester, the fetus is covered by the vernix caseosa (VC), a protective coat secreted by the sebaceous glands and composed of protein (10%), lipids (10%) and water (80%) [9]. The VC is uniquely human, with no counterpart identified in animal models. This coat was initially posited to function as a lubricant in the birthing process. However, as the fetus continues to mature, part of the vernix sloughs from the skin surface into the surrounding amniotic fluid [10]. This physiologic decrease of vernix with advancing gestational age renders this role unlikely. More recent studies of the VC suggest the layer helps to facilitate the transition from an aqueous in utero environment to the dry, extrauterine environment [11]. The vernix helps to protect the fetal epidermis from maceration while immersed in amniotic fluid and permits epidermal cornification and stratum corneum formation [9]. The VC also contains high levels of lysozyme, lactoferrin, linoleic acid, as well as, other anti-infective properties [9, 12].
Full-term infants at birth have skin that is anatomically mature when examined histologically with all five layers present. These includefrom deep to superficial: stratum basale, stratum spinosum, stratum granulosum, stratum licidum and stratum corneum[13]. As epidermal cells mature, their morphology changes from the columnar stratum basale to the tightly overlapping squamous keratinocytes of the stratum corneum [5, 6]. The time to fully mature, keratinize and form this protective horny layer varies depending on body site[14]. This occurs more rapidly in facial skin than in the trunk and limbs[6]. Neonatal skin has a relatively coarse texture compared to older infants and proceeds to develop a more homogeneous smooth structure during the first 30 days of life [15]. Infants have smaller corneocytes and a significantly thinner stratum corneum until two years of age[13].
During the next developmental period from infancy to puberty, there is little difference in skin between males and females patients. Both genders demonstrate a steady increase in dermal thickness, with males developing thicker epidermal and dermal layers[16]. At the onset of puberty, there is significant hormonal influence on the skin. After age twelve, females accumulate a thick layer of subcutaneous fat, which is absent in males. On the other hand, males exhibit a gradual thinning of their thick epidermal and dermal layers. These layers remain a constant thickness in females throughout adolescence and adulthood until menopause [16]. Additionally, dermal composition begins to change with advancing age starting at puberty, with both sexes showing similar rates of linear decrease in skin collagen content with age. As females start with baseline lower collagen density, they appear to age earlier than men [4, 16].
Skin Function
Skin has multiple functions including regulation of body temperature and protection against physical, chemical and biological insults [17, 18]. At birth, in full-term neonates, the skin is histologically mature, however it remains functionally immature. Neonatal barrier functions are in a constant state of flux, in contrast to mature adult skin[5]. It has been proposed that this changing infant skin barrier is not a deficit but beneficial as adaptive flexibility allowing constant optimization, balancing growth, thermoregulation, water barrier and protective functions.
Biophysical Skin Parameters
Skin can be defined by a variety of biophysical skin parameters, which permit the study of skin in a non-invasive manner. Multiple parameters, including transepidermal water loss, hydration, and skin acidity, are affected in skin diseases such as atopic dermatitis, psoriasis, and allergic or irritant contact dermatitis[2].
Transepidermal Water Loss
Transepidermal water loss (TEWL) describes the amount of water loss through the epidermis through evaporation and is dependent on multiple factors, including skin temperature, skin blood flow, local hemodynamics, degree of corneocyte formation and stratum corneum lipid content[19]. TEWL is measured by electrical skin impedance, where lower impedance indicates higher skin hydration[20]. There is a direct relationship between TEWL and skin development. In full term infants, there is a significant decrease in TEWL indicating a functional barrier to evaporative water loss. At birth, the sweat glands are anatomically mature, however only a small fraction of these glands are functionally mature with secretory activity[20]. During the first few months of life, impedance values begin to decrease corresponding to the recruitment and maturation of sweat glands. These values stabilize at four months in full term infants [20].
Premature infants, less than 32 weeks gestation, have lower impedance and high TEWL at birth. This excess loss is a result of immature barrier function and thinner epidermal layers [20], relatively increased blood flow to the skin compared to other infants as well as a high ratio of total body surface area to volume. These factors lead to increased insensible water loss. TEWL in these premature infants can exceed 30% of their total body weight within a 24 hr period. TEWL decrease as skin continues to mature, with improved barrier function. The barrier function regulating evaporative losses varies according to anatomic location. In general, TEWL is higher in the facial region, compared the body [19]. The highest TEWL is reported in the nasolabial fold and perioral regions, with the lowest TEWL over the cheeks. There are no gender differences noted in the neonatal or pediatric period[21].
Skin Hydration
At birth, skin is transitioned from continuous immersion in an aqueous solution to exposure to relatively low humidity ambient air. Maintenance of skin hydration is critical for skin function, including plasticity, flexibility, prevent fissuring and proper desquamation [22].. Neonatal skin responds with a dramatic decrease in hydration at birth [9]. Skin hydration then quickly increases for up to 90 days following birth, as the eccrine glands function matures [23]. This increased skin hydration persists for up to the first year of life [5] and subsequently stabilizes to adult levels[24].
The sebaceous glands also play an important role in maintaining optimal skin hydration, secreting lipid-rich sebum. The highest sebum levels in the face are located in the nasolabial area [19]. These glands are hormonally regulated and are active in utero, producing the vernix caseosa [9]. Following birth, there are low levels of sebum protection until puberty, where it markedly increased to adult levels, particularly in males[25] [6, 24].
Skin Acidity
Fully mature skin is characterized by a physiologic “acid mantle” with the pH maintained between 4.5 to 6.0 [2, 26]. This acid mantle is an important mechanism in the skin’s defense against infection. The enzymes in the upper epidermis are optimized to function at pH 5.6. Neonatal skin is characterized by a higher pH compared to older pediatric and adult patients, regardless of gestational age, sex, mode of delivery or body weight [27]. This newborn skin is shown to have a different chemical composition of skin surface lipids [2]. Maturation and maintenance of the acid mantle is dependent on lactic acid, free amino acids and fatty acids found in sebum and sweat. Once established, the acid mantle is more uniformly distributed anatomically in the early pediatric population[2]. At the onset of puberty, the intertriginous areas such as the axilla and inguinal region approach a neutral or even alkaline pH, as found in adults [28].
Therapeutic Options
As the survival rate of premature infants continues to increase with earlier gestation ages, the issue of immature pediatric skin becomes more prevalent. The therapeutic options focus on supporting the barrier function of the infant skin, including thermoregulation, hydration and attenuating risk of infection. In the neonatal period, thermoregulation can be supported by the use of low energy infrared radiation, which warms the ambient air [17]. Skin temperature can vary greatly depending on anatomic location with the skin overlying the liver correlates most closely with core temperature [17]. Maintenance of a “normal” core temperature decreases the mortality rate of these premature infants and also helps to minimize insensible water loss by optimizing the TEWL. Though barrier function becomes physiologically mature early in the pediatric period, the increased surface area to volume ratio of younger children continues to put them at a higher risk of excess water loss and dehydration due to insensible losses compared to adults and adolescents. Cognizance of this risk and regular oral hydration is necessary to maintain homeostasis.
From birth to puberty, pediatric skin is at risk of desiccation with lower levels of sebum production. Decreased skin hydration is associated with impairment of the skin’s barrier function and pathology such as atopic dermatitis. Desiccation of the skin leads to the early appearance of fine cracks and fissures in pediatric skin [9]. These defects are a route to infection and irritants. Maintenance of hydration can be facilitated by continual use of moisturizers.
Clinical Outcomes
Skin development continues from birth throughout life. With interim support of skin function in the neonatal period, even the most premature infant progresses to normal skin capable of thermoregulation, maintenance of hydration and protection against infection.
Complications and Concerns
Pediatric skin has increased permeability with higher TEWL and increased skin hydration. This increased permeability leads to the tendency to develop xerosis, excessively dry skin which presents as white patches with fine white scales, particularly on the exposed facial skin[6]. This xerosis leads to an increased development irritant or allergic contact dermatitis[2, 6].
Without support of thermoregulation, hydration and pathogen barrier in the neonatal and early pediatric period, immature skin can result in lethal consequences secondary to extensive transcutaneous fluid loss or infection[18].
Summary
Skin development is a continuous process, beginning in utero and continuing throughout life. The skin is anatomically mature at birth, but continues to functionally develop through the first year of life. The glandular components of skin are strongly influenced by hormonal changes, with further development seen at the onset of puberty.
Key Points.
Skin is anatomically mature at birth but continues functional maturity during the first year of life.
In contrast to adults, infant skin is in a constant state of flux with changes in transepidermal water loss, hydration, lipid content and skin acidity.
Mature barrier function is critical for maintenance of thermoregulation, hydration and protection against infection.
Impaired barrier function and skin desiccation increases risk of pathology including atopic or contact dermatitis, infection and, even, lethal excess water loss.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no disclosures.
References
- 1.Leung A, Crombleholme TM, Keswani SG. Fetal wound healing: implications for minimal scar formation. Curr Opin Pediatr. 2012;24:371–378. doi: 10.1097/MOP.0b013e3283535790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giusti F, Martella A, Bertoni L, et al. Skin barrier, hydration, and pH of the skin of infants under 2 years of age. Pediatr Dermatol. 2001;18:93–96. doi: 10.1046/j.1525-1470.2001.018002093.x. [DOI] [PubMed] [Google Scholar]
- 3.Ersch J, Stallmach T. Assessing gestational age from histology of fetal skin: an autopsy study of 379 fetuses. Obstet Gynecol. 1999;94:753–757. doi: 10.1016/s0029-7844(99)00379-8. [DOI] [PubMed] [Google Scholar]
- 4.Satish L, Kathju S. Cellular and Molecular Characteristics of Scarless versus Fibrotic Wound Healing. Dermatol Res Pract. 2010:790234. doi: 10.1155/2010/790234. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikolovski J, Stamatas GN, Kollias N, et al. Barrier function and water-holding and transport properties of infant stratum corneum are different from adult and continue to develop through the first year of life. J Invest Dermatol. 2008;128:1728–1736. doi: 10.1038/sj.jid.5701239. [DOI] [PubMed] [Google Scholar]
- 6.Tagami H. Location-related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int J Cosmet Sci. 2008;30:413–434. doi: 10.1111/j.1468-2494.2008.00459.x. [DOI] [PubMed] [Google Scholar]
- 7.Coolen NA, Schouten KC, Boekema BK, et al. Wound healing in a fetal, adult, and scar tissue model: a comparative study. Wound Repair Regen. 2010;18:291–301. doi: 10.1111/j.1524-475X.2010.00585.x. [DOI] [PubMed] [Google Scholar]
- 8.Coolen NA, Schouten KC, Middelkoop E, et al. Comparison between human fetal and adult skin. Arch Dermatol Res. 2010;302:47–55. doi: 10.1007/s00403-009-0989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visscher MO, Utturkar R, Pickens WL, et al. Neonatal skin maturation--vernix caseosa and free amino acids. Pediatr Dermatol. 2011;28:122–132. doi: 10.1111/j.1525-1470.2011.01309.x. [DOI] [PubMed] [Google Scholar]
- 10.Narendran V, Wickett RR, Pickens WL, et al. Interaction between pulmonary surfactant and vernix: a potential mechanism for induction of amniotic fluid turbidity. Pediatr Res. 2000;48:120–124. doi: 10.1203/00006450-200007000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Hoath SB. Physiologic development of the skin. In: RA P, Fox W, Abman S, editors. Fetal and Neonatal Physiology. Elsevier; Philadelphia, PA: 2004. [Google Scholar]
- 12.Walker VP, Akinbi HT, Meinzen-Derr J, et al. Host defense proteins on the surface of neonatal skin: implications for innate immunity. J Pediatr. 2008;152:777–781. doi: 10.1016/j.jpeds.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Stamatas GN, Nikolovski J, Luedtke MA, et al. Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level. Pediatr Dermatol. 2010;27:125–131. doi: 10.1111/j.1525-1470.2009.00973.x. [DOI] [PubMed] [Google Scholar]
- 14.Spearman RI. Vertebrate skin. Nature. 1978;276:442. doi: 10.1038/276442a0. [DOI] [PubMed] [Google Scholar]
- 15.Stamatas GN, Nikolovski J, Mack MC, et al. Infant skin physiology and development during the first years of life: a review of recent findings based on in vivo studies. Int J Cosmet Sci. 2011;33:17–24. doi: 10.1111/j.1468-2494.2010.00611.x. [DOI] [PubMed] [Google Scholar]
- 16.Tur E. Physiology of the skin--differences between women and men. Clin Dermatol. 1997;15:5–16. doi: 10.1016/s0738-081x(96)00105-8. [DOI] [PubMed] [Google Scholar]
- 17.Friedman F, Adams FH, Emmanouilides G. Regulation of body temperature of premature infants with low-energy radiant heat. J Pediatr. 1967;70:270–273. doi: 10.1016/s0022-3476(67)80422-0. [DOI] [PubMed] [Google Scholar]
- 18.Has C, Bruckner-Tuderman L. Molecular and diagnostic aspects of genetic skin fragility. J Dermatol Sci. 2006;44:129–144. doi: 10.1016/j.jdermsci.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Marrakchi S, Maibach HI. Biophysical parameters of skin: map of human face, regional, and age-related differences. Contact Dermatitis. 2007;57:28–34. doi: 10.1111/j.1600-0536.2007.01138.x. [DOI] [PubMed] [Google Scholar]
- 20.Emery MM, Hebert AA, Aguirre Vila-Coro A, et al. The relationship between skin maturation and electrical skin impedance. J Dermatol Sci. 1991;2:336–340. doi: 10.1016/0923-1811(91)90026-t. [DOI] [PubMed] [Google Scholar]
- 21.Ramos-e-Silva M, Boza JC, Cestari TF. Effects of age (neonates and elderly) on skin barrier function. Clin Dermatol. 2012;30:274–276. doi: 10.1016/j.clindermatol.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Visscher MO, Chatterjee R, Munson KA, et al. Changes in diapered and nondiapered infant skin over the first month of life. Pediatr Dermatol. 2000;17:45–51. doi: 10.1046/j.1525-1470.2000.01711.x. [DOI] [PubMed] [Google Scholar]
- 23.Hoeger PH, Enzmann CC. Skin physiology of the neonate and young infant: a prospective study of functional skin parameters during early infancy. Pediatr Dermatol. 2002;19:256–262. doi: 10.1046/j.1525-1470.2002.00082.x. [DOI] [PubMed] [Google Scholar]
- 24.Firooz A, Sadr B, Babakoohi S, et al. Variation of biophysical parameters of the skin with age, gender, and body region. ScientificWorldJournal. 2012:386936. doi: 10.1100/2012/386936. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Man MQ, Xin SJ, Song SP, et al. Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large Chinese population. Skin Pharmacol Physiol. 2009;22:190–199. doi: 10.1159/000231524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fluhr JW, Darlenski R, Lachmann N, et al. Infant epidermal skin physiology: adaptation after birth. Br J Dermatol. 2012;166:483–490. doi: 10.1111/j.1365-2133.2011.10659.x. [DOI] [PubMed] [Google Scholar]
- 27.Yosipovitch G, Maayan-Metzger A, Merlob P, et al. Skin barrier properties in different body areas in neonates. Pediatrics. 2000;106:105–108. doi: 10.1542/peds.106.1.105. [DOI] [PubMed] [Google Scholar]
- 28.Behrendt H, Green M. The relationship of skin pH pattern to sexual maturation in boys. AMA Am J Dis Child. 1955;90:164–172. doi: 10.1001/archpedi.1955.04030010166006. [DOI] [PubMed] [Google Scholar]