Abstract
Objective
The aim of this study was to examine the cortical and segmental excitability changes during fatigue of the soleus muscle.
Methods
Ten healthy young subjects performed 45 plantar flexion maximal voluntary contractions (MVCs) (7-s on/3-s off) in 9 epochs of five contractions. Motor evoked potentials (MEPs) using transcranial magnetic stimulation and H-reflexes were assessed during the task.
Results
The torque and the soleus EMG activity both showed the greatest decline during the 1st epoch, followed by a gradual, but significant decrease by the end of the task (~70% pre-fatigue). The H-reflex sampled at rest after each epoch decreased to 66.6 ± 18.3% pre-fatigue after the first epoch, and then showed no further change. The MEP on 10% pre-fatigue MVC after each epoch increased progressively (252.9 ± 124.2% pre-fatigue). There was no change in the MEPs on the 3rd MVC in each epoch. The silent period on the MVC increased (109.0 ± 9.2% pre-fatigue) early with no further changes during the task.
Conclusions
These findings support that the motor cortex increases excitability during fatigue, but with a concomitant inhibition.
Significance
These findings are in contrast to upper extremity muscles and may reflect a distinct response specific to postural, fatigue-resistant muscle.
Keywords: Muscle fatigue, Transcranial magnetic stimulation, H-reflex, Soleus
1. Introduction
Muscle fatigue varies according to the muscle studied, the task, and the individuals studied (age, gender, activity level). Sites of muscle fatigue include peripheral as well as central mechanisms characterized by changes in muscle properties, electromyography (EMG), segmental excitability, and cortical excitability (Taylor et al., 2006). Postural muscles, like the soleus, may demonstrate distinct mechanisms of fatigue from upper extremity muscles. Fatigued postural muscles may be important contributors to altered function with injury or age.
Several methods are available to examine the excitability of specific central nervous system pathways in humans. The motor evoked potential (MEP) using transcranial magnetic stimulation (TMS), provides an index of motor cortex and motoneuron pool excitability (Burke et al., 1993). Corticospinal tract stimulation, such as cervicomedullary motor evoked potentials (CMEPs), isolates the influence of a descending volley on the motor neuronal pool (Taylor, 2006). The Hoffmann or H-reflex has been used extensively to measure the strength of the excitation achieved by a same-sized compound Ia afferent action potential on its homonymous motoneuron pool (Magladery et al., 1951). The size of the single H-reflex alone does not measure the motoneuron pool excitability because the amount of the neurotransmitter released from the Ia afferent terminal can be modulated pre-synaptically through both GABA-mediated primary afferent depolarization (Rudomin, 1999) and/or homosynaptic post-activation depression (Hultborn et al., 1996). Double pulse stimulation with a long inter-pulse interval (500 ms) offers an opportunity to focus on the homosynaptic post-activation depression mechanism (Hultborn et al., 1996).
Various mechanisms contribute to fatigue of limb musculature. In the elbow flexors, the MEP size increases during both a sustained maximal voluntary contraction (MVC) and a sustained sub-maximal contraction, whereas the CMEP size decreases with a sustained MVC (Butler et al., 2003), but increases during a sustained sub-maximal contraction (Levenez et al., 2008). Intermittent MVCs have been reported to decrease the H-reflex size, which may be explained, in part, by an increase in post-activation depression in the human triceps surae (Nordlund et al., 2004). Collectively, the central and peripheral contributors to soleus fatigue have not been established during intermittent MVC contractions.
The soleus muscle may have distinct central versus segmental influences during muscle fatigue when compared to upper extremity muscles. The soleus motoneuron pool receives weaker corticospinal projections compared to other muscles such as the tibialis anterior (Bawa et al., 2002) and the biceps brachii (de Noordhout et al., 1999). The soleus muscle is primarily composed of type I fibers (Johnson et al., 1973), and recruits motor units close to MVC (Oya et al., 2009), while other muscles such as the biceps brachii modulates firing frequency after early recruitment of all motor units (Kukulka and Clamann, 1981). These factors may contribute to differences between central and segmental contributions to fatigue of postural muscles. Importantly, fatigue-induced changes in cortical and segmental excitability have not been fully explored for the soleus during intermittent maximal contractions. Hoffman and colleagues reported that, at task failure, both the MEP and CMEP from the human triceps surae increased during a sustained sub-maximal fatigue task (Hoffman et al., 2009). Task dependency (e.g., sustained sub-maximal contraction versus intermittent MVCs) during fatigue has been well established (Taylor and Gandevia, 2008). Even the specific knee flexion angle varies the mechanisms associated with fatigue of the ankle plantar flexors (Kawakami et al., 2000). The soleus muscle plays an important role in posture control (Kouzaki and Shinohara, 2010), but also generates repetitive forces during high velocity activities. Accordingly, the mechanisms contributing to fatigue of the soleus during intermittent MVCs warrants further examination.
The purpose of the present study was to determine the degree to which the excitability of the motor cortex and segmental moto-neuronal pool modulates during intermittent MVCs in the soleus. We hypothesized that cortical excitability (MEP size) would increase, whereas the motoneuronal responsiveness to Ia afferent stimulation (the H-reflex size) would decrease. Post-activation depression would increase supporting a pre-synaptic contribution to the reduced H-reflex. Because of distinct physiologic and functional characteristics of the soleus, we expect a more robust change at the spinal level than at the cortical level. These responses will vary from that reported for upper limb muscles.
2. Methods
2.1. Subjects
A total of 10 male subjects were recruited in the study. Their age, height, and weight were 28.3 ± 6.8 (mean ± standard deviation, SD) yrs old, 177.8 ± 8.5 cm, and 78.7 ± 13.0 kg, respectively. Only males were recruited because it has been shown that greater fatigue resistance is present in females compared with males (Hicks et al., 2001). Subjects had no known neurologic disease or ankle joint musculoskeletal problems. Subjects were asked to refrain from exhaustive exercise and intake of caffeine and alcohol 24 h prior to participation in the study. The study was approved by the internal review board at the University of Iowa, and all subjects gave written informed consent prior to participation.
2.2. Instrumentation and experimental set-up
2.2.1. Torque recording
Ankle plantar flexion torque was measured using a force transducer (1500 ASK-300 Interface, AZ, USA). Subjects sat on a custom-designed chair with a padded seat and metal frame. The knee joint was flexed at 90° (0° being full extension) and the ankle joint was placed at 0° between plantar and dorsiflexion, so the foot was parallel to the floor. The majority of the plantar flexion torque generated in this position is attributed to the soleus muscle (Sale et al., 1982). The transducer was mounted to a rigid metal plate on an adjustable metal bar extending from the chair. The subject’s left foot was secured to this component. Two non-elastic straps were used to hold the foot in position: one at the ankle and the other from the bottom of the plate to the anterior aspect of the distal femur to prevent upward vertical movement of the lower leg with plantar flexion. The analog signal from the transducer was amplified (500×) and sampled at 1 kHz.
2.2.2. Electromyographic (EMG) recordings
EMG was recorded using active bipolar surface electrodes (silver-silver chloride disks of 8 mm in diameter spaced 20 mm between centers) for the soleus (Sol) and tibialis anterior (TA) of the left leg. The locations of these electrodes were as follows: about 1 cm medial to the midline of the posterior calf and immediately distal to the insertion of the medial gastrocnemius into the Achilles’ tendon (Sol), and at upper 1/3 on the line between the tip of the fibular head and the tip of the medial malleolus (TA). The skin at these locations was cleaned with an alcohol swab and sandpaper. A common ground electrode was placed on the anterior aspect of the tibia of the left leg. The signal was amplified (1 k for both TA and Sol), band-pass filtered (15-4 kHz) (Therapeutics Unlimited, Iowa City, IA, USA), and sampled at 4 kHz for Sol and TA.
2.2.3. Tms
Stimulation of the primary motor cortex was accomplished using a Magstim 2002 (Magstim Company Ltd., Whitland, Dyfed, UK) with a large (11 cm) curved figure-of-eight (double cone) coil. The coil was placed on the head, and was oriented so that the current in the conjunction of the two coils traveled in the anterior posterior direction. The best position where the largest MEP was observed in Sol was found by repeated trials while moving the coil over the head via a grid and then the location was marked on a swim cap fit tightly over the subject’s head. The coil was held manually, and a Velcro® strap was used to help stabilize the coil at the desired position. The active motor threshold (AMT) was determined as the minimum intensity required eliciting MEPs whose amplitude was 50–100 µV above the background volitional EMG in four out of eight trials in the Sol muscle, each separated by more than 6 s while subjects were holding a 10% MVC target torque. Pilot data supported that 10% background activity was important to triggering reproducible MEPs. Supra-threshold stimulus intensity was decreased by 5% maximal stimulator output until the AMT was found. The AMT was found to be 59.5 ± 9.6% maximal stimulator output. Then the stimulus intensity was set at 1.2 times the AMT, and this intensity was used throughout the experiment for both SP and MEP measurements on MVCs, and MEP measurements on sub-maximal target contractions (see below).
2.2.4. Peripheral nerve electrical stimulation
The posterior tibial nerve was electrically stimulated using a constant current stimulator (DS7A, Digitimer, UK). A cathode (1.0 cm diameter) was placed at the left popliteal fossa, and an anode (8.0 × 8.0 cm stainless steel plate covered with water-soaked gauze) was placed on the anterior aspect of the femur immediately superior to the patella of the right leg. A square 1-ms pulse was used to record both direct compound muscle action potential (M wave) and H-reflex. At the beginning of each experiment, stimulus intensity for maximal M wave (M max) and H-reflex was determined. To obtain the M max, the stimulus intensity was gradually increased until no further increase in peak-to-peak amplitude was observed, and that stimulus intensity was multiplied by 1.2 to ensure the maximal stimulus intensity throughout the experiment. To record H-reflex, the stimulus intensity was increased gradually from the sub-threshold intensity for the H-reflex until the H-reflex became close to its maximal amplitude, but still on the ascending limb of the H-reflex recruitment curve (~80% of maximal H-reflex). At this stimulus intensity, a small M wave preceded the H-reflex. This small M wave was monitored throughout the study and shown to be consistent throughout the experiment. All the electrical stimulations were applied while the muscle was at rest, and muscle relaxation was ensured through audio feedback of the Sol EMG. When the H-reflex was recorded, the electrical stimulation was always applied in pairs (500-ms interval). The first H-reflex (H1) size can be affected both post- and pre-synaptically. However, the size of the second H-reflex (H2), relative to the H1 (H2/H1), is assumed to be predominantly be mediated by the longer term post-activation depression (Nordlund et al., 2004). The classical GABA-mediated pre-synaptic inhibition can last only up to 500 ms (Eccles, 1964), whereas the homosynaptic post-activation depression can last over 10 s (Hultborn et al., 1996). Thus, this protocol focused primarily on the post-activation depression mechanism by using the 500 ms interval between pulses.
2.3. Experimental procedure
After subjects sat on the custom-designed chair, EMG recording, ground electrodes, and stimulating electrodes were placed on the tested leg. Then the foot was strapped to the foot device, and the trunk was stabilized using belts at the waist and chest. The optimal position for TMS was found while subjects were holding short term intermittent constant torque contractions (~10% MVC; 3 s on, 30 s off; ~20 contractions). The position was marked, and pre-fatigue measurements were taken.
2.3.1. Maximal voluntary contraction (MVC) measurements
After 3–5 near-maximal warm-up contractions, subjects performed three MVCs into plantar flexion followed by another two into dorsiflexion. Subjects were given verbal encouragement and visual feedback of the exerted torque on a computer screen placed in front of them. Each MVC was held for 5 s and a 1-min rest period was given between contractions. Using the highest torque among the three MVCs in plantar flexion, a target torque of 10% was calculated and displayed as a horizontal line for the subject to view on the computer screen.
2.3.2. MEP measurements
The AMT with the 10% MVC target torque contraction was determined, and the stimulus intensity was set at 1.2 times the AMT. In order to obtain pre-fatigue MEP size, three single pulses of TMS were applied while subjects were holding the target torque, separated by more than 5 s. MEP responses were measured during muscle activation to better control for both the cortical and motoneuron pool excitability, and therefore the MEP size becomes less variable (Darling et al., 2006). Moreover, holding a low constant muscle activity ensures that changes in response to TMS are not simply a result of differences in sub-threshold activation of neurons (Ridding and Rothwell, 1995).
2.3.3. Silent period (SP) measurements
Subjects were asked to perform two to three 5-s MVCs with a rest of ~1 min between. While they were exerting the maximal torque, one single pulse TMS was superimposed on each MVC in order to obtain pre-fatigue SP duration. Subjects were asked to bring their torque back to their maximal level as quickly as possible after TMS, and verbal encouragement was given during MVCs.
2.3.4. Maximal M wave and H-reflex recordings
Two maximal M waves were recorded first, followed by three pairs of H-reflex with the muscle at rest.
2.3.5. Fatigue task
After all the pre-fatigue measurements described above had been obtained, subjects performed a 9-epoch (cycle) fatigue task with each epoch consisting of five MVCs (Fig. 1A). Subjects performed five 7-s MVCs, each separated by a complete rest of 3 s (Fig. 1B). A single pulse TMS was superimposed on the 3rd MVC. After the 5th MVC of each epoch, subjects were asked to hold the target torque (pre-fatigue 10% MVC) for 10 s, and another TMS pulse was delivered. Then subjects were asked to relax and a pair of H-reflexes was induced (500 ms inter-pulse interval). This was the end of one epoch. The exact same procedure was repeated for a total of 9 epochs. After subjects received the electrical stimulation for the H-reflex in the 1st and the 9th (last) cycles, supramaximal electrical stimulation was delivered to repeat M max measurements. Subjects were verbally encouraged throughout the task. The computer screen showed the exerted torque for 10 s with grids indicating every 1 s, so subjects knew the timing for when to contract and when to relax. Investigators also provided verbal cues to help subjects perform the task.
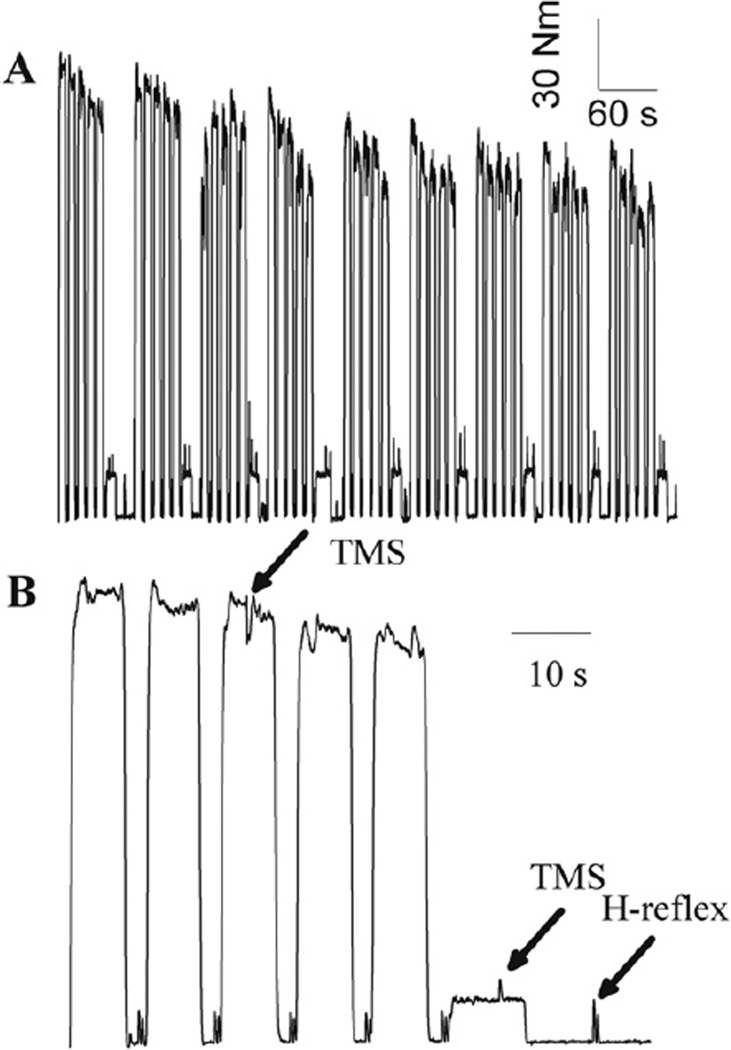
Fig. 1.
A representative example of the volitional torque during the fatigue task (A) and an expanded signal showing only the first cycle (B). Subjects performed five 7-s MVCs and a 10-s target contraction at pre-fatigue 10% MVC, and then they were asked to relax for 15 s each cycle. The timings of TMS and electrical stimulation for the H-reflex are indicated by arrows in B. The small increases in torque before each MVC in B are due to electrical stimulation used to ensure that the size of the preceding small M waves is constant.
2.3.6. Post-fatigue task
In order to examine recovery, post-fatigue measurements were collected at 1 and 10 min. Subjects were asked to perform a 7-s MVC on which TMS was superimposed. Then a 10-s target contraction (10% MVC) with TMS, followed by a rest of 10 s with the H-reflex recording, was repeated. Finally, the M max was repeated at the conclusion of the 10 min recovery period.
2.4. Data reduction and analysis
All the analog signals were digitized and analyzed using Datapac 2K2 ver. 3.18 (Run Technologies Co., Vallejo, CA, USA). Pre-fatigue and post 1- and 10-min MVC torque and EMG activity were determined by taking the mean and root mean square (RMS) amplitude of the torque and EMG signal respectively, over a 2-s window where the torque was most stable. For trials on which TMS was superimposed (post 1- and 10-min MVCs), two separate time windows were used before and after TMS, so TMS-induced extra torque (if any) and stimulus artifacts were not included. The higher value from the two windows was chosen to represent the maximal torque and EMG for that trial. Volitional torque and EMG during the fatigue task were calculated as mean and RMS amplitude, respectively, over the middle 4 s for each 7-s MVC, and five values from each epoch were averaged.
MEP size was quantified using integrated amplitude due to its multi-phasic shape. The time window was always 25 ms, but 30 ms after TMS delivery was used for five subjects, and 35 ms was used for the rest of the subjects as the starting time of the window to adjust for slight differences in the MEP onset latency across subjects. Pre-TMS Sol EMG activity level was calculated as the RMS amplitude of a 100 ms window immediately before TMS. SP duration was visually determined as the time between the TMS delivery and the recurrence of continuous voluntary EMG activity after TMS. It has been recently reported (Damron et al., 2008) that the visual approach to quantitate the SP duration resulted in slightly lower between-visit variability than an automated mathematical approach described elsewhere (Daskalakis et al., 2003).
The size of M wave and H-reflex was quantified as peak-to-peak amplitude, and the ratio of H2 and H1 (H2/H1) was calculated. The twitch associated with M max was used to obtain maximal peak torque and peak relaxation rate. The peak relaxation rate was determined by calculating the slope of a regression line (25 ms) computed for successive moving intervals over the duration during which the torque was declining after the peak torque. The minimal slope of the regression line was chosen as the peak relaxation rate. The peak relaxation rate was then normalized to the peak torque in order to account for the different rate with different peak torque levels.
Whenever possible, values obtained during and after the fatigue task were expressed as a percentage of the corresponding values from the pre-fatigue measurements. In the text, the results are expressed as mean ± SD, whereas in figures, the error bars represent standard error.
2.4.1. Statistical analyses
Variables obtained before, during and after the fatigue task were analyzed using one-way repeated measures analysis of variance (ANOVA) to determine whether time had a significant effect. In the event of a significant effect, post hoc analysis was carried out using paired t tests (Bonferroni). For multiple regression analyses, a stepwise method was used with 0.05 for the probability to enter and 0.10 for the probability to remove variables.
3. Results
3.1. Voluntary torque, maximal M wave amplitude, and EMG activity
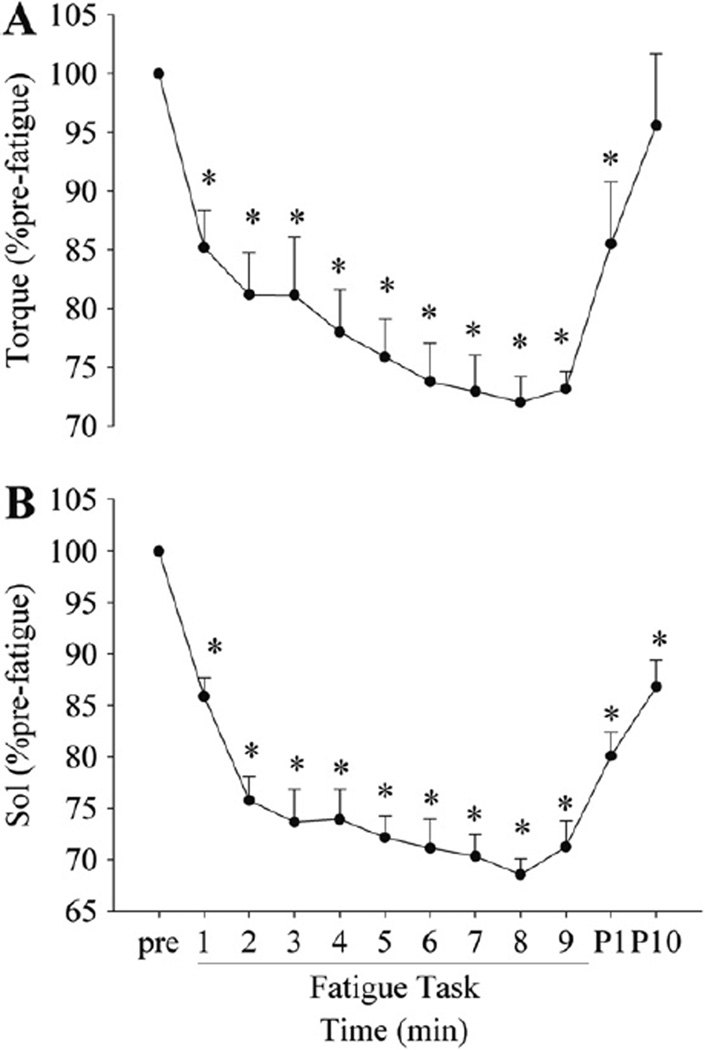
The voluntary torque before fatigue was 140.9 ± 43.6 N m, and decreased to 85 ± 6.5% of pre-fatigue after the 1st epoch (p < 0.01: pre-fatigue torque versus epoch 1 torque). There was a progressive decrease in torque from epoch 1 through epoch 9 with a final torque of 73.2 ±4.5% of pre-fatigue (p < 0.05: epoch 1 versus epoch 9; Fig. 2A). The torque recovered from fatigue after the 10 min of rest (95.6 ± 19.2% pre-fatigue, p = 0.25 for the pre-fatigue versus 10-min post comparison). The peak-to-peak amplitude of the maximal M wave recorded before fatigue (8.12 ± 2.09 mV) did not change with fatigue and stayed stable during recovery (p = 0.20 for the main effect of time from pre-fatigue to 10 min post, ranging from 7% decrease to 9% increase). Sol EMG showed similar changes with torque: decreasing significantly after the 1st epoch (p < 0.01), followed by a gradual, but significant change from epoch 1 to epoch 9 (p < 0.05) (Fig. 2B).
Fig. 2.
Changes in the voluntary torque (A) and voluntary Sol EMG activity (B) during and after the fatigue task. Pre, P1 and P10 indicate pre-fatigue, post 1- and 10-min fatigue task, respectively. The greatest decline in torque and EMG activity was found during the 1st cycle, followed by a more gradual, but significant decrease by the end of the task for both torque and EMG. *p < 0.05 from pre-fatigue values.
While the subjects were performing the plantar flexion MVCs, the antagonist (TA) showed less than 10% pre-fatigue MVC activity at the beginning of the fatigue task, and did not change over time (p = 0.35 for the main effect of time).
3.2. H-reflex (H1) size and H2/H1 ratio
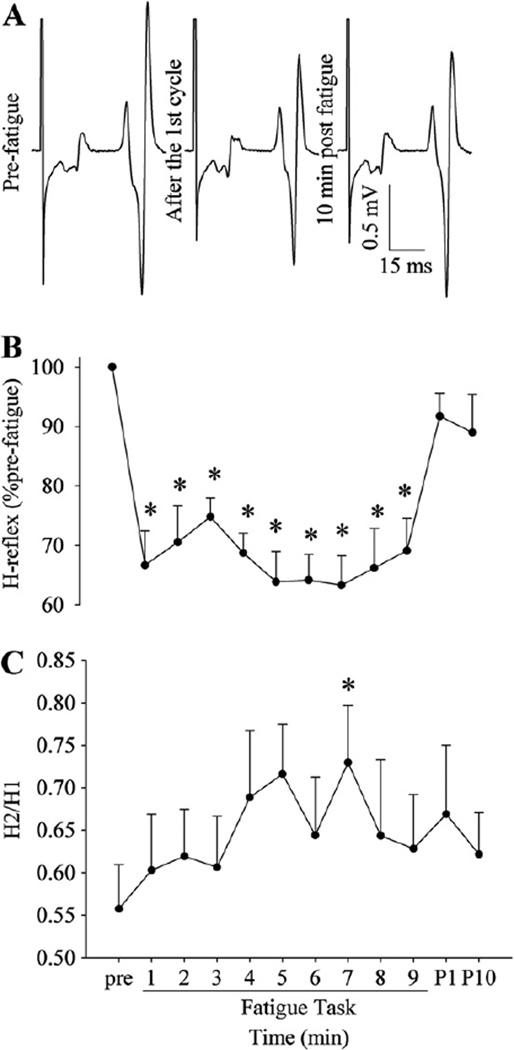
No change in the small M wave amplitude preceding the H-reflex was observed (p = 0.17; coefficient of variations across time within subjects was <2%), indicating that the stimulus intensity was successfully maintained to ensure the consistent electrical stimulation. The peak-to-peak amplitude of the H-reflex before fatigue (34.5 ± 11.6% M max) decreased by the end of the 1st epoch (group of five MVCs, 66.6 ± 18.3% pre-fatigue), and it stayed at that level (no significant effect of time from the 1st cycle to the 9th cycle; Fig. 3B). The size of the H-reflex recovered with the 1-min rest after the fatigue task (p > 0.23 for pre-fatigue versus post 1-min comparison).
Fig. 3.
A representative example of the H-reflex (H1) (A) and changes in the size of H1 (B) and H2/H1 (C) during and after the fatigue task. The H-reflex size was decreased after the 1st cycle, and then showed no change toward the end of the task, whereas H2/H1 showed a trend of increasing with fatigue. *p < 0.05 from pre-fatigue values.
H2/H1 ratio before fatigue (0.56 ±0.16) was relatively stable throughout the fatigue task with no significant effect of time (p = 0.11; Fig. 3C). However, there was a trend of the ratio to increase with fatigue: after the 7th epoch, the ratio was significantly higher than that of the pre-fatigue condition (p < 0.05) supporting reduced post-activation suppression.
3.3. MEP size with target contractions
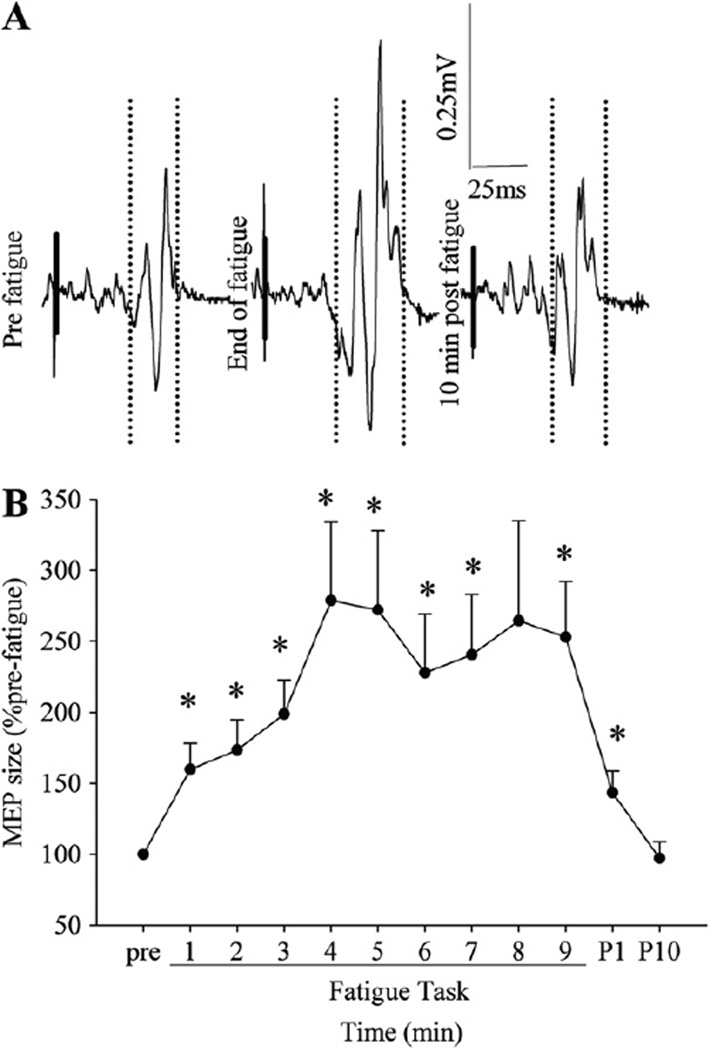
While the subjects were holding the pre-fatigue 10% MVC target contractions, the Sol EMG level was about 9% of the pre-fatigue MVC level, and did not change with fatigue (no main effect of time). The MEP size with the target contraction increased with fatigue progressively toward the end of the task (252.9 ± 124.2% pre-fatigue at the end, Fig. 4B). When the MEP size on target contractions was normalized to the pre-stimulus EMG activity or to the maximal M wave, the results were unchanged (data not presented).
Fig. 4.
A representative example of the TMS responses (motor evoked potentials) recorded while a subject was holding a pre-fatigue 10% MVC target torque contraction (A) and changes in the MEP size (B) during and after the fatigue task. The vertical dotted lines in A indicate onsets and offsets of the time windows used to calculate the integrated amplitude of the MEP size, and short thick solid lines were superimposed on the stimulus artifacts on the EMG signal for clarity purpose. Fatigue increased the MEP size on the target contraction. *p < 0.05 from pre-fatigue values.
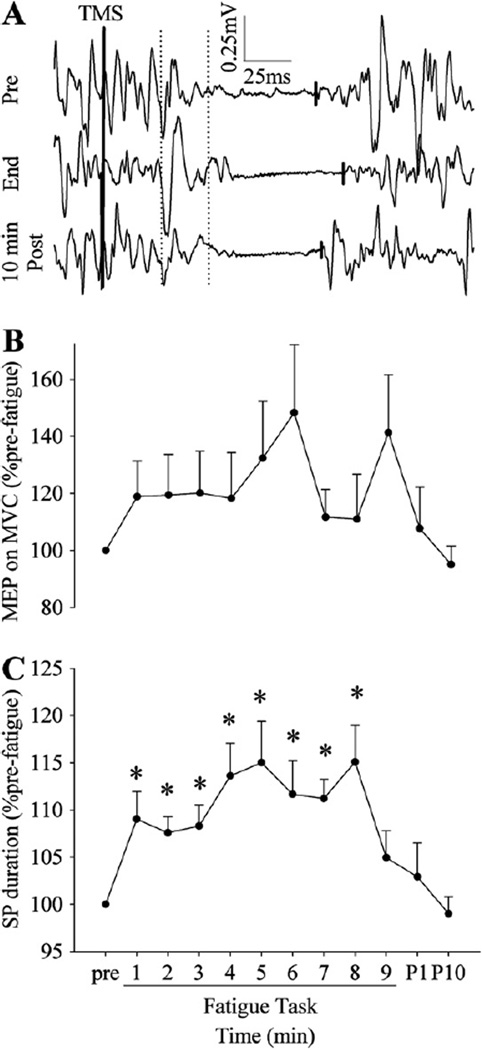
3.4. MEP size and SP duration with MVCs
The MEP size on MVCs did not change with fatigue (no main effect of time from pre-fatigue to the 9th cycle, p = 0.09, Fig. 5B), and a similar result was obtained when normalized to the maximal M wave. When the MEP size was normalized to pre-TMS Sol EMG, because the volitional EMG activity with MVCs decreased with fatigue (Fig. 2B), the normalized MEP size on MVCs increased with fatigue.
Fig. 5.
A representative example of the TMS responses recorded during MVCs (A) and changes in the MEP size (B) and SP duration (C) during and after the fatigue task. The vertical dotted lines indicate onsets and offsets of the time windows used to calculate the integrated amplitude of the MEP, and the short solid lines indicate the end of the SP. The MEP size on MVCs did not significantly change with fatigue, whereas the SP duration became longer during the 1st cycle, and then showed no change toward the end of the task. *p < 0.05 from pre-fatigue values.
The duration of the silent period in the Sol EMG was longer after the first epoch (113.7 ± 14.2 versus 123.5 ± 16.6 ms for pre-fatigue and the 1st epoch; p < 0.02; Fig. 5C). However, the SP duration did not change after the 1st epoch (no effect of time from the 1st epoch to the 9th epoch).
3.5. Twitch peak torque and relaxation rate
The peak twitch torque associated with the maximal M wave decreased at the end of the fatigue task compared to that at the end of the 1st epoch (34.6 ± 9.0 versus 30.2 ± 7.5 N m; p < 0.01). The maximal relaxation rate of the twitch was similar at the end of the 1st epoch and at the end of the entire task (p = 0.21).
3.6. Relationships among dependent variables
Pre-fatigue variables and the voluntary torque at the end of the fatigue task (expressed as absolute and % pre-fatigue, respectively) were correlated. It was found that the pre-fatigue MVC torque showed a significant negative correlation with fatigue (r2 = 0.38; p = 0.05), indicating that subjects who presented with high pre-fatigue MVC torque showed a large decrease in torque with time during the fatigue task.
The relative decrease in torque at each cycle of the task was correlated with other variables also measured at the same time. It was found that the volitional EMG showed a positive correlation with the torque (r2 = 0.85, 0.65 and 0.49 for the 3rd, the 4th and 6th cycles, respectively; p < 0.05 for all), but no other significant univariate associations were found between torque and other variables.
Multiple regression analysis was completed to explore the best model to predict fatigue (a decrease in torque with time during the fatigue task). The best model included volitional EMG activity, SP duration, and H-reflex size with the adjusted r2 of 0.54. However, much of this change was explained by the EMG activity (see discussion).
4. Discussion
We designed this study to examine the cortical and segmental excitability changes during fatiguing intermittent MVCs. We aspired to study the soleus muscle because of its known slow fiber phenotype, role as a postural muscle, and the limited information on segmental/central components of fatigue in postural muscles when asked to drive high intensity but intermittent contractions.
4.1. The extent of torque decline and peripheral fatigue
The decline in voluntary torque was about 30% in the present study. This decline was greater than that observed by Kawakami and colleagues after 100 MVCs using a similar knee and ankle position (about 19% decrease) (Kawakami et al., 2000). However, our work rest cycle was more stringent (7 s on/3 s off) than that used by Kawakami et al. (1 s on/1 s off). Interestingly, both Kawakami et al. and our study showed no significant change in the maximal M wave amplitude indicating that there was minimal neuromuscular transmission failure. The intermittent nature of the protocol likely enabled full recovery of neuromuscular junction and sarco-lemmal membrane propagation during the rest periods. This finding also indicates that the changes seen in the other EMG recordings (the volitional EMG activity level and MEP size, etc.) were not attributable to changes at and/or beyond the neuromuscular junction.
Twitch peak torque decreased during the fatigue task, which is not consistent with the only comparable study (Kawakami et al., 2000). The discrepancy in the fatigue-induced change in twitch peak torque is also likely explained by the greater work/rest ratio used in the present study.
Although fatigue is often associated with slowing of the contractile properties of the muscle (Bigland-Ritchie et al., 1983), there was no significant decrease in the peak relaxation rate of the twitch. This is also in line with a previous finding (Kawakami et al., 2000). However, the significant decrease in the peak torque with no slowing of the contractile properties of the twitch may be associated with a potentiation process that occurred during the 1st epoch of the fatigue protocol and has been observed by others (Garner et al., 1989).
4.2. Segmental excitability with fatigue
Many mechanisms may change the excitability of the alpha motoneuron pool during fatigue. The decrease in responsiveness of the motoneurons through a change in their intrinsic properties (Kernell and Monster, 1982a, b; Spielmann et al., 1993), decreased facilitation with reduced muscle spindle firing rates (Bongiovanni and Hagbarth, 1990; Griffin et al., 2001; Macefield et al., 1991), an increased inhibition from increased activity in the group III and IV muscle afferents (through an increase in the GABAergic pre-synaptic inhibition of the Ia afferents (Pettorossi et al., 1999; Rossi et al., 1999), and/or through reflexive inhibition (Garland, 1991; Woods et al., 1987)) are all plausible mechanisms contributing to decreased H-reflex excitability. In the present study, the H-reflex (H1) decreased with fatigue, a finding consistent with previous reports (Enoka et al., 1980; Etnyre et al., 1990; Manca et al., 1998; Nordlund et al., 2004). Whether the decreased excitability is predominantly pre-synaptic and/or post-synaptic is less discern-able. The extent of pre-synaptic inhibition was previously shown to be reduced with fatigue (Nordlund et al., 2004), suggesting that most of the change in excitability is post-synaptic. The greatest change in H-reflex in this study was after the 1st epoch, with little change for the remainder of the fatigue task. It has been shown that high force muscle contractions are followed by a marked and persistent increase in its sensory discharge (Enoka et al., 1980; Hutton et al., 1973), leading to an accommodation and associated decrease in the H-reflex. However, we did not observe a robust change in the H2/H1 ratio in the present study, suggesting that the amount of post-activation depression was stable. Therefore, another explanation would be that the first five 7-s MVCs may have caused cutaneous afferent stimulation leading to inhibition and the resultant decrease in the H-reflex size (Floeter, 2003).
The volitional EMG activity continued to decline from the 1st to the last cycles, indicating that some of the motoneurons stopped firing and/or decreased their firing rates with fatigue. Decreased surface EMG activity may be explained, at least partly, by the development of central fatigue (Gandevia, 2001). If the segmental excitability stayed relatively constant during the fatigue task as suggested by no significant change in the H-reflex size from epoch 2 through 9, then it is likely that the descending drive to the motoneuron pool became sub-optimal in the present study similar to that reported for the elbow flexors during fatiguing intermittent MVCs (Hunter et al., 2006).
4.3. Motor cortex excitability during fatigue
Although the early part of the SP is attributed to the inhibition occurring at both cortical and segmental levels, the latter part of the SP is thought to be predominantly due to intracortical inhibition (Brasil-Neto et al., 1995; Fuhr et al., 1991; Inghilleri et al., 1993). The contribution of the cortex to the latter part of the SP duration is still supported in soleus (Ziemann et al., 1993), despite the finding that SP duration in the muscle has been reported to be relatively short (Roick et al., 1993). Therefore, the increase in SP duration after the 1st cycle seen in the present study supports an increase in cortical inhibition. However, unlike other muscles such as the first dorsal interosseous (Benwell et al., 2007) and the biceps brachii (Hunter et al., 2006), no additional increase in SP duration with fatigue was observed after the 1st epoch in the soleus. Similarly, the MEP size on target contractions showed a gradual increase throughout the task, but, the MEP during the MVCs did not increase significantly.
The progressive increase in MEP size on the target contractions is in line with a previous finding in the triceps surae for sub-maximal contractions (Hoffman et al., 2009). However, the fatigue task used in the present study consisted of repetitive fatiguing MVCs with MEPs evoked intermittently during a 10% background contraction. As a check, we examined the volitional EMG activity level during the 10% background contractions throughout the fatigue trial and found no change. Hence, taken together, these findings support that cortical excitability had to be increased in order to maintain the similar net output from a motoneuronal pool that was segmentally becoming inhibited, as supported by the H-reflex findings.
The fatigue-induced increase in MEP size on the target contractions in our study is consistent with previous findings for the elbow flexors (Taylor et al., 2006). However, our results support that the fatigue-induced increase in MEP occurred to a lesser extent in the soleus compared to the biceps brachii. This may be due to the finding that new motor units have been reported to be recruited up to ~88% MVC in the biceps brachii, and further increases in volitional force is primarily achieved by increasing firing rates of the recruited motor units (Kukulka and Clamann, 1981). Conversely, the motor unit recruitment has been reported to continue close to MVC in the soleus (Oya et al., 2009). This variance in control of a postural versus an extremity muscle may explain the differences observed in the cortex excitability during maximal repetitive contractions.
We used 120% of the AMT for TMS on both MVCs and the 10% target contractions in this study. It is possible that the fatigue-related changes in the MEP size on MVCs are stimulus intensity dependent. Alternatively, it is possible that although the volitional torque and EMG activity level decreased significantly throughout the task, for this fatigue-resistant muscle, the fatigue task was not intense enough to cause significant changes in the excitability at the motor cortex. This possibility needs to be explored using a higher stimulus intensity and more stringent fatigue task (e.g., sustained MVC) in future studies.
4.4. Important factors when interpreting mechanisms
Several factors may have contributed to fatigue in this study. First, as reviewed elsewhere (Nielsen et al., 1999), there are several limitations such as different responsible pathways, and presence/ absence of pre-synaptic inhibition when comparing the MEP, volitional EMG, and H-reflex.
First, the H-reflex was elicited with the muscle at rest, therefore, the decreased excitability of the pathway (the decrease in H1 size) observed does not necessarily explain the excitability of the pathway while subjects were performing MVCs, as it has been reported that post-activation depression is reduced under volitional contractions compared to at rest (Stein et al., 2007). In addition, the MEP was elicited on a background 10% contraction. Therefore, the increase in MEP could reflect an increase in central drive to maintain the 10% background contraction given the concurrent segmental inhibition (H-reflex). Indeed, in the absence of an increase in the resting MEP, the increased MEP observed in this study may support that the motor cortex is trying to overcome the enhanced segmental inhibition as a result of fatigue.
Second, an increase in the small diameter group III and IV muscle afferent activity increases the GABAergic pre-synaptic inhibition of the Ia afferents (Pettorossi et al., 1999; Rossi et al., 1999), which increases fusimotor discharge in the cat (Jovanovic et al., 1990), and spindle discharge during muscle fatigue (Ljubisavljevic et al., 1992). Indeed, when the group III and IV muscle afferent activity is increased, the excitability of the motoneuron pools were found to be increased (Martin et al., 2008). Logically, the group III and IV muscle afferent activity may have increased during fatigue and modulated the central motor drive during exercise (Amann et al., 2009).
Third, the stimulus intensity to elicit an H-reflex during the fatigue task was verified by the small preceding M wave, commonly performed to ensure stable stimulation conditions. However, the extent of activity-dependent hyperpolarization differs for sensory and motor axons in peripheral nerves (Burke et al., 1997; Vagg et al., 1998), and, therefore, maintaining the efferent volley by eliciting the preceding M wave of a similar size does not guarantee that the afferent volley stayed constant.
4.5. Predictive model of soleus muscle fatigue
Because various measurements characterized the cortical, segmental, and muscle activity, we explored which component best predicted the change in torque during fatigue. Interestingly, the volitional EMG alone explained over 47% of the change in torque. Specific measures of cortical and segmental changes only raised our ability to predict the change in torque to 50%. Theoretically this makes intuitive sense because the surface EMG activity reflects an integrated summary of all excitatory and inhibitory mechanisms contributing to muscle fatigue.
5. Conclusions
When the human soleus muscle was fatigued through intermittent MVCs, the motoneuron pool excitability was found to be decreased at the early part of fatigue while the volitional torque and EMG activity level continued to decrease toward the end of the task. Meanwhile, the cortical excitability was found to increase progressively throughout the task when MEPs were assessed with low level background activity (10% MVC). Collectively, these findings indicate that the neuronal processes at the motor cortex and spinal cord interact uniquely in postural muscle when contrasted with non-postural extremity muscles.
HIGHLIGHTS.
Fatigue decreases spinal excitability in human soleus, but increases cortical excitability with concomitant inhibition at rest.
Cortical excitability assessed with maximal effort stayed constant with fatigue.
These findings may reflect distinct responses specific to postural muscle.
Acknowledgments
The authors thank Andrew E. Littmann, Shuo-Hsiu Chang, Stephanie A. Miller, Nicole E. Lokenvitz, and Catherine E. Herman for their assistance with the data collections. This study was supported in part by awards to RKS from the National Institutes of Health, The U.S. Department of Veterans Affairs, and the Craig H. Neilsen Foundation.
References
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587:271–283. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa P, Chalmers GR, Stewart H, Eisen AA. Responses of ankle extensor and flexor motoneurons to transcranial magnetic stimulation. J Neurophysiol. 2002;88:124–132. doi: 10.1152/jn.2002.88.1.124. [DOI] [PubMed] [Google Scholar]
- Benwell NM, Mastaglia FL, Thickbroom GW. Differential changes in long-interval intracortical inhibition and silent period duration during fatiguing hand exercise. Exp Brain Res. 2007;179:255–262. doi: 10.1007/s00221-006-0790-2. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Johansson R, Lippold OC, Woods JJ. Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. J Neurophysiol. 1983;50:313–324. doi: 10.1152/jn.1983.50.1.313. [DOI] [PubMed] [Google Scholar]
- Bongiovanni LG, Hagbarth KE. Tonic vibration reflexes elicited during fatigue from maximal voluntary contractions in man. J Physiol. 1990;423:1–14. doi: 10.1113/jphysiol.1990.sp018007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cammarota A, Valls-Sole J, Pascual-Leone A, Hallett M, Cohen LG. Role of intracortical mechanisms in the late part of the silent period to transcranial stimulation of the human motor cortex. Acta Neurol Scand. 1995;92:383–386. doi: 10.1111/j.1600-0404.1995.tb00151.x. [DOI] [PubMed] [Google Scholar]
- Burke D, Hicks R, Gandevia SC, Stephen J, Woodforth I, Crawford M. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. J Physiol. 1993;470:383–393. doi: 10.1113/jphysiol.1993.sp019864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Kiernan M, Mogyoros I, Bostock H. Susceptibility to conduction block: differences in the biophysical properties of cutaneous afferents and motor axons. In: Kimura J, Kaji R, editors. Physiology of ALS and related diseases. Amsterdam: Elsevier; 1997. pp. 43–53. [Google Scholar]
- Butler JE, Taylor JL, Gandevia SC. Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci. 2003;23:10224–10230. doi: 10.1523/JNEUROSCI.23-32-10224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damron LA, Dearth DJ, Hoffman RL, Clark BC. Quantification of the corticospinal silent period evoked via transcranial magnetic stimulation. J Neurosci Methods. 2008;173:121–128. doi: 10.1016/j.jneumeth.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Darling WG, Wolf SL, Butler AJ. Variability of motor potentials evoked by transcranial magnetic stimulation depends on muscle activation. Exp Brain Res. 2006;174:376–385. doi: 10.1007/s00221-006-0468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Molnar GF, Christensen BK, Sailer A, Fitzgerald PB, Chen R. An automated method to determine the transcranial magnetic stimulation-induced contralateral silent period. Clin Neurophysiol. 2003;114:938–944. doi: 10.1016/s1388-2457(03)00038-5. [DOI] [PubMed] [Google Scholar]
- de Noordhout AM, Rapisarda G, Bogacz D, Gerard P, De Pasqua V, Pennisi G, et al. Corticomotoneuronal synaptic connections in normal man: an electrophysiological study. Brain. 1999;122(Pt 7):1327–1340. doi: 10.1093/brain/122.7.1327. [DOI] [PubMed] [Google Scholar]
- Eccles J. Berlin. Heidelberg: New York Springer; 1964. The physiology of synapses. [Google Scholar]
- Enoka RM, Hutton RS, Eldred E. Changes in excitability of tendon tap and Hoffmann reflexes following voluntary contractions. Electroencephalogr Clin Neurophysiol. 1980;48:664–672. doi: 10.1016/0013-4694(80)90423-x. [DOI] [PubMed] [Google Scholar]
- Etnyre BR, Kinugasa T, Abraham LD. Post-contraction variations in motor pool excitability. Electromyogr Clin Neurophysiol. 1990;30:259–264. [PubMed] [Google Scholar]
- Floeter MK. Cutaneous silent periods. Muscle Nerve. 2003;28:391–401. doi: 10.1002/mus.10447. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Garland SJ. Role of small diameter afferents in reflex inhibition during human muscle fatigue. J Physiol. 1991;435:547–558. doi: 10.1113/jphysiol.1991.sp018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner SH, Hicks AL, McComas AJ. Prolongation of twitch potentiating mechanism throughout muscle fatigue and recovery. Exp Neurol. 1989;103:277–281. doi: 10.1016/0014-4886(89)90051-4. [DOI] [PubMed] [Google Scholar]
- Griffin L, Garland SJ, Ivanova T, Gossen ER. Muscle vibration sustains motor unit firing rate during submaximal isometric fatigue in humans. J Physiol. 2001;535:929–936. doi: 10.1111/j.1469-7793.2001.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks AL, Kent-Braun J, Ditor DS. Sex differences in human skeletal muscle fatigue. Exerc Sport Sci Rev. 2001;29:109–112. doi: 10.1097/00003677-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Hoffman BW, Oya T, Carroll TJ, Cresswell AG. Increases in corticospinal responsiveness during a sustained submaximal plantar flexion. J Appl Physiol. 2009;107:112–120. doi: 10.1152/japplphysiol.91541.2008. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Butler JE, Todd G, Gandevia SC, Taylor JL. Supraspinal fatigue does not explain the sex difference in muscle fatigue of maximal contractions. J Appl Physiol. 2006;101:1036–1044. doi: 10.1152/japplphysiol.00103.2006. [DOI] [PubMed] [Google Scholar]
- Hutton RS, Smith JL, Eldred E. Post-contraction sensory discharge from muscle and its source. J Neurophysiol. 1973;36:1090–1103. doi: 10.1152/jn.1973.36.6.1090. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles An autopsy study. J Neurol Sci. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Jovanovic K, Anastasijevic R, Vuco J. Reflex effects on gamma fusimotor neurones of chemically induced discharges in small-diameter muscle afferents in decerebrate cats. Brain Res. 1990;521:89–94. doi: 10.1016/0006-8993(90)91528-o. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Amemiya K, Kanehisa H, Ikegawa S, Fukunaga T. Fatigue responses of human triceps surae muscles during repetitive maximal isometric contractions. J Appl Physiol. 2000;88:1969–1975. doi: 10.1152/jappl.2000.88.6.1969. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Motoneurone properties and motor fatigue. An intracellular study of gastrocnemius motoneurones of the cat. Exp Brain Res. 1982a;46:197–204. doi: 10.1007/BF00237177. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Time course and properties of late adaptation in spinal motoneurones of the cat. Exp Brain Res. 1982b;46:191–196. doi: 10.1007/BF00237176. [DOI] [PubMed] [Google Scholar]
- Kouzaki M, Shinohara M. Steadiness in plantar flexor muscles and its relation to postural sway in young and elderly adults. Muscle Nerve. 2010;42:78–87. doi: 10.1002/mus.21599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukulka CG, Clamann HP. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Res. 1981;219:45–55. doi: 10.1016/0006-8993(81)90266-3. [DOI] [PubMed] [Google Scholar]
- Levenez M, Garland SJ, Klass M, Duchateau J. Cortical and spinal modulation of antagonist coactivation during a submaximal fatiguing contraction in humans. J Neurophysiol. 2008;99:554–563. doi: 10.1152/jn.00963.2007. [DOI] [PubMed] [Google Scholar]
- Ljubisavljevic M, Jovanovic K, Anastasijevic R. Changes in discharge rate of fusimotor neurones provoked by fatiguing contractions of cat triceps surae muscles. J Physiol. 1992;445:499–513. doi: 10.1113/jphysiol.1992.sp018936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield G, Hagbarth KE, Gorman R, Gandevia SC, Burke D. Decline in spindle support to alpha-motoneurones during sustained voluntary contractions. J Physiol. 1991;440:497–512. doi: 10.1113/jphysiol.1991.sp018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magladery JW, Porter WE, Park AM, Teasdall RD. Electrophysiological studies of nerve and reflex activity in normal man IV The two-neurone reflex and identification of certain action potentials from spinal roots and cord. Bull Johns Hopkins Hosp. 1951;88:499–519. [PubMed] [Google Scholar]
- Manca M, Cavazzini L, Cavazza S, Salvadori T, DeGrandis D, Basaglia N. H reflex excitability following voluntary muscle contraction of different duration. Electromyogr Clin Neurophysiol. 1998;38:381–384. [PubMed] [Google Scholar]
- Martin PG, Weerakkody N, Gandevia SC, Taylor JL. Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol. 2008;586:1277–1289. doi: 10.1113/jphysiol.2007.140426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Morita H, Baumgarten J, Petersen N, Christensen LO. On the comparability of H-reflexes and MEPs. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:93–101. [PubMed] [Google Scholar]
- Nordlund MM, Thorstensson A, Cresswell AG. Central and peripheral contributions to fatigue in relation to level of activation during repeated maximal voluntary isometric plantar flexions. J Appl Physiol. 2004;96:218–225. doi: 10.1152/japplphysiol.00650.2003. [DOI] [PubMed] [Google Scholar]
- Oya T, Riek S, Cresswell AG. Recruitment and rate coding organisation for soleus motor units across entire range of voluntary isometric plantar flexions. J Physiol. 2009;587:4737–4748. doi: 10.1113/jphysiol.2009.175695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettorossi VE, Della Torre G, Bortolami R, Brunetti O. The role of capsaicin-sensitive muscle afferents in fatigue-induced modulation of the monosynaptic reflex in the rat. J Physiol. 1999;515(Pt 2):599–607. doi: 10.1111/j.1469-7793.1999.599ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Reorganisation in human motor cortex. Can J Physiol Pharmacol. 1995;73:218–222. doi: 10.1139/y95-032. [DOI] [PubMed] [Google Scholar]
- Roick H, von Giesen HJ, Benecke R. On the origin of the postexcitatory inhibition seen after transcranial magnetic brain stimulation in awake human subjects. Exp Brain Res. 1993;94:489–498. doi: 10.1007/BF00230207. [DOI] [PubMed] [Google Scholar]
- Rossi A, Decchi B, Ginanneschi F. Presynaptic excitability changes of group la fibres to muscle nociceptive stimulation in humans. Brain Res. 1999;818:12–22. doi: 10.1016/s0006-8993(98)01253-0. [DOI] [PubMed] [Google Scholar]
- Rudomin P. Presynaptic selection of afferent inflow in the spinal cord. J Physiol Paris. 1999;93:329–347. doi: 10.1016/s0928-4257(00)80061-3. [DOI] [PubMed] [Google Scholar]
- Sale D, Quinlan J, Marsh E, McComas AJ, Belanger AY. Influence of joint position on ankle plantarflexion in humans. J Appl Physiol. 1982;52:1636–1642. doi: 10.1152/jappl.1982.52.6.1636. [DOI] [PubMed] [Google Scholar]
- Spielmann JM, Laouris Y, Nordstrom MA, Robinson GA, Reinking RM, Stuart DG. Adaptation of cat motoneurons to sustained and intermittent extracellular activation. J Physiol. 1993;464:75–120. doi: 10.1113/jphysiol.1993.sp019625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB, Estabrooks KL, McGie S, Roth MJ, Jones KE. Quantifying the effects of voluntary contraction and inter-stimulus interval on the human soleus H-reflex. Exp Brain Res. 2007;182:309–319. doi: 10.1007/s00221-007-0989-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL. Stimulation at the cervicomedullary junction in human subjects. J Electromyogr Kinesiol. 2006;16:215–223. doi: 10.1016/j.jelekin.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol. 2008;104:542–550. doi: 10.1152/japplphysiol.01053.2007. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Todd G, Gandevia SC. Evidence for a supraspinal contribution to human muscle fatigue. Clin Exp Pharmacol Physiol. 2006;33:400–405. doi: 10.1111/j.1440-1681.2006.04363.x. [DOI] [PubMed] [Google Scholar]
- Vagg R, Mogyoros I, Kiernan MC, Burke D. Activity-dependent hyperpolarization of human motor axons produced by natural activity. J Physiol. 1998;507(Pt 3):919–925. doi: 10.1111/j.1469-7793.1998.919bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JJ, Furbush F, Bigland-Ritchie B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. J Neurophysiol. 1987;58:125–137. doi: 10.1152/jn.1987.58.1.125. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Netz J, Szelenyi A, Homberg V. Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex. Neurosci Lett. 1993;156:167–171. doi: 10.1016/0304-3940(93)90464-v. [DOI] [PubMed] [Google Scholar]