Abstract
Cell specific gene transfer and sustained transgene expression are goals of cutaneous gene therapy for tissue repair and regeneration. Adeno-associated virus serotype 2 (AAV2/2) mediated gene transfer to the skin results in stable transgene expression in the muscle fascicles of the panniculus carnosus in mice, with minimal gene transfer to the dermal or epidermal elements. We hypothesized that pseudotyped AAV vectors may have a unique and characteristic tropism and transduction efficiency profile for specific cells in the cutaneous wounds. We compared transduction efficiencies of cells in the epidermis, cells in the dermis, and the fascicles of the panniculus carnosus by AAV2/2 and three pseudotyped AAV vectors, AAV2/5, AAV2/7 and AAV2/8 in a murine excisional wound model. AAV2/5 and AAV2/8 result in significantly enhanced transduction of cells both in the epidermis and the dermis compared to AAV2/2. AAV2/5 transduces both the basilar and supra-basilar keratinocytes. In contrast, AAV2/8 transduces mainly supra-basilar keratinocytes. Both AAV2/7 and AAV2/8 result in more efficient gene transfer to the muscular panniculus carnosus compared to AAV2/2. The capsid of the different pseudotyped AAV vectors produces distinct tropism and efficiency profiles in the murine wound healing model. Both AAV2/5 and AAV2/8 administration result in significantly enhanced gene transfer. To further characterize cell specific transduction and tropism profiles of the AAV pseudotyed vectors, we performed in vitro experiments using human and mouse primary dermal fibroblasts. Our data demonstrates that pseudotyping strategy confers a differential transduction of dermal fibroblasts, with higher transduction of both human and murine cells by AAV2/5 and AAV2/8 at early and later time points. At later time points, AAV2/2 demonstrates increased transduction. Interestingly, AAV2/8 appears to be more efficacious in transducing human cells as compared to AAV2/5. The pseudotype-specific pattern of transduction and tropism observed both in vivo and in vitro suggests that choice of AAV vectors should be based on the desired target cell and the timing of transgene expression in wound healing for gene transfer therapy in dermal wounds.
Keywords: Gene Therapy, Wound Healing, Adeno-associated Virus, Pseudotyped Vectors
INTRODUCTION
Chronic non-healing wounds represent a significant cause of morbidity and a substantial annual health care expense (1). Standard wound management is often inadequate to completely heal these challenging wounds. Growth factor therapy is known to stimulate missing or dysfunctional components in the chronic wound setting, and may therefore represent an attractive strategy to heal these wounds. However, treatment of these wounds with topically applied recombinant growth factors has been shown to require large and repetitive doses to achieve even a modest improvement (2), thereby minimizing the clinical utility. In this context, the use of gene transfer to overexpress vulnerary transgenes is an appealing strategy for the treatment of chronic non-healing wounds. We have previously reported the use of adenoviral-mediated gene transfer to deliver growth factor transgenes with promising results in animal models (3, 4). The potential drawbacks in the clinical translation of this work are the inflammatory response to the adenovirus and the limited duration of transgene expression. Adenoviral-mediated gene transfer in wounds results in transgene expression limited to, at most seven days, in an immuno-competent host (3). While this duration of transgene expression may be adequate to stimulate initial healing of the wounds, it offers no long-term protection. Prolonged gene expression may be necessary to maintain tissue integrity and improve treatment efficacy in certain clinical settings, where repeated tissue breakdown is observed at characteristically pre-disposed sites, such as in patients with diabetes, venous stasis and complex decubitus ulcers (1).
Gene transfer with more durable transgene expression has been reported in a variety of tissues including brain, retina, liver, lung, and muscle, using lentiviral and adeno-associated virus (AAV)-based vectors (5-7). Efficient and long-term gene transfer in murine skin has been demonstrated using both HIV-based recombinant lentiviral vectors (8) and AAV vectors (serotype 2) (9). However, lentiviruses are associated with an increased risk of oncogenic transformation from insertional mutagenesis (10). In contrast, AAV vectors have a better safety profile and a lower risk for insertion events (6). This led us to investigate AAV vectors as a durable gene transfer strategy. Although several reports demonstrate AAV-mediated gene transfer in the skin with vulnerary effects, transgene expression has been limited to the panniculus carnosus, a thin muscular layer deep to the dermal elements in loose skinned animals (9, 11, 12). In vivo gene transfer with AAV2/2 to the epidermal and dermal elements of the skin has been particularly inefficient (13). While the results of these reports highlight the tropism of AAV2/2 for muscle fibers and suggest that AAV2/2 can be used for targeted muscle therapy, its clinical application is limited where the epidermal and dermal cell types are the target populations.
The capsid is a major determinant of vector tropism. An AAV pseudotyping strategy is one that replaces the capsid of AAV2 with the capsid of another AAV serotype. For example, an AAV2/5 vector has the genome of AAV2 but has the capsid of AAV5. This change in the AAV serotype capsid could potentially result in a unique tropism for each pseudotyped vector. Previous studies have reported that pseudotype AAV2/5 has significantly greater transduction of muscle when compared to AAV2/2. Additional pseudotypes AAV2/7 and AAV2/8 have demonstrated robust and tissue specific transduction profiles in liver, pancreas and cardiac tissue (14-16). However, there have been no previous reports on the tropism of these pseudotyped AAV vectors with respect to their ability to transduce specific cell types in cutaneous wounds. Taken together, we hypothesize that each pseudotyped AAV vector may have a unique characteristic tropism and transduction efficiency for specific cells within cutaneous wounds. To test this hypothesis, we compared transduction efficiencies in vivo to cells in the epidermis, cells in the dermis and the fascicles of the panniculus carnosus between AAV2/2 and three pseudotyped AAV vectors, AAV2/5, 2/7 and 2/8, in a murine excisional wound model. To establish a clinical relevance of our findings, we further characterized and compared cell specific transduction efficiencies of the AAV2/2 and pseudotyed AAV2 vectors in vitro using primarily isolated murine and human dermal fibroblasts.
METHODS AND MATERIALS
Adeno-associated virus production
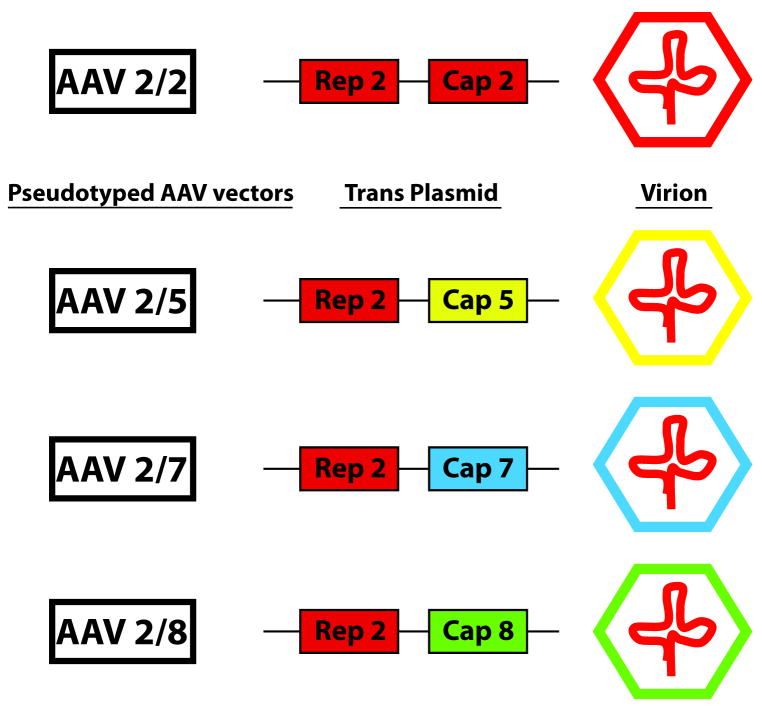
Single strand recombinant adeno-associated viral vectors AAV2/2, 2/5, 2/7 and 2/8 were obtained via a Material Transfer Aggrement from the Vector Core at the University of Pennsylvania (Kindly made available by James Wilson, MD, PhD). Vectors were produced as previously described (14). The AAV2/2 serotype was constructed by standard transfection protocols and purified by single step heparin chromatography. A pseudotyping strategy was used to produce AAV vectors packaged with the capsid proteins of AAV5, AAV7 and AAV8 (Figure 1). Recombinant AAV genomes equipped with AAV2/2 inverted terminal repeats (ITRs) were packaged by triple transfection of 293 cells with cis-plasmid, adenovirus helper plasmid and a chimeric packaging construct where the AAV2/2 rep gene is fused with cap genes of other AAV serotypes. To create the chimeric packaging constructs, the XhoI site of p5E18 plasmid at 3,169 bp was ablated, and the modified plasmid was restricted with XbaI and XhoI in a complete digestion to remove the AAV2/2 cap gene and replace it with a 2,267-bp SpeI/XhoI fragment containing AAV5, AAV7 or AAV8 cap gene. For all AAV vectors, the cDNA bacterial β-galactosidase or green fluorescent protein (GFP) was inserted as a reporter and vectors were driven by a CMV promoter. Pseudotyped recombinant vectors were purified by the standard CsCl2 or iodixinol sedimentation method. Genome copy (GC) titers of AAV vectors were determined by TaqMan (Applied Biosystems, Foster City, CA) analysis, using probes and primers targeting SV40 poly (A) region.
Figure 1.
AAV is composed of single stranded DNA and contains two genes: a “Rep” gene (that codes for proteins which control viral replication, structural gene expression, and integration into the host genome) and a “Cap” gene (that codes for capsid structural proteins). A pseudotyping strategy was used to produce three AAV vectors with the genome of AAV2 (REP) packaged with the capsid (CAP) proteins of AAV5, AAV7 and AAV8 to obtain AAV2/5, AAV2/7, and AAV2/8 vectors. The capsid is a major determinant of vector tropism, therefore the change in the AAV serotype capsid could potentially result in a unique tropism for each pseudotyped vector.
Wound Healing Model
All animal procedures were approved by the Instituational Animal Care and Usage Committee of the Children’s Hospital of Philadelphia. C57BL/6J mice (Charles River Laboratories, Wilmington, MA) at 8 weeks of age were anesthetized with methoxyflurane inhalation (0.5ml titrated), shaved and skin prepared with betadine (Purdue, Stamford, CT). Using an 8mm dermal punch biopsy (Miltex, Beth Page, NY) the skin was marked and excised sharply leaving the panniculus carnosus intact. Two 8 mm excisional wounds were created in the dorsum per animal. A transparent sterile dressing was then applied circumferentially around the trunk of the animal (3M Healthcare, St. Paul, MN). Animals were housed in separate cages until harvest. Animals were euthanized by CO2 inhalation, followed by cervical dislocation at 28 days post injection.
Experimental Design
To compare the transduction efficiencies of AAV2/2 and the pseudotyped vectors, bilateral 8 mm wounds were created on the dorsum of C57BL/6J mice (n=25). 1 × 1011 Genome Copies of AAV2/2, AAV2/5, AAV 2/7 or AAV 2/8 in 30μl of PBS (Gibco, Long Island, NY) or 30 μl PBS alone for control (n=10 wounds per vector and control) were injected onto the wound bed with a Hamilton 50 μl syringe (Hamilton Company, Reno, NV) and 30 ½ gauge needle. Injections were mixed with 5 μl of India ink to facilitate histologic identification of the wound. At 28 days post injection, wounds were analyzed for transgene expression in the cells of the epidermis, cells in the dermis and fascicles of the panniculus carnosus.
Tissue Processing and Histology
After the mice were euthanized, wounds were harvested, and stained for β-galactosidase activity. Wounds were rinsed in cold PBS, kept separately and fixed in 0.5% glutaraldehyde for 10 minutes, and X-gal stained with a solution containing 1 mg/mL of 5-bromo-4-chloro-3-indoyl-β-D-galactopyronidase, 5 mmol/L K3Fe(CN)6, 5 mmol/L K4Fe(CN)6, and 1 mmol/L MgCl2 in PBS, pH 7.4, overnight at 37 °C. Wounds were then fixed in 10% neutral buffered formalin (Sigma, St Louis, MO) for 16 hours. Post-fixed X-gal stained tissues were mechanically processed and paraffin embedded. Serial 5 μm sections from the paraffin embedded wounds were obtained using a RM 2035 microtome (Leica, Heidelberg, Germany), collected on Superfrost Plus slides (Fisher, Pittsburgh, PA) and counterstained with 0.5% nuclear fast red.
Quantification of transduction efficiencies
Photomicrographic images were acquired using a DMRBE light microscope (Leica, Heidelberg, Germany) and Carl Zeiss lens at magnifications of 5x, 20x and 40x and the lacZ positive cells were counted with computer assisted image analysis software (Scanalytics, Fairfax, VA). The presence of India ink particles confirmed the site of the prior viral injection. A minimum of five sections per wound were analyzed for transgene expression signified by cell specific β-gal staining. The techniques for quantification of transduction efficiency were modified based on the characteristics of the three different cellular targets being examined. For quantification of transduction efficiencies of cells in the epidermis, the number of β-gal positive cells were counted and expressed as percentage of the total number of epidermal cells in the re-epithelialized wound. The margins of the wound bed were defined by the presence of India ink particles in the underlying dermis and the typical morphologic changes characteristic of scar formation, including a dropout of dermal appendages. In addition, the epidermis is typically hypertrophied at the wound margins and flattened over the wound bed. The transduction of supra-basilar and/or basilar keratinocytes was also noted. Basilar keratinocytes were defined as cells with typical keratinocyte morphology with contact to the basement membrane at the epidermal-dermal junction. The transduction of the fascicles of the panniculus carnosus were determined similarly by defining the extent of wound bed and then counting the number of β-gal positive fascicles which is then expressed as percentage of the total number of fascicles in the defined wound bed. To appropriately assess transduction efficiency to the cells in the dermis, the mean number of β-gal positive cells was calculated from 10 high- powered fields (40x magnification images) evenly distributed throughout the similarly defined wound bed. The assessment of transduction efficiency for cells of the dermis was modified because of the much larger region of interest, as well as, to control for topographic variability in the dermis of the wound bed. All quantification was performed on blinded slides by a research fellow trained in wound morphology, to ensure consistant analysis of the different compartments of skin as per the above stated methodology.
Cell Culture
Human dermal fibroblasts were obtained from Life Technologies (Grand Island, NY). Primary adult murine dermal fibroblasts were isolated in our laboratory from skin of C57BL/6J mice, (purchased from Jackson Labs, Bar Harbor, ME). Protocol was approved by Cincinnati Children’s Hospital Medical Center Institutional Animal Care and Usage Committee. The fibroblast cultures was maintained in Dulbecco’s modified Eagle’s Media (DMEM) (GIBCO, Carlsbad, CA) supplemented with 10% bovine growth serum (BGS) (Hyclone, Logan, UT) and penicillin 100 units / streptomycin 100 μg / amphotericin B 0.25 μg (PSF) (Invitrogen, Carlsbad, CA) at 37° C with 5% CO2, and cells before passage 10 were used for experiments.
In vitro Comparision of transduction efficiencies of AAV and pseudotyped vectors
Human or mouse fibroblasts were transduced by seeding 20000 cells each to 4-well chamber slides in normal culture media. After 24 hrs, cells were transduced with AAV2/2 and pseudotyped AAV2/5, 2/7 and 2/8 GFP reporter vectors. AAV vector preparations were added at multiplicity of infection (MOI of 100000), in fresh media. Cells were infected for 24 hrs, at which time the AAV preparations were removed and chambers slides were rinsed. These cells were expanded for 7 days and GFP positive cells were counted in each AAV preparation using Nikon 80I microscope and Nikon Elements analysis software. A total of 150-200 cells were counted in 6-10 20x magnification fields per AAV preparation. The mean of percentage of transduced cells per AAV prep are reported.
Statistical Analysis
All data are presented as mean±SEM. Student’s t-test and ANOVA were used for assessing transduction efficiencies of the AAV vectors to the epidermis, dermal wound matrix and panniculus carnosus. Probability values of p<0.05 were interpreted to denote statistical significance.
RESULTS
Effects of AAV Vectors on Wound Healing
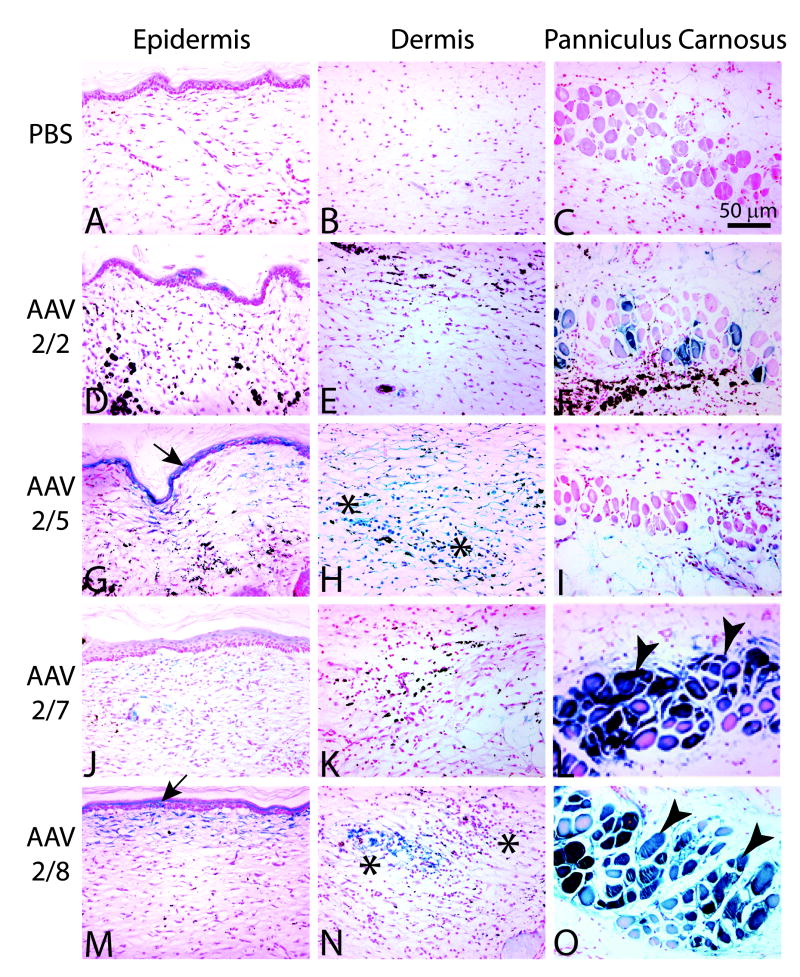
In the current study, none of the AAV treated animals displayed any adverse effects as a result of vector administration. Both vector treated and PBS treated control animals heal their wounds by 7 days post wounding, which is consistent with previous reports of wounds of this size and type (4, 17). In addition, there are no observable deficiencies and no histologic differences in wound healing in AAV treated animals compared to PBS controls at 28 days post wounding (Figure 2). Both AAV treated and PBS treated control wounds demonstrate characteristic scar formation consistent with normal healing of excisional wounds, with flattened epidermis and loss of dermal appendages. Histological examination demonstrated no apparent difference in the degree of scarring between wounds treated with AAV vectors or PBS control.
Figure 2.
Wounds at 28 days post injury in C57BL/6J mice. Wounds were injected with PBS (A-C), or 1×1011 Genome Copies of AAV2/2 (D-F), AAV2/5 (G-I), AAV2/7 (J-L) or AAV2/8 (M-O). Assessment of transduction efficiencies was assessed in three different wound compartments, the cells in the epidermis (A,D,G,J,M), the cells of the dermis (B, E, H, K, N), or the fascicles of the panniculus carnosus (C, F, I, L, O). Magnification 20x.
Tropism and Transduction Efficiency of AAV2/2 in Excisional Wounds
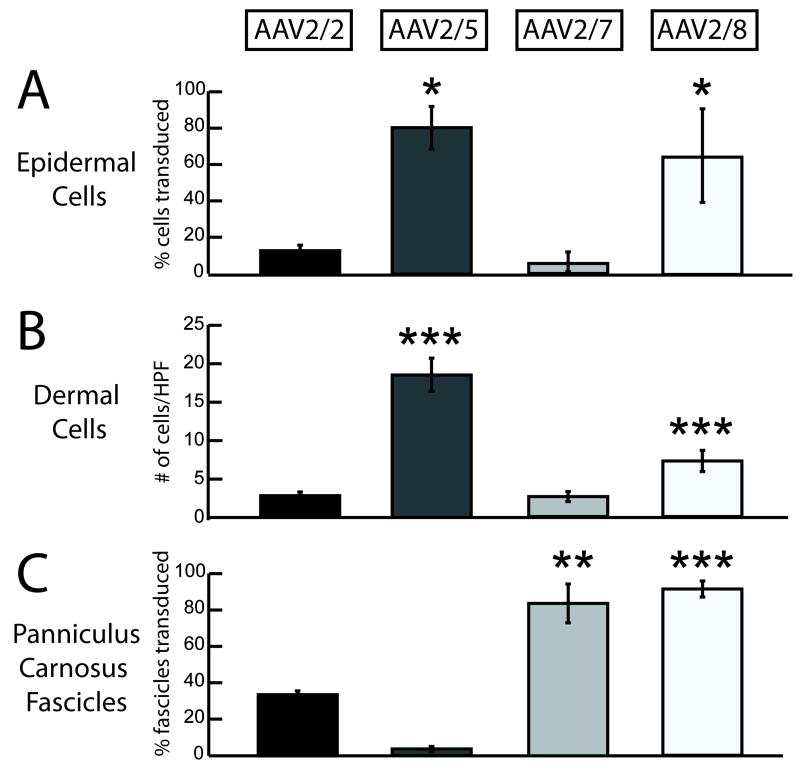
To compare tropism and transduction efficiency profiles of the pseudotyped AAV vectors, we first needed to define the tropism and transduction efficiency profile for AAV2/2. To assess vector tropism, the wound was divided into three cellular compartments for gene transfer, the cells in the epidermis, the cells in the dermis and the fascicles of the panniculus carnosus. AAV2/2 administration results in a low rate of gene transfer to the epidermis (11.7%±2.9) and minimal gene transfer to the dermis (2.7±0.5 cells/hpf). The majority of epidermal cells transduced by AAV2/2 consist primarily of supra-basilar keratinocytes. However, AAV2/2 administration results in efficient gene transfer to the panniculus carnosus, transducing 33.1%±2.1 of fascicles (Figure 3).
Figure 3.
Comparison of transduction efficiencies of AAV2/2 and pseudotyped vectors AAV2/5, AAV2/7 and AAV2/8 in wounds. In the cells of the epidermis, both AAV2/5 and AAV2/8 resulted in significantly increased transduction efficiencies compared to AAV2/2 (A). Similarly, in the cells of the dermis, AAV2/5 and AAV2/8 resulted in higher gene transfer than AAV2/2 (B). In the skeletal muscle layer panniculus carnosus, AAV2/7 and AAV2/8 resulted in more muscles fascicles transduced than AAV2/2 (C). High powered fields (HPF- 40x magnification images) (* p<0.05, ** p<0.001, *** p<0.0001).
Tropism and Transduction Efficiency of Pseudotyped AAV Vectors in Excisional Wounds
We next assessed the tropism and transduction efficiency profiles of the three pseudotyped AAV vectors, AAV2/5, AAV2/7 and AAV2/8. At 28 days post wounding, AAV2/5 is highly efficient in transducing the cells in the epidermis (80.3%±11.6) with expression in both basilar and supra-basilar keratinocytes. However, AAV2/5 results in less transduction of the dermal cells (18.4±2.1 cells/hpf) and minimal gene transfer to the fascicles of the panniculus carnosus (3.6±1.3%). Gene transfer to the panniculus carnosus was limited to myocytes actively regenerating in the wound bed. In contrast, AAV2/7 results in limited gene transfer to the epidermis (5.5% 5.4) and the dermis (2.6±0.6 cells/hpf), but efficient gene transfer to fascicles of the panniculus carnosus (83.6%±10.6). AAV2/8 administration results in efficient gene transfer to cells in the epidermis (64.3%±25.3), dermis (7.2±1.4 cells/hpf), and the fascicles of the panniculus carnosus (91.2%±4.4). In contrast to AAV2/5, expression in the epidermis with AAV2/8 is mainly observed in the supra-basilar keratinocytes (Figure 3).
Comparison of AAV2/2 and Pseudotyped AAV Vectors in Wound Healing
In the epidermis, AAV2/5 (AAV2/5 80.3%±11.6 vs. AAV2/2 11.7%±2.9, p<0.05) and AAV2/8 (AAV2/8 64.3%±25.3 vs. AAV2/2 11.7%±2.9, p<0.05) results in significantly more efficient gene transfer compared to AAV2/2. AAV2/5 results in a seven-fold, and AAV2/8 in a nearly six-fold increase in gene transfer compared to AAV2/2. In comparing the efficiencies of AAV2/5 and AAV2/8, AAV2/5 transduced significantly more cells in the epidermis than AAV2/8 (AAV2/5 80.3%±11.6 vs. AAV2/8 64.3%±25.3, p<0.05). AAV2/5 is also unique in its ability to transduce both basilar and supra-basilar keratinocytes. In contrast, AAV2/8 mainly results in transduction of supra-basilar keratinocytes. In the cells in the dermis, both AAV2/5 (AAV2/5 18.4±2.1 cells/hpf vs. AAV2/2 2.7±0.5 cells/hpf, p<.0001) and AAV2/8 (AAV2/8 7.2±1.4 cells/hpf vs. AAV2/2 2.7±0.5 cells/hpf, p<.0001) are significantly more efficient in gene transfer than AAV2/2. AAV2/5 results in a seven-fold and AAV2/8 in an over twofold increase in gene transfer efficiency compared to AAV2/2 in dermal cells. AAV2/5 administration results in significantly higher transduction in cells of the dermis compared to AAV2/8 (AAV2/5 18.4%±2.1 vs. AAV2/8 7.2%±1.4, p<0.05). AAV2/7 (AAV2/7 83.6%±10.6 vs. AAV2/2 33.6%±2.1, p<.001) and AAV2/8 (AAV2/8 91.2%±4.4 vs. AAV2/2 33.6%±2.1, p<.0001) administration results in a nearly threefold greater transduction efficiency in the panniculus carnosus than AAV2/2. These results define a unique tropism profile for each of the pseudotyped AAV vectors, as summarized in Table 1. Representative images are displayed in Figure 2.
Table 1.
Pseudotyping AAV Vectors Have Unique Tropism Profiles in Wound Healing
| AAV Transduction in Wound Healing | |
|---|---|
| Epidermis | AAV2/5 ≫ AAV2/8 ≫ AAV2/2 ≈ AAV2/7 |
| Dermal Cells | AAV2/5 ≫ AAV2/8 ≫ AAV2/2 ≈ AAV2/7 |
| Muscle | AAV2/8 ≈ AAV2/7 ≫ AAV2/2 ≫ AAV2/5 |
Comparison of AAV2/2 and Pseudotyped AAV Vectors transduction efficacy using human and mouse fibroblasts in vitro culture
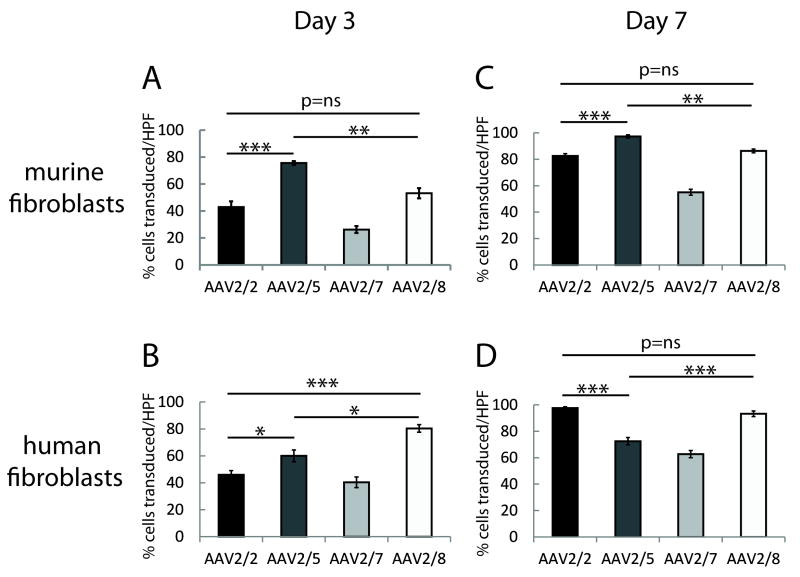
There is no evidence of vector toxicity at high MOI of 100000 in any culture. In murine fibroblasts at day 3 post transduction, AAV2/5 had maximal gene transfer (75.6%±1.46), followed by AAV2/8 (53.2%±3.9) and AAV2/2 (43.0%±4.2). Both AAV2/5 and AAV2/8 demonstrate significantly higher transduction efficiencies compared to AAV2/7 (26.2%±2.5) (Figure 4A). Human fibroblasts are also differentially transduced by the AAV pseudotyped vectors. AAV2/8 had maximal transduction efficiency in human fibroblasts (80.4%±2.8), followed by AAV2/5 (60.0%±4.4). Similar to murine transduction efficiencies, both AAV2/5 and AAV2/8 transduced significantly more human fibroblasts, as compared to AAV2/2 (46.0%±3.1) and AAV2/7 (40.4%±4.0) (Figure 4B).
Figure 4.
Comparison of transduction efficiencies of AAV2/2 and pseudotyped vectors AAV2/5, AAV2/7 and AAV2/8 in in vitro cultures of murine and human fibroblasts at day 3 and day 7 post vector administration. In murine fibroblasts at day 3 post transduction, AAV2/5 had maximal gene transfer (A). In human fibroblasts, AAV2/5 and AAV2/8 had higher transduction efficiencies (B). At 7 days after AAV administration, the percentage of transduced cells in all the vector preparations increased significantly compared to day 3. AAV2/5 continued to demonstrate higher transduction efficiency in murine cells (4C). In human fibroblasts, both AAV2/2 and AAV2/8 show higher gene transfer rates (4D). High powered fields (HPF- 20x magnification images) (* p<0.05, ** p<0.001, *** p<0.0001).
At 7 days after AAV administration, the percentage of transduced cells in all the vector preparations increased significantly compared to day 3. Notably in murine fibroblasts, AAV2/5 (97.3%±1.2) demonstrate higher transduction efficiency compared to AAV2/2 (82.6%±1.7), AAV2/7 (55.0%±2.2), and AAV2/8 (86.3%±1.4) (Figure 4C). In human fibroblasts, AAV2/2 (97.6%±1.0) and AAV2/8 (93.3%±2.1) result in significantly higher gene transfer rates compared to AAV2/5 (72.5%±2.8) and AAV2/7 (62.7%±2.7) (Figure 4D).
DISCUSSION
The purpose of this study is to determine if a pseudotyping strategy with AAV vectors can result in distinct tropism profiles for the individual AAV vectors. Our results demonstrate that pseudotyping recombinant AAV vectors produces distinct tropism and efficiency profiles in the three cellular compartments of the skin in a murine wound healing model. AAV2/5 and AAV2/8 result in gene transfer to the cells in the epidermis and dermal matrix, with significant enhancement of gene transfer when compared to AAV2/2. These results suggest that the pseudotyping strategy can potentially be used as a targeted gene transfer treatment for specific disease states characterized by deficiencies in individual skin compartments.
Altering gene transfer efficiencies and tropism profiles by pseudotyping or mutating AAV vector capsids have been previously reported, but AVV2/5, 2/7, and 2/8 have not been examined in wound healing (18-22). Our results suggest that the transduction of cells in an excisional wound with AAV2/2 and these pseudotyped AAV vectors have no deleterious effects on the wound healing process, which is consistent with AAV vector administration in other applications of wound repair (11, 23, 24). This is an advantage over adenoviral based vectors, which can impair wound healing in the absence of a vulnerary transgene due to the augmented inflammatory response to the adenovirus (25).
Proof of concept of the effectiveness of AAV-mediated gene transfer therapy has been demonstrated in work by other groups. An AAV construct carrying the vascular endothelial growth factor (VEGF) transgene was administered to diabetic mice wounds and resulted in significantly improved wound neovascularization and accelerated wound healing (11). However, the authors conceded that the VEGF transgene expression was limited to the muscular panniculus carnosus. This tissue acted as a platform for transgene expression in the bed of the wound. While these results in diabetic mice were promising, they have limited clinical applicability because of the lack of a muscle “platform” in humans to facilitate growth factor production in the wound bed. In the current study, AAV2/5 and AAV2/8 result in efficient gene transfer, not only in the panniculus carnosus but also in the cells of the dermal and epidermal compartments. It should be noted that the authors intentionally did not use a vulnerary transgene, as the goal of this study is to determine if the pseudotyping strategy can result in unique vector tropism profiles. The tropism exhibited by these pseudotyped AAV vectors makes them more attractive as vectors for clinical translation in wound healing applications.
There is one previous report utilizing AAV2/5 in vivo, in which the vector was injected subcutaneously into intact murine skin, with transgene expression limited to the panniculus carnosus (26). In contrast, in our study, we found minimal gene transfer to the panniculus carnosus. Interestingly, the few cells of the panniculus carnosus that were transduced by AAV2/5 consisted of newly formed muscle fibers at the regenerating edge of the panniculus carnosus, indicating little to no transduction of differentiated myocytes. Additionally, we also observed excellent gene expression in the cells of the dermis and epidermis. A noteworthy advantage of AAV2/5 is its ability to transduce the basilar keratinocytes. Basilar keratinocytes are highly active in the re-epithelialization process, and have also been demonstrated to contain a putative epidermal stem cell population (27). This enhanced transduction efficiency and distinct tropism profile may be due to the fact that the wound represents a dynamic microenvironment with high cellular turnover. The cells are not in their normal extra cellular environment and are exposed to cytokines, chemokines and other mediators that largely differ from uninjured skin. The wound itself may provide a unique exposure of cellular receptors and the rolling migration of keratinocytes in the process of re-epithelialization may make the keratinocytes more vulnerable to AAV-mediated gene transfer than in intact skin.
In vitro transduction of human and mouse primary fibroblasts demonstrate that, similar to the in vivo murine model, AAV2/5 and AAV2/8 had higher transduction efficiency rates compared to AAV2/2 or AAV2/7. Similar to the in vivo wound experiments, AAV2/5 maximally transduced mouse fibroblasts in in vitro culture experiments. Notably, AAV2/8 is more efficacious r in gene transfer to human cells, as compared to the other pseudotyped AAV vectors. An interesting observation is that AAV2/8 transduces about 75% cells within 3 days after infection, and continues to increase in transduction efficiency. This suggests that depending on the nature of the wounds and the requirements of transgene expression, AAV2/8 is optimal for delivery for gene transfer applications that require early transgene expression, as well as for stable long term expression. However, by day 7, AAV2/2 demonstrates similarly high transduction efficacy, suggesting that AAV2/2 can be used for therapy where sustained expression of targeted genes/ factors are required. However, immunologic tolerance may be developed to the vectors in vivo, rendering multiple vector administrations impossible. Boutin et al. (28) reported that sero-prevalence of antibodies against AAV8 is only moderate in human population when compared to AAV2, potentially facilitating immune escape of AAV8 vectors in vivo. Pseudotyping strategy can provide the additional benefit of repeated administration of the transgenes using different capsid serotypes to minimize immunologic reaction. This strategy can facilitate gene therapy for the treatment of chronic skin diseases, where multiple administrations of transgenes may be necessary to promote healing. Lastly, high MOIs were utilized for all vectors for the in vitro transduction of dermal fibroblasts. This was recommended for maximal vector transduction, because AAV vectors do not function well in in vitro cultures. It is established that AAV vectors have maximal transduction efficacy in vivo, as they need other supporting cytokines and enzymes for efficacious transduction. In this context, the experimental conditions of the in vitro experiments should mimic the in vivo environment to fully appreciate the benefits of AAV pseudotyping strategy for targeted gene therapy.
When using AAV vectors, the possibility of insertional mutagenesis must be considered. It has been reported that the rate of insertion events with recombinant AAV vectors may be higher than previously appreciated (29). It would be important to know if the increased transcriptional activity of re-epithelializing keratinocytes in wound healing predisposes them to insertion events. Lastly, a 28-day time point may considerably underestimate transduction efficiency. In a rapidly proliferating cell population like the epidermis, it is possible that transduced epidermal cells may have already been sloughed. Similarly, in the dermal compartment, as normal wounds complete re-epithelialization many of the cells within the granulation tissue undergo apoptosis, which may further underestimate transduction efficiency. It is interesting to note that we performed the in vivo transduction and tropism experiments in normal/acute cutaneous wounds, which are known to heal at a maximal rate. Even in this maximal healing wound model, the AAV pseudotyped vectors demonstrated specific tropism and stably expressed reporter genes until 28 days, demonstrating their potential for the treatment of chronic wounds. Chronic wounds such as diabetic and pressure ulcers and epidermolysis bullosa are known to reoccur at predisposed sites, as a direct consequence of a less than satisfactory healing of the initial wound incidence. Sustained and long term targeted growth factor delivery using pseudotyped vectors may provide the necessary strength and protection to these wounds and decrease wound reoccurrence incidences.
We have demonstrated that pseudotyping recombinant AAV vectors produces distinct tropism and efficiency profiles in the three cellular compartments of the skin in a wound healing model. Both AAV2/5 and AAV2/8 administration results in significantly enhanced gene transfer in target cellular compartments and may have advantages for gene transfer applications in wound repair, as well as other gene therapy applications in skin.
Acknowledgments
The authors thank Dr. Julie Johnston and Dr. Arbans Sandhu from University of Pennsylvania, Vector Core.
This work is supported in part by grants from the NIDDK R01-DK074055, R01-DK072446 and R01-DK59242 (TMC); Wound Healing Foundation 3M Award, and K08 GM098831-01 (SGK); and NIDDK P30 grant P30 DK47757-19 (JMW).
List of Abbreviations
- AAV
adeno-associated virus
- CMV
cytomegalovirus
- HIV
human-immunodeficiency virus
- hpf
high power field
- tet
tetracycline
- VEGF
vascular endothelial growth factor
- β-gal
beta-galactosidase
References
- 1.Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361(9368):1545–51. doi: 10.1016/S0140-6736(03)13169-8. [DOI] [PubMed] [Google Scholar]
- 2.Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study Diabetes Care. 1998;21(5):822–7. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- 3.Liechty KW, Nesbit M, Herlyn M, Radu A, Adzick NS, Crombleholme TM. Adenoviral-mediated overexpression of platelet-derived growth factor-B corrects ischemic impaired wound healing. J Invest Dermatol. 1999;113(3):375–83. doi: 10.1046/j.1523-1747.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 4.Keswani SG, Katz AB, Lim FY, Zoltick P, Radu A, Alaee D, Herlyn M, Crombleholme TM. Adenoviral mediated gene transfer of PDGF-B enhances wound healing in type I and type II diabetic wounds. Wound Repair Regen. 2004;12(5):497–504. doi: 10.1111/j.1067-1927.2004.12501.x. [DOI] [PubMed] [Google Scholar]
- 5.Buchschacher GL, Jr, Wong-Staal F. Development of lentiviral vectors for gene therapy for human diseases. Blood. 2000;95(8):2499–504. [PubMed] [Google Scholar]
- 6.Lu Y. Recombinant adeno-associated virus as delivery vector for gene therapy--a review. Stem Cells Development. 2004;13(1):133–45. doi: 10.1089/154732804773099335. [DOI] [PubMed] [Google Scholar]
- 7.Allocca M, Mussolino C, Garcia-Hoyos M, Sanges D, Iodice C, Petrillo M, Vandenberghe LH, Wilson JM, Marigo V, Surace EM, Auricchio A. Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J Virol. 2007;81(20):11372–80. doi: 10.1128/JVI.01327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serrano F, Del Rio M, Larcher F, Garcia M, Munoz E, Escamez MJ, Muñoz M, Meana A, Bernad A, Jorcano JL. A comparison of targeting performance of oncoretroviral versus lentiviral vectors on human keratinocytes. Hum Gene Ther. 2003;14(16):1579–85. doi: 10.1089/104303403322495089. [DOI] [PubMed] [Google Scholar]
- 9.Donahue BA, McArthur JG, Spratt SK, Bohl D, Lagarde C, Sanchez L, Kaspar BA, Sloan BA, Lee YL, Danos O, Snyder RO. Selective uptake and sustained expression of AAV vectors following subcutaneous delivery. J Gene Med. 1999;1(1):31–42. doi: 10.1002/(SICI)1521-2254(199901/02)1:1<31::AID-JGM3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 10.Connolly JB. Lentiviruses in gene therapy clinical research. Gene Ther. 2002;9(24):1730–4. doi: 10.1038/sj.gt.3301893. [DOI] [PubMed] [Google Scholar]
- 11.Galeano M, Deodato B, Altavilla D, Cucinotta D, Arsic N, Marini H, Torre V, Giacca M, Squadrito F. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia. 2003;46(4):546–55. doi: 10.1007/s00125-003-1064-1. [DOI] [PubMed] [Google Scholar]
- 12.Jazwa A, Kucharzewska P, Leja J, Zagorska A, Sierpniowska A, Stepniewski J, Kozakowska M, Taha H, Ochiya T, Derlacz R, Vahakangas E, Yla-Herttuala S, Jozkowicz A, Dulak J. Combined vascular endothelial growth factor-A and fibroblast growth factor 4 gene transfer improves wound healing in diabetic mice. Genet Vaccines Ther. 2010;8:6. doi: 10.1186/1479-0556-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hengge UR, Mirmohammadsadegh A. Adeno-associated virus expresses transgenes in hair follicles and epidermis. Mol Ther. 2000;2(3):188–94. doi: 10.1006/mthe.2000.0118. [DOI] [PubMed] [Google Scholar]
- 14.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(18):11854–9. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang AY, Peng PD, Ehrhardt A, Storm TA, Kay MA. Comparison of adenoviral and adeno-associated viral vectors for pancreatic gene delivery in vivo. Hum Gene Ther. 2004;15(4):405–13. doi: 10.1089/104303404322959551. [DOI] [PubMed] [Google Scholar]
- 16.Craig AT, Gavrilova O, Dwyer NK, Jou W, Pack S, Liu E, Pechhold K, Schmidt M, McAlister VJ, Chiorini JA, Blanchette-Mackie EJ, Harlan DM, Owens RA. Transduction of rat pancreatic islets with pseudotyped adeno-associated virus vectors. Virol J. 2009;6:61. doi: 10.1186/1743-422X-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim I, Mogford JE, Chao JD, Mustoe TA. Wound epithelialization deficits in the transforming growth factor-alpha knockout mouse. Wound Repair Regen. 2001;9(5):386–90. doi: 10.1046/j.1524-475x.2001.00386.x. [DOI] [PubMed] [Google Scholar]
- 18.Perabo L, Endell J, King S, Lux K, Goldnau D, Hallek M, Büning H. Combinatorial engineering of a gene therapy vector: directed evolution of adeno-associated virus. J Gene Med. 2006;8(2):155–62. doi: 10.1002/jgm.849. [DOI] [PubMed] [Google Scholar]
- 19.Perabo L, Huber A, Marsch S, Hallek M, Buning H. Artificial evolution with adeno-associated viral libraries. Comb Chem High Throughput Screen. 2008;11(2):118–26. doi: 10.2174/138620708783744507. [DOI] [PubMed] [Google Scholar]
- 20.Work LM, Buning H, Hunt E, Nicklin SA, Denby L, Britton N, Leike K, Odenthal M, Drebber U, Hallek M, Baker AH. Vascular bed-targeted in vivo gene delivery using tropism-modified adeno-associated viruses. Mol Ther. 2006;13(4):683–93. doi: 10.1016/j.ymthe.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Perabo L, Buning H, Kofler DM, Ried MU, Girod A, Wendtner CM, Enssle J, Hallek M. In vitro selection of viral vectors with modified tropism: the adeno-associated virus display. Mol Ther. 2003;8(1):151–7. doi: 10.1016/s1525-0016(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 22.Girod A, Ried M, Wobus C, Lahm H, Leike K, Kleinschmidt J, Deléage G, Hallek M. Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2. Nat Med. 1999;5(9):1052–6. doi: 10.1038/12491. [DOI] [PubMed] [Google Scholar]
- 23.Chang DS, Su H, Tang GL, Brevetti LS, Sarkar R, Wang R, Kan YW, Messina LM. Adeno-associated viral vector-mediated gene transfer of VEGF normalizes skeletal muscle oxygen tension and induces arteriogenesis in ischemic rat hindlimb. Mol Ther. 2003;7(1):44–51. doi: 10.1016/s1525-0016(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 24.Melo LG, Agrawal R, Zhang L, Rezvani M, Mangi AA, Ehsan A, Griese DP, Dell’Acqua G, Mann MJ, Oyama J, Yet SF, Layne MD, Perrella MA, Dzau VJ. Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation. 2002;105(5):602–7. doi: 10.1161/hc0502.103363. [DOI] [PubMed] [Google Scholar]
- 25.Crombleholme TM. Adenoviral-mediated gene transfer in wound healing. Wound Repair Regen. 2000;8(6):460–72. doi: 10.1046/j.1524-475x.2000.00460.x. [DOI] [PubMed] [Google Scholar]
- 26.Hildinger M, Auricchio A, Gao G, Wang L, Chirmule N, Wilson JM. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J Virol. 2001;75(13):6199–203. doi: 10.1128/JVI.75.13.6199-6203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303(5656):359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21(6):704–12. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 29.Nakai H, Montini E, Fuess S, Storm TA, Grompe M, Kay MA. AAV serotype 2 vectors preferentially integrate into active genes in mice. Nat Genetics. 2003;34(3):297–302. doi: 10.1038/ng1179. [DOI] [PubMed] [Google Scholar]