Abstract
We examined male and female adolescents (8–18 years of age) that were scanned with structural brain MRI and looked for a correlation between volume of the right or the left amygdala and parent-reported ability of emotional control. A sex difference was found in the correlation between emotional control and the corrected volume of the left amygdala (that is the amygdala volume adjusted for total cranial volume). In girls, smaller left amygdala volumes were associated with better emotional control. In boys, larger left amygdala volumes were associated with better emotional control. These findings suggest that healthy girls and boys show a difference in the correlation between parental reports of emotional control and the left amygdala volume.
Keywords: adolescents, amygdala volume, MRI
Introduction
The development of emotion regulation (including the ability to control emotions to meet situational demands) is an important developmental task [1]. Emotion regulation is particularly important in adolescence, a time of changes in self, peer, and family domains that require youth to regulate emotions to meet environmental demands [1,2]. Indeed, the improvements in affective regulation and identification of emotional cues occur during the transition of adolescence [3] and are thought to involve the maturation of orbitofrontal [4] and dorsolateral areas of the frontal lobe [5]. This maturation of frontal areas is thought to allow for the regulation of subcortical nuclei such as the amygdala [6]. The amygdala, in particular, through its connections with the autonomic nervous system, leads to physiological arousal during emotional situations through the activation of β-adrenergic receptors and aids in the facilitation of memory consolidation, learning, and attention to emotional qualities [7].
Although the important role of the amygdala in emotional behaviors has been characterized and emotion regulation is clearly important, few studies describe the role of the amygdala in emotion regulatory ability during adolescence and with a focus on sex differences [8]. Research with adults suggests that an increase in BOLD functional MRI (fMRI) signal of frontal regions such as the right ventrolateral frontal lobe negatively correlates with a functional deactivation, or decrease in BOLD fMRI signal, of the amygdala to aid in top–down modulation of emotional states. Thus, the deactivation of the amygdala may be part of a process associated with improved emotional control [9]. Furthermore, presentation of emotional distractors involves fMRI deactivation in areas of the brain involved in cognitive control such as the dorsolateral frontal lobe and a fMRI increase in activation of the amygdala [10] highlighting a role for both the amygdala and prefrontal cortex in emotional arousal.
In adolescents, although there are few studies that have examined sex differences, there is a different pattern of fMRI BOLD signal activity during emotional tasks as compared to children and adults. Adolescents show an increase in fMRI activation of the amygdala relative to children and adults during an emotional go/no-go task [6]. The fMRI activation of the amygdala that occurs when viewing fearful faces is also more pronounced in adolescents than in adults [11]. Studies also suggest that the changes in both fMRI activity and volume of the amygdala may result in the risk for or present as a pathology during adolescence. For example, adolescents at risk for depression show increased amygdala activation during the processing of fearful faces as compared to those low in risk [12]. In addition, impairments in emotion regulation along with changes in amygdala volume are described in adolescents with bipolar disorder [13]. In adolescents, the volume of the amygdala was correlated with the measurements of fearfulness. Parent-reported fearfulness correlated positively with left amygdala volume in healthy girls with a positive family history of depression but not in healthy boys [14]. These studies indicate the importance of studying emotional behaviors associated with the amygdala during adolescence.
Hypotheses
Given that the regulation of affective states through fMRI deactivation of the amygdala and activation of frontal subregions occur in adults and frontal subregions are still maturing during adolescence, we tested the hypothesis that parental reports of emotional control would correlate to volumes of the amygdala. Furthermore, given the sex differences in brain morphology and function and in emotion-related psychopathologies (such as depression; [15]), we examined whether associations between the amygdala volume and emotional control differ for boys versus girls.
Methods
Participants
Forty healthy girls (ages: 8.77–18.26 years; median: 11.90 years) and 32 healthy boys (ages: 9.16–18.35 years; median: 13.53 years) were included in the study. Handedness information was not included for this study. These data were the first scans that were available from a larger cohort of the Pediatric MRI Data Repository created by the NIH MRI Study of Normal Brain Development [16]. This is a multi-site, longitudinal study of typically developing children, from birth to young adulthood, conducted by the Brain Development Cooperative Group.
Pubertal status
The pubertal development scale was administered to each participant [17]. This questionnaire is a self-report measurement of pubertal stage that shows good validity. Participants that were in prepubertal, midpubertal, or postpubertal stages were included in the study. Median pubertal stage for girls and boys was midpubertal. For girls, 24.4% were prepubertal, 39% were midpubertal, and 36.6% were postpubertal. For boys, 34.4% were prepubertal, 53.1% were midpubertal, and 12.5% were postpubertal.
Emotional control
The Behavioral Rating Inventory of Executive Functioning [18] was used to assess adolescents’ emotional control through parent report. The Behavioral Rating Inventory of Executive Functioning is comprised of several Likert questions such as ‘mood changes frequently’, ‘becomes tearful easily’, ‘overreacts to small problems’, and ‘mood is easily influenced by the situation’. Increasing scores represent low-emotional control whereas lower scores represent high-emotional control. For girls, the median emotional control score was 42 (minimum–maximum: 36–59) and for boys the median emotional control score was 43 (minimum–maximum: 36–65).
MRI and region-of-interest analyses
High-resolution three-dimensional T1-weighted SPGR structural MRI scans were collected from all participants. Image acquisition sequence parameters included a sagittal plane acquisition (1mm slice thickness), TR= 22–25 ms, TE=10–11 ms, and one excitation. The image pre-processing involved the correction for image intensity non-uniformity [19], identification of a binary brain mask in native space, and registration of data to stereotaxic space using ANIMAL software [20]. Total intracranial volumes (total cranial volume) of gray, white, and cerebrospinal fluid tissue types were automatically generated using INSECT [21].
Amygdala nuclei
The left and right amygdala were manually traced by a rater blind to pubertal stage, age, emotional control scores, and sex in successive 1mm slices in the coronal viewing plane using Multitracer (http:www.loni.ucla.edu/software). For details of guidelines for neuroanatomic delineation of the amygdala, please see [22]. In brief, the amygdala nuclei were traced in a posterior-to-anterior sequence with the first slice being that which grey matter adjacent and superior to the temporal horn of the lateral ventricle and superior to the anterior hippocampi was first visualized. The first slice of the amygdala is generally at the level in which the hippocampus has folded upon itself with the fimbria wrapping around the temporal horn. The temporal horn of the lateral ventricle and the fimbria were used to delineate hippocampus from amygdala with all grey matter superior to the temporal horn and fimbria being the amygdala. This tracing was continued successively until the temporal horn could no longer be seen and all grey matter thereafter was traced as the amygdala. Both the axial and sagittal viewing planes were utilized to corroborate neuroanatomy. The coefficient of variation for intra-rater reliability was 3% and inter-rater reliability showed an intraclass correlation coefficient of 0.890.
Statistical analyses
Sex differences in amygdala volumes were assessed using an independent t-test. In addition, sex was used as the independent factor and left and right amygdala volumes as the dependent measures in an analysis of covariance to test for sex differences in amygdala volumes with total cranial volumes as the covariate to account for possible differences because of the head size.
For correlation analyses, we first examined the data to assess the normality of the data distributions. For girls, emotional control showed slight skewness (1.036), the total cranial volume of white matter (median=251.401) was found to have both a slight skewness (1.265) and kurtosis (3.276), and age (median=11.900 years) was found to show slight kurtosis (−1.178). For boys, only age (median=13.530 years) was found to have slight kurtosis (−1.377). All other data were normally distributed. As the emotional control data for girls did not fit a Gaussian distribution, the data were converted to rank-ordered data before the statistical analysis and non-parametric correlations were used when possible. Non-parametric tests were also used for boys when possible so that boys’ data would be comparable with girls’ data. When non-parametric tests were not available (as in the case of partial correlations co-varying for total cranial volume), parametric tests were run on the rank transformed data. Thus, in boys, to examine the relationships between left and right volumes of the amygdala, emotional control, pubertal stage, and age, a Spearman’s rho correlation coefficient analysis was used. A Pearson’s partial correlation analysis, using total cranial volume as a covariate, was used to test for the relations between emotional control and amygdala volumes.
For girls, a Spearman’s rho correlation coefficient analysis was calculated to examine the relationships between amygdala volumes, emotional control, pubertal stage, and age variables. A Pearson’s partial correlation analysis, using total cranial volume as a covariate, was used to test for relations between emotional control and amygdala volumes in girls.
A Wilcoxon-rank sum test was used to test sex differences using sex as the independent variable and emotional control, pubertal stage, and age as the dependent variables. To make statistical comparisons between girls and boys in the independent correlations of the corrected volume of the amygdala (that is the amygdala volume adjusted for total cranial volume) and emotional control measurements, a Fisher’s r-to-z transformation was used.
Results
Sex differences in amygdala volume, pubertal stage, and emotional control
Girls showed larger uncorrected right amygdala volumes [t(70)=2.780, P=0.007] relative to boys. However, after co-varying for total cranial volume there were no sex differences in amygdala volumes. Girls were more advanced in pubertal stage relative to boys [Ws (n1=40, n2=32)=996.500, z= −2.091, P=0.037] but there were no sex differences in age. There were no sex differences in the levels of emotional control.
Correlations between amygdala volume and emotional control
Girls showed a different independent correlation between emotional control and corrected volume of the left amygdala as compared with boys (girls: r=0.320; boys: r= −0.360; z-obtained=2.730, P<0.004). Below we present the correlation analyses separately for girls and boys.
Girls
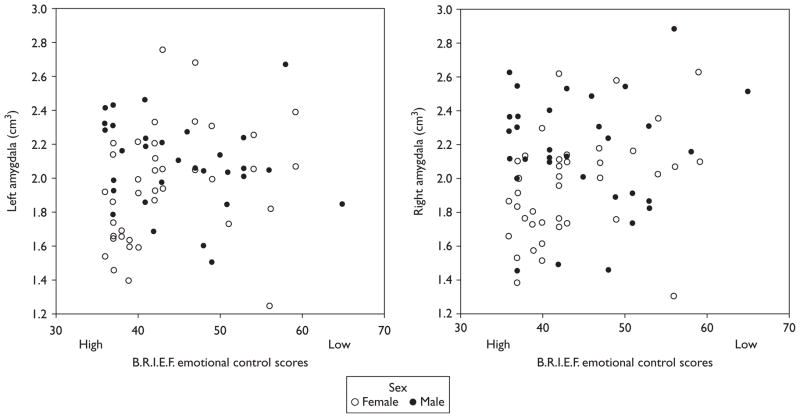
A positive correlation was found between emotional control scores and both the left and right amygdala volumes for girls [rs(n=40)=0.453, P=0.003; rs (n= 40)=0.420, P=0.007, respectively] (Fig. 1). Girls with better emotional control (i.e. lower scores) had smaller amygdala. Conversely, girls with poor emotional control (i.e. higher scores) were found to have larger amygdala volumes. Emotional control was also found to positively correlate with total cranial volume of grey matter [rs (n=40)=0.420, P=0.007] and total cranial volume [rs (n=40)=0.368, P=0.019], with worse emotional control scores associated with greater total cranial volume of grey matter and total cranial volume. Neither age nor pubertal status was correlated with emotional control or volumes of the right or left amygdala.
Fig. 1.
A significant correlation was found between the uncorrected left and right amygdala volumes and emotional control scores in adolescent females but not males. B.R.I.E.F., Behavioral Rating Inventory of Executive Functioning.
Corrected volumes
After co-varying for total cranial volume both the left and right amygdala continued to positively correlate with levels of emotional control in girls [r(37)=0.317, P= 0.049; r(37)=0.318, P=0.049].
Boys
There were no correlations between any of the uncorrected brain measurements and emotional control in boys. Moreover, neither age nor pubertal status was correlated with emotional control or volumes of the right or left amygdala.
Corrected volumes
After co-varying for total cranial volume, emotional control was negatively correlated with the volume of the left amygdala in boys [r(29)= −0.363, P=0.045]. Boys with poorer emotional control (i.e. higher scores) had smaller amygdala.
Discussion
Our results indicate sex differences in the correlation between parental ratings of emotional control and the corrected volume of the left amygdala. In girls, better emotional control scores were associated with smaller left amygdala volumes whereas in boys, better emotional control scores were related to larger left amygdala volumes. There were no significant sex differences in emotional control ratings. There were no significant associations between pubertal stage nor age with ratings of emotional control or with amygdala volumes.
Significant sex differences in the relation between parental ratings of emotional control and the left amygdala volume were observed. Banks et al. [23] has suggested that improved emotional control is associated with the attenuation of left amygdala reactivity through connections with prefrontal regions and that it is the connectivity that allows for the regulation of emotions and is part of an ‘emotion generation regulation’ circuit. Furthermore, fMRI studies suggest that during adolescent maturation there is a decline in left amygdala activity alongside an increase in dorsalateral prefrontal cortex activity during emotional tasks in girls with a reverse pattern seen in boys [8]. Our anatomical results are consistent with these functional findings and suggest that in girls, but not in boys, there is a correlation between smaller left amygdala volumes and parental reports of emotional control.
Our finding may also suggest sex differences in the pattern of development of amygdala volumes during adolescence that correlates with emotional control. For example, pruning in the amygdala, that may co-occur with the interdependent maturation of the prefrontal cortex, could allow for the regulation of more complex emotional feelings in girls. Pruning might include a decrease in the number of dendrites, terminal dendritic spine densities or neuronal pruning. Thus, lack of pruning and larger amygdala volumes in girls may then be a risk factor for poorer emotional control and could potentially lead to the development of affective disorders. Enlarged amygdala volumes in pediatric populations are associated with depression, which preferentially affects female populations during adolescence [15] and increased fearfulness in girls [14].
Interestingly in boys, less pruning during adolescence could preserve neuronal processes that are necessary in the male brain to achieve emotion regulation and lead to improved emotional control. Therefore, if amygdala processes are pruned leading to smaller volumes it may lead to a risk for psychopathology. For example, smaller amygdala volumes have been reported in the disorders that preferentially affect boys, such as conduct disorders [24].
We did not find a relationship between emotional control and age nor emotional control and stage of pubertal development in either girls or boys, although past research suggests an increase in emotional control ability across adolescence [3]. This is in agreement with thoughts that adolescence as a time of ‘storm and stress’ might be pertinent only for adolescents that are at risk [25] and that adolescence does not engender problems in emotion regulation. A potential reason why we did not find relations between age and emotional control is that our assessment of emotional control was based on parental ratings, which may not capture the more internal regulation of emotion that youth may experience, particularly as they enter later adolescence. Future studies may benefit from using both parental and self-report assessments of emotional control.
A potential limitation to our study was that it was cross-sectional in design. A longitudinal sampling would benefit in the assessment of individual changes in emotional regulatory ability. Moreover, timing of puberty was not measured and several studies have provided evidence that early onset of puberty may be a risk factor for emotion dysregulation and development of disorders such as depression and substance abuse. Nonetheless, the current findings are the first to document sex differences in the relations of amygdala volumes and emotional control in adolescence, an important stage for the development of emotion regulation.
Conclusion
These data suggest sex differences in the correlation of parental reports of emotional control and the corrected volume of the amygdala in adolescents. A smaller left amygdala volume is correlated with parental reports of better emotional control in girls and a larger left amygdala volume is correlated with parental reports of better emotional control in boys.
Acknowledgments
This research was supported by grants from the NIH, P50-DA016556 (R.S.), Ul1-DE019586 (R.S.), PL1-DA024859 (R.S.) and K01-DA024759 (T.C.). This scientific research was also supported by the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract Nos. N01-HD02-3343, N01-MH9-0002, and N01-NS-9-2314, N01-NS-2315, N01-NS-2316, N01-NS-2317, N01-NS-2319 and N01-NS-2320). This study reflects the views of the authors and may not reflect the opinions or views of all the Study Investigators or the NIH.
Footnotes
There are no conflicts of interest.
References
- 1.Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandura A, Caprara GV, Barbaranelli C, Gerbino M, Pastorelli C. Role of affective self-regulatory efficacy in diverse spheres of psychosocial functioning. Child Develop. 2003;74:769–782. doi: 10.1111/1467-8624.00567. [DOI] [PubMed] [Google Scholar]
- 3.Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Current Opinions Neurobiol. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Shaw P, Lalonde F, Lepage C, Rabin C, Eckstrand K, Sharp W, et al. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2009;66:888–896. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanton RE, Levitt JG, Thompson PM, Narr KL, Capetillo-Cunliffe L, Nobel A, et al. Mapping cortical asymmetry and complexity patterns in normal children. Psych Res Neuroimag. 2001;107:29–43. doi: 10.1016/s0925-4927(01)00091-9. [DOI] [PubMed] [Google Scholar]
- 6.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psych. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 8.Herba C, Phillips M. Annotation: development of facial recognition from childhood to adolescence: behavioural and neurological perspectives. J Child Psychol Psych Allied Disciplines. 2004;45:1185–1198. doi: 10.1111/j.1469-7610.2004.00316.x. [DOI] [PubMed] [Google Scholar]
- 9.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotional regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, et al. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, III, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 13.Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 14.Van der Plas EAA, Boes AD, Wemmie JA, Tranel D, Nopoulos P. Amygdala volume correlates positively with fearfulness in normal healthy girls. Social, Cognitive and Affective Neuroscience. 2010 doi: 10.1093/scan/nsq009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch Gen Psychiatry. 2000;57:21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- 16.Evans AC The Brain Development Cooperative Group. The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 17.Peterson AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 18.Gioia GA, Isquith PK. Ecological assessment of executive function in traumatic brain injury. Develop Neuropsychol. 2004;25:135–158. doi: 10.1080/87565641.2004.9651925. [DOI] [PubMed] [Google Scholar]
- 19.Sled JG, Zijdenbos AP, Evans AC. A non-parametric method for automatic correction of intensity non-uniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 20.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 21.Cocosco CA, Zijdenbos AP, Evans AC. A fully automatic and robust MRI tissue classification method. Med Image Anal. 2003;7:513–527. doi: 10.1016/s1361-8415(03)00037-9. [DOI] [PubMed] [Google Scholar]
- 22.Levitt JG, Blanton RE, Caplan R, Asarnow R, Guthrie D, Toga AW, et al. Medial temporal lobe in childhood-onset schizophrenia. Psychiatry Research Neuroimaging. 2001;107:29–43. doi: 10.1016/s0925-4927(01)00108-1. [DOI] [PubMed] [Google Scholar]
- 23.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Social, Cognitive and Affective Neuroscience. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huebner T, Vloet TD, Marx I, Konrad K, Fink GR, Herpertz SC, et al. Morphometric brain abnormalities in boys with conduct disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:540–547. doi: 10.1097/CHI.0b013e3181676545. [DOI] [PubMed] [Google Scholar]
- 25.Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address Ann N Y Acad Sci. 2006;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]