Abstract
Using nanoparticles for therapy and imaging holds tremendous promise for the treatment of major diseases such as cancer. However, their translation into the clinic has been slow because it remains difficult to produce nanoparticles that are consistent ‘batch-to-batch’, and in sufficient quantities for clinical research. Moreover, platforms for rapid screening of nanoparticles are still lacking. Recent microfluidic technologies can tackle some of these issues, and offer a way to accelerate the clinical translation of nanoparticles. In this Progress Article, we highlight the advances in microfluidic systems that can synthesize libraries of nanoparticles in a well-controlled, reproducible and high-throughput manner. We also discuss the use of microfluidics for rapidly evaluating nanoparticles in vitro under microenvironments that mimic the in vivo conditions. Furthermore, we highlight some systems that can manipulate small organisms, which could be used for evaluating the in vivo toxicity of nanoparticles or for drug screening. We conclude with a critical assessment of the near- and long-term impact of microfluidics in the field of nanomedicine.
Nanomedicine is the application of nanotechnology to medicine, specifically involving the use of engineered nanomaterials for therapy and diagnosis of major diseases such as cancer, cardiovascular and infectious diseases1. The first generation of nanoparticles with applications in medicine dates back to the 1970s, when drug-loaded nanoscale liposomes were developed to deliver their cargo to diseased cells in a ‘Trojan horse’ fashion2. Since then, a new generation of targeted drug delivery vehicles (for example, polymeric nanoparticles)3, contrast agents (such as iron oxide nanoparticles)4, diagnostic tools5, and antennas for photothermal therapy (for example, gold nanoparticles)6 have emerged. This is driven in part by further understanding of the biology of diseased states, and by technological advances in imaging techniques and synthesis of novel biocompatible and biodegradable materials7. Now, nanomedicine promises the precise delivery of drugs to disease sites (such as tumours and atherosclerotic plaques) without off-target toxicities, and the early detection of diseases using selective contrast agents and sensitive diagnostic tools8.
Nanoparticles are attractive in medicine because their surfaces can be chemically modified for targeting specific disease tissues, or for in vivo stability. For therapy, drugs can be encapsulated inside nanoparticles and released in a controlled manner over time. For imaging, nanoparticles can provide higher contrast (for example, iron oxide nanoparticles for magnetic resonance imaging) or higher brightness (for example, quantum dots (QDs) for fluorescence imaging) than conventional small-molecule agents9. Despite these advantages and several decades of research, only a handful of nanoparticles have received approval from the US Food and Drug Administration. Examples include iron oxide nanoparticles for magnetic resonance imaging (for example, Feridex and Resovist), liposomes encapsulating the anticancer drug, doxorubicin, for chemotherapy (known as Doxil), and the protein-based nanoparticle encapsulating paclitaxel for chemotherapy (called Abraxane)10.
In fact, translation of nanoparticles to the clinic has been slow compared with small-molecule drugs, with the majority of nanoparticles not even reaching the point of in vivo evaluation, and even fewer reaching clinical trials. This is due to a combination of factors. It remains difficult to reproducibly synthesize batches of nanoparticles that have identical properties and in sufficient quantities for clinical applications11. Moreover, knowledge on the fate of nanoparticles at the body-, organ- and cell-level remains limited12; this makes rational design of nanoparticles difficult and necessitates the use of screening-based approaches for synthesis. Furthermore, there are few platforms that can rapidly evaluate the biological behaviour of nanoparticles in vitro under conditions that can be correlated with their performance in vivo11. For example, there is a need for high-throughput methods for evaluating the binding and internalization of nanoparticles by cells, or the interaction of nanoparticles with plasma proteins and the complement system, among others. Finally, there is insufficient understanding of the biophysical and chemical interactions of nanoparticles with proteins, membranes, DNA and organelles. These interactions could have either beneficial or adverse outcomes13. It is expected that technologies tackling some of these challenges could significantly accelerate the discovery and clinical translation of nanomedicines.
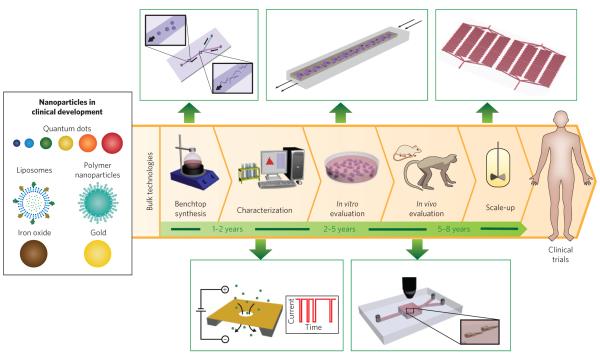
Microfluidics — the science and technology of manipulating nanolitre volumes in microscale fluidic channels — has impacted a range of applications, including biological analysis, chemical synthesis, single-cell analysis and tissue engineering14. Building on its origins in semiconductor technology and chemical separations, the expansion of microfluidics has been driven by its ability to process small sample volumes and access biologically relevant length scales and microscale transport phenomena. This expansion has been largely facilitated by techniques, such as soft lithography, that enable rapid design and prototyping of microfluidic devices using a variety of materials14. Recent advances and innovations in microfluidics are expected to improve the synthesis of nanoparticles and accelerate their transition to clinical evaluation (Fig. 1). Although many of these microfluidic systems are still being developed, they have the potential to become widely adopted because they are economical, reproducible, amenable to modifications and can be integrated with other technologies15. In this Progress Article, we highlight some of these technologies and discuss their impact on accelerating the clinical translation of nanoparticles.
Figure 1. Nanoparticles in clinical development, steps for their translation (with average timescales) and microfluidic methods (green boxes) that could improve or complement current technologies.
Synthesis is carried out in large reaction flasks, whereas microfluidic synthesis is carried out at micro and nano length scales that allow for improved control over reaction conditions. Characterization often involves taking a small sample of nanoparticles and measuring their properties offline, whereas nanopores embedded in microfluidic devices allow for real-time, in-line characterization. In vitro evaluation in plate wells produces a microenvironment far from that in vivo, whereas continuous flow in microfluidic systems result in conditions closer to those in vivo. In vivo evaluation in large animals is helpful for estimating the pharmacology of nanoparticles. To complement these studies microfluidic systems could enable real-time tracking of nanoparticles in large numbers of small organisms. Scale-up is generally carried out in reactor vessels several times larger than benchtop flasks, whereas parallelization of microfluidic channels can increase the production rate of nanoparticles with properties identical to the one at bench scale.
Well-controlled synthesis of nanoparticles
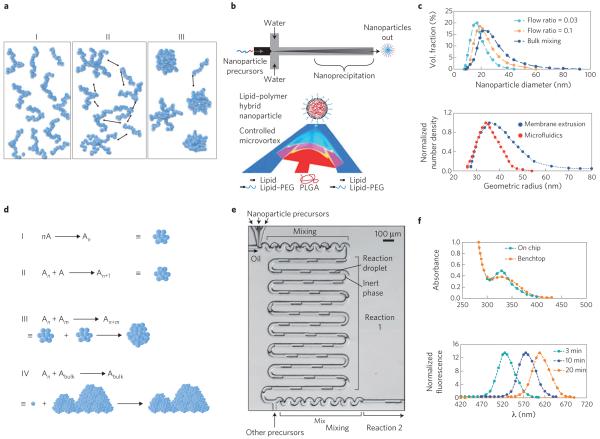
Amphiphilic molecules such as block copolymers and lipids can self-assemble into nanoparticles when they experience a change in solvent quality (for example, from organic solvent to aqueous) (Fig. 2a). A common and flexible way to accomplish a change in solvent quality is by mixing the solvent with the anti-solvent, where mixing time directly influences the final size and size distribution of the nanoparticles formed16. If the mixing timescale, τmix, is longer than the characteristic timescale for chains to nucleate and grow (τagg, ~10–100 ms depending on the molecular weight of the chain), the nanoparticles begin to assemble under varying degrees of solvent quality. This heterogeneous environment prevents effective stabilization of the nanoparticles by the hydrophilic portion of the amphiphilic molecule and facilitates their aggregation, leading to the formation of larger, polydisperse nanoparticles. However, if τmix < τagg, particle self-assembly occurs primarily when the solvent change is complete. This homogenous solvent environment for nanoparticle assembly allows the hydrophilic portion of the molecule to stabilize the nanoparticles more effectively, and this yields smaller nanoparticles with uniform size17. Although conventional bulk mixing occurs at the timescale of seconds, in microfluidic devices the mixing time of solvents is controllable and tunable from the millisecond to microsecond scale (reaching τmix < τagg)16,18.
Figure 2. Microfluidic synthesis of nanoparticles.
a, Schematic of the self-assembly mechanism of organic nanoparticles. On mixing with anti-solvent, polymers (or lipids) are brought to the vicinity of each other (I) then nucleate (II), subsequently aggregating into nanoparticles (III). b, Schematic of microfluidic synthesis of organic nanoparticles by rapid mixing through hydrodynamic flow focusing (top) and microvortices (bottom). Red and dark blue indicate organic and aqueous streams, respectively, while pink and light blue indicate their degree of mixing. PEG, polyethylene glycol; PLGA, poly(lactic-co-glycolic acid). c, Size distribution of polymeric nanoparticles (top) and liposomes (bottom) prepared in microfluidics compared with bulk synthesis. In both cases, narrower particle-size distributions are produced through microfluidics. d, Schematic of the self-assembly mechanism of inorganic nanoparticles. Individual molecules first nucleate (I and II), followed by aggregation of nuclei into nanoparticles (III). If the reaction is not quenched or stabilized, nanoparticles tend to agglomerate into bulk material (IV). A refers to individual molecules forming the nanoparticle, and An and Am refer to nuclei formed of n and m number of A molecules, respectively. e, Microfluidic synthesis of inorganic nanoparticles by rapid mixing through two-phase flow where reagents are embedded in fluid droplets carried by an inert fluid. f, Top: sharp versus broad absorption maximum of QDs synthesized in microchannels and bulk, respectively. Bottom: control of the absorption spectra of QDs as function of reaction time. Figure reproduced with permission from: a, ref. 16, © 2003 APS; b, Top: ref. 18, © 2008 ACS; Bottom: ref. 35, © 2012 ACS; c, Top: ref. 18, © 2008 ACS; Bottom: ref. 23, © 2008 Springer; d, ref. 24, © 2005 ACS; e, ref. 27, © 2004 RSC; f, Top: ref. 28, © 2010 Wiley; Bottom: ref. 27, © 2004 RSC.
In recent years, several microfluidic systems that enable rapid mixing without the need of external actuators, such as stirrers or electric fields, have been developed19. The most widely used include flow-focusing mixers20, droplet mixers21 and those with micromixing structures embedded inside the channel22. Flow focusing squeezes the solvent stream between two anti-solvent streams, resulting in rapid solvent exchange via diffusion (Fig. 2b). Droplets and three-dimensional microchannel geometries result in complex folding of fluid flows, which can completely mix two or more streams in milliseconds (Fig. 2b). The implementation of these mixing techniques for the formation of organic nanoparticles in continuous flow has resulted in polymeric and lipid nanoparticles with tunable nanoparticle size, narrower size distribution, higher drug loadings and greater batch-to-batch reproducibility relative to those made with conventional bulk techniques23 (Fig. 2c).
Similarly, inorganic nanoparticles comprising transition metals such as gold, iron and cadmium, among others, undergo self-assembly where metal solutes nucleate, grow and agglomerate into nanoclusters (Fig. 2d)24. Obtaining narrow particle-size distribution requires rapid nucleation followed by growth of nanoparticles to the desired size in the absence of further nucleation, which can be accomplished by controlling the mixing time of reagents, reaction temperature and reaction time25. In bulk, these parameters are difficult to control, leading to uneven mixing, local temperature fluctuations and uncontrolled reaction times25. In contrast, microfluidic devices allow for control over the mixing time by varying solvent flow rates or channel geometry. Moreover, better heat transfer owing to large surface areas enables better temperature control, preventing the formation of large temperature gradients. Finally, as the channel length directly corresponds to the time taken by the reactants to flow through it in continuous flow synthesis, the reaction time can be controlled by tuning the channel length or by adding reagents at precise downstream locations during the particle formation process to quench the reaction26.
Two-phase droplet mixers where reagents are encapsulated in droplets and separated by inert fluids are commonly used for the synthesis of inorganic nanoparticles (Fig. 2e)27. In this configuration, rapid mixing of the solutions occurs inside the droplets, which serve as identical microscale reactors providing homogeneous conditions for nanoparticle nucleation and growth. Both droplet-based and single-phase systems have been used to synthesize QDs that exhibit narrow size distributions, which translates into sharper absorption peaks and better luminescence qualities27, with control over size to tune the absorption spectra28 (Fig. 2f). Finally, similar systems have been implemented in the controlled synthesis of gold nanoparticles of defined size and shape, iron oxide nanoparticles with higher magnetization, and QDs with controlled size and biocompatible coatings29.
Over the past four years, several examples showing the use of microfluidics for the synthesis of nanoparticles with different size, shape and surface compositions have emerged30. At present, there are microfluidic systems capable of characterizing the nanoparticle size and stability following synthesis in a single platform31,32. Moreover, large numbers of distinct nanoparticles can be obtained through combinatorial synthesis33, and production rates of identical nanoparticles can be increased through parallelization or re-design of the devices34,35. Similar to high-throughput synthesis of libraries of small molecules, these advantages could potentially enable screening and optimization of libraries of nanoparticles with distinct properties. One of the challenges in gene therapy, for example, is finding the right formulation for delivering nucleic acids to specific sites in the body. By mixing different precursors at varying ratios, microfluidic systems have enabled a one-step combinatorial synthesis of libraries of polymeric and lipid nanoparticles that encapsulate DNA and small-interfering RNA, respectively. Screening these libraries of nanoparticles has helped identify superior formulations for gene transfection, compared with conventional transfection agents such as lipofectamine 2000. Furthermore, using this method, potent lipid-based small-interfering RNA formulations for in vivo delivery to the liver have also been discovered33,34.
Evaluation and screening of nanoparticles
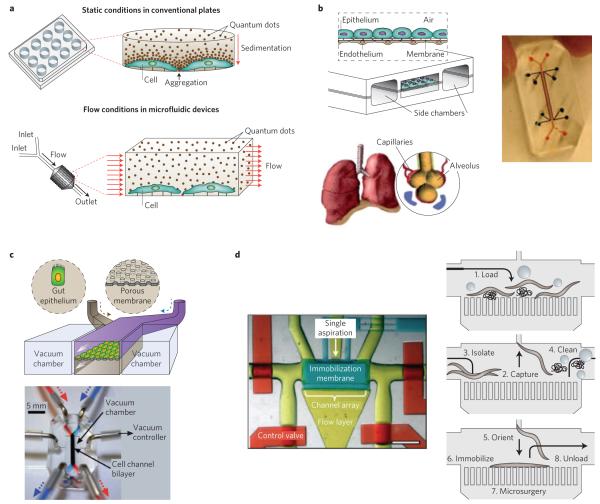
Another challenge in nanoparticle development is the lack of in vitro models capable of predicting in vivo behaviour36. Conventionally, nanoparticles are evaluated in vitro using cells cultured in well plates, which does not capture the complexity of nanoparticle–cell interactions in vivo. For instance, a recent study showed that sedimentation of gold nanoparticles in well plates could lead to misinterpretation of results, such as increased nanoparticle uptake37. Microfluidics provides significant advantages over conventional methods for cell and tissue culture by displaying structures and networks at relevant physiological length scales, and by incorporating fluid flow and mechanical forces that bring the cell-based assays a step closer to mimicking the in vivo microenvironment38 (Fig. 3a). This is especially advantageous, for instance, when investigating the toxic effects related to the cell uptake of nanoparticles. A recently developed microfluidic system for evaluating QD toxicity on mouse fibroblasts revealed increased cell viability under flow conditions compared with static incubation, possibly due to the absence of QD sedimentation39.
Figure 3. Microfluidic systems for in vitro evaluation and screening of nanoparticles.
a, Schematic of nanoparticle sedimentation in conventional plates, which could result in misinterpretation of results. In contrast, flow conditions in microfluidics provide a more-accurate method for evaluating nanoparticles in vitro. b, Left: schematic of the lung-on-a-chip that reconstitutes the critical functional alveolar-capillary interface of the human lung through a stretchable membrane containing an epithelium layer on one side and an endothelium layer on the other. Right: photograph of actual device. c, Top: schematic of the gut-on-a-chip made by flexible, porous, extracellular matrix-coated membrane lined by gut epithelial cells. The blue and brown arrows indicate two different streams of culture medium separated by a membrane, entering the channel from the top and bottom, respectively. Bottom: photograph of the gut-on-a-chip device made of polydimethylsiloxane elastomer. A syringe pump was used to perfuse dyes (red and blue) for channel visualization. d, Left: photograph of a dye-filled microfluidic system designed to handle C. elegans worms. Red, control valve layer; yellow, flow layer; blue, immobilization layer. Scale bar, 1 mm. Right: schematic showing load, capture, orient, immobilization and unload of the worm. Figure reproduced with permission from: a, ref. 39, © 2010 AIP; b, ref. 41, © 2010 AAAS; c, ref. 42, © 2012 RSC; d, ref. 48, © 2010 NAS.
Recently, researchers have focused on developing biomimetic microfluidic technologies capable of portraying organ-level functions on a chip, such as those observed in the lung, liver and kidneys, among others38,40. For instance, microfluidic systems that reconstitute the critical functional alveolar-capillary liquid/air interface of the human lung have been recently fabricated by growing alveolar epithelial cells and microvascular endothelial cells on different sides of a perforated silicone membrane. The membrane was pneumatically actuated to mimic the physiological expansion–contraction motion due to breathing. It was found that cyclic mechanical strain accentuates toxic and inflammatory responses in the lung when exposed to silica nanoparticles, which could not have been observed with other conventional in vitro systems41 (Fig. 3b). Using a similar design approach, a ‘gut-on-a-chip’ was developed by coating both sides of a membrane separating two microfluidic devices with extracellular matrix and lined by human intestinal epithelial cells. It was demonstrated that by subjecting the membrane to flow and cyclic strains similar to those encountered in the gut, villi-like structures were formed and the co-culture of the intestinal microbes was made possible42 (Fig. 3c).
Expansion of these technologies to other organs, for instance ‘liver-on-a-chip’43, could lead to platforms for evaluating and screening nanoparticle toxicity in organs where nanoparticles tend to accumulate and toxicity is likely to be a major concern (for example, liver, spleen and kidney). Although nanoparticles would still need to be evaluated in animals, such microfluidic systems could take in vitro nanoparticle screening to a new level of utility by selecting promising candidates with higher probabilities of success from a large pool of nanoparticle formulations, and eliminating those that would otherwise have failed in larger animal studies. Furthermore, coupling these technologies with microfluidic devices used for nanoparticle synthesis opens the possibility of rapid combinatorial screening of a large number of different nanoparticles under various conditions (for example, concentrations, pH values, under the presence of specific proteins and so on).
Nanoparticles exhibiting promising results in vitro are subsequently evaluated in vivo, which is considerably more expensive and resource intensive, especially in non-human primates. Although most of the parameters, such as pharmacokinetics, biodistribution and efficacy, are evaluated in mice and larger animals, tracking physiological effects of nanoparticles on animal development could potentially be obtained using a large number of smaller organisms. The zebrafish and Caenorhabditis elegans worms are well-known models for studying fundamental mechanisms and progression of human diseases, and for drug screening44. For example, the zebrafish was recently used as an in vivo model to develop a hazard ranking for engineered nanoparticles based on their impact on mortality rate and morphological defects in zebrafish embryos exposed to these materials45. However, current methods for manipulating these organisms generally suffer from low throughput, low automation and imprecise delivery of external stimuli46. To solve these challenges, engineered microfluidic systems with dimensions comparable to small organisms and containing valves and suction points have been developed. These systems enable precise manipulation of these organisms with respect to placement and orientation for high-throughput screening46,47 (Fig. 3d). Other microfluidic systems are being developed that are capable of imaging dynamic cellular processes in small organisms, such as cell division and migration, degeneration, aging and regeneration48. With such technologies in place, it might be possible to use real-time microscopy to track physiological responses to fluorescently labelled nanotherapeutics and nano-imaging agents, as well as assess the distribution and efficacy of nanoparticles at both the organ and body level. Furthermore, real-time tracking of nanoparticle-induced toxicity at different concentrations and conditions in small organisms could enable rapid selection of nanoparticles (especially those made with novel synthetic materials) that are more likely to be non-toxic in larger animals.
Future prospects
At present, the field of microfluidics applied to nanomedicine is still in its infancy. Although nanoparticles have a relatively small footprint in the pharmaceutical industry, it is anticipated that as these products bring in revenue, industry-led research and development efforts would probably adopt technologies, such as microfluidics, to accelerate their development. Nevertheless, microfluidic technologies, such as organ-on-a-chip and small-animal screening, are likely to be adopted first for the screening of small-molecule drug candidates, where the need for such tools is evident.
There are a few key directions at the intersection of microfluidics and nanomedicine that are likely to be pursued in the near future (Table 1). Although the quantity of nanoparticles synthesized by microfluidic devices is often in the micro- to milligram range, parallel and stackable microfluidic systems could continuously produce nanoparticles on the gram to kilogram scale with the same properties as those prepared at the bench scale. Similarly, the use of microfluidic platform technologies to reproducibly synthesize and screen libraries of nanoparticles with different chemical compositions and/or physical and chemical properties could potentially advance nanoparticle discovery analogously to how the high-throughput screening of small molecules in medicinal chemistry advanced small-molecule discovery. With respect to the design and development of novel nanoparticle constructs, the use of microfluidics could enable the synthesis of nanoparticles with properties not accessible by conventional synthesis, similar to what has already occurred for microparticle synthesis49.
Table 1.
Advantages, disadvantages/challenges, stage of development and potential impact of microfluidic systems on different steps in the clinical translation of nanoparticles.
| Advantages | Disadvantages/challenges | Stage of development | Potential impact | |
|---|---|---|---|---|
| Synthesis |
|
|
***** | Rapid combinatorial, controlled and reproducible synthesis of libraries of distinct nanoparticles for a specific application, and/or reference nanoparticles for toxicology studies |
| Characterization |
|
|
* | In-line rapid characterization and optimization of nanoparticles |
| In vitro |
|
|
**** | High-throughput studies of nanoparticle toxicity, efficacy, tumour penetration and organ distribution, using ‘organ-on- a-chip’ systems |
| In vivo |
|
|
** | Real-time tracking of the distribution or toxicity of nanoparticles on small-scale organisms |
| Large-scale synthesis |
|
|
*** | Synthesis of nanoparticles for human administration using stackable parallel microfluidic units |
Rank: Most advanced in development
to least advanced in development, based on the amount of research carried out on each category, as well as the potential ease of adoption by industry.
Another avenue of future research will be the integration of different steps of nanoparticle development into a single system (for example, nanoparticle synthesis characterization and evaluation), together with feedback control through a combination of microfluidics, robotics and automation, thus significantly cutting the time and cost of nanoparticle development. Finally, mass-produced microfluidic devices and well-defined nanoparticle precursors can aid in the synthesis of identical batches of nanoparticles with little to no variations introduced by user handling. This could lead to the use and commercialization of ‘nanoparticle synthesis kits’ composed of calibrated devices that can reproducibly synthesize a specific class of nanoparticle with well-defined properties for use as standards in conventional toxicological assays. Considering the large number of nanoparticles being made of novel synthetic materials or of unusual shapes, such standardization would be highly useful for regulatory purposes, among others.
Microfluidic technologies are capable of accelerating the discovery and translation of nanoparticles, and could serve as a tool for nanotherapeutics to reach a similar ‘tipping point’ reached by genome sequencing in the past decade after high-throughput sequencing technologies were developed50. Among all the microfluidic technologies, those developed for synthesis and in vitro screening of nanoparticles have the highest probability of making an impact in the near future (Table 1). Specifically, microfluidic synthesis may be adopted as a second-generation manufacturing technology after the initial success of US Food and Drug Administration-approved nanoparticles in cases where the advantages of microfluidic synthesis are significant. Microfluidic synthesis may also be adopted as a screening tool to identify optimal nanoparticles in academic and industrial research laboratories. Alternatively, the impact of microfluidics might be observable in the medium- to long-term future for nanoparticle characterization and in vivo evaluation. For nanoparticle characterization, the use of microfluidics would probably increase once more-advanced technologies are developed to characterize several nanoparticle properties (for example, size, charge, surface composition and stability) in a single system. Similarly, in vivo evaluation of nanoparticles in microfluidics would probably mature once both easily adoptable microfluidic systems for manipulating small organisms and methods for translating data obtained from these organisms to larger animals are developed.
Overall, the use of microfluidic technologies in nanomedicine brings exciting opportunities to expand the body of knowledge in the field, advance the clinical translation of nano-based therapeutics and imaging agents, and demonstrate innovative ways to develop other classes of drugs.
Acknowledgements
This work was supported by the Koch-Prostate Cancer Foundation Award in Nanotherapeutics (R.L. and O.C.F.), the National Cancer Institute Center of Cancer Nanotechnology Excellence at MIT-Harvard (U54-CA151884, R.L. and O.C.F.), and the National Heart, Lung, and Blood Institute Programs of Excellence in Nanotechnology (HHSN268201000045C; R.L. and O.C.F.). P.M.V. is supported by the National Science Foundation graduate research fellowship. We thank B. Timko and F. Karim for assistance in drafting Figs 1 and 2, respectively. We also thank A. Radovic-Moreno, C. Alabi and E. Pridgen for their insightful comments.
Footnotes
Additional information The authors declare competing financial interests: O.C.F. and R.L. disclose financial interest in BIND Biosciences and Selecta Biosciences, two biotechnology companies developing nanoparticle technologies for medical applications. BIND and Selecta did not support the aforementioned work, and at present these companies have no rights to any technology or intellectual property developed as part of this work.
Reprints and permissions information is available online at www.nature.com/reprints.
References
- 1.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nature Rev. Drug. Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 2.Gregoriadis G. Drug entrapment in liposomes. FEBS Lett. 1973;36:292–296. doi: 10.1016/0014-5793(73)80394-1. [DOI] [PubMed] [Google Scholar]
- 3.Hrkach J, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 2012;4:128ra39. doi: 10.1126/scitranslmed.3003651. This article describes the translation of the first targeted polymeric nanoparticle for drug delivery from discovery to clinical trials.
- 4.Qiao R, Yang C, Gao M. Superparamagnetic iron oxide nanoparticles: from preparations to in vivo MRI applications. J. Mater. Chem. 2009;19:6274–6293. [Google Scholar]
- 5.Haun JB, et al. Micro-NMR for rapid molecular analysis of human tumor samples. Sci. Transl. Med. 2011;3:71ra16. doi: 10.1126/scitranslmed.3002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim BY, Rutka JT, Chan WC. Nanomedicine. N. Engl. J. Med. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 7.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotech. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 9.Barreto JA, et al. Nanomaterials: applications in cancer imaging and therapy. Adv. Mater. 2011;23:H18–H40. doi: 10.1002/adma.201100140. [DOI] [PubMed] [Google Scholar]
- 10.Shi J, Xiao Z, Kamaly N, Farokhzad OC. Self-assembled targeted nanoparticles: evolution of technologies and bench to bedside translation. Acc. Chem. Res. 2011;44:1123–1134. doi: 10.1021/ar200054n. [DOI] [PubMed] [Google Scholar]
- 11.Murday JS, Siegel RW, Stein J, Wright JF. Translational nanomedicine: status assessment and opportunities. Nanomedicine. 2009;5:251–273. doi: 10.1016/j.nano.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Chou LY, Ming K, Chan WC. Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 13.Nel AE, et al. Understanding biophysicochemical interactions at the nano–bio interface. Nature Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 14.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. An excellent classic review on the present and future of microfluidics by one of the fathers of the field, George Whitesides.
- 15.DeMello AJ. Control and detection of chemical reactions in microfluidic systems. Nature. 2006;442:394–402. doi: 10.1038/nature05062. [DOI] [PubMed] [Google Scholar]
- 16.Johnson BK, Prud’homme RK. Mechanism for rapid self-assembly of block copolymer nanoparticles. Phys. Rev. Lett. 2003;91:118302. doi: 10.1103/PhysRevLett.91.118302. This article describes the mechanism of nanoparticle self-assembly and explains how rapid mixing is key in controlling nanoparticle size.
- 17.Chen T, Hynninen AP, Prud’homme RK, Kevrekidis IG, Panagiotopoulos AZ. Coarse-grained simulations of rapid assembly kinetics for polystyrene-b-poly(ethylene oxide) copolymers in aqueous solutions. J. Phys. Chem. B. 2008;112:16357–16366. doi: 10.1021/jp805826a. [DOI] [PubMed] [Google Scholar]
- 18.Karnik R, et al. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008;8:2906–2912. doi: 10.1021/nl801736q. [DOI] [PubMed] [Google Scholar]
- 19.Capretto L, Cheng W, Hill M, Zhang X. Micromixing within microfluidic devices. Top. Curr. Chem. 2011;304:27–68. doi: 10.1007/128_2011_150. [DOI] [PubMed] [Google Scholar]
- 20.Rhee M, et al. Synthesis of size-tunable polymeric nanoparticles enabled by 3D hydrodynamic flow focusing in single-layer microchannels. Adv. Mater. 2011;23:H79–H83. doi: 10.1002/adma.201004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu K, et al. A digital microfluidic droplet generator produces self-assembled supramolecular nanoparticles for targeted cell imaging. Nanotechnology. 2010;21:445603. doi: 10.1088/0957-4484/21/44/445603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valencia PM, et al. Single-step assembly of homogenous lipid-polymeric and lipid-quantum dot nanoparticles enabled by microfluidic rapid mixing. ACS Nano. 2010;4:1671–1679. doi: 10.1021/nn901433u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahn A, et al. Preparation of nanoparticles by continuous-flow microfluidics. J. Nanopart. Res. 2008;10:925–934. [Google Scholar]
- 24.Besson C, Finney EE, Finke RG. A mechanism for transition-metal nanoparticle self-assembly. J. Am. Chem. Soc. 2005;127:8179–8184. doi: 10.1021/ja0504439. [DOI] [PubMed] [Google Scholar]
- 25.Song Y, Hormes J, Kumar CS. Microfluidic synthesis of nanomaterials. Small. 2008;4:698–711. doi: 10.1002/smll.200701029. [DOI] [PubMed] [Google Scholar]
- 26.Gu FX, et al. Targeted nanoparticles for cancer therapy. Nano Today. 2007;2:14–21. [Google Scholar]
- 27.Shestopalov I, Tice JD, Ismagilov RF. Multi-step synthesis of nanoparticles performed on millisecond time scale in a microfluidic droplet-based system. Lab Chip. 2004;4:316–321. doi: 10.1039/b403378g. [DOI] [PubMed] [Google Scholar]
- 28.Kikkeri R, Laurino P, Odedra A, Seeberger PH. Synthesis of carbohydrate-functionalized quantum dots in microreactors. Angew. Chem. Int. Ed. 2010;49:2054–2057. doi: 10.1002/anie.200905053. [DOI] [PubMed] [Google Scholar]
- 29.Marre S, Jensen KF. Synthesis of micro and nanostructures in microfluidic systems. Chem. Soc. Rev. 2010;39:1183–1202. doi: 10.1039/b821324k. [DOI] [PubMed] [Google Scholar]
- 30.Zhao CX, He LZ, Qiao SZ, Middelberg APJ. Nanoparticle synthesis in microreactors. Chem. Eng. Sci. 2011;66:1463–1479. [Google Scholar]
- 31.Fraikin JL, Teesalu T, McKenney CM, Ruoslahti E, Cleland AN. A high-throughput label-free nanoparticle analyser. Nature Nanotech. 2011;6:308–313. doi: 10.1038/nnano.2011.24. [DOI] [PubMed] [Google Scholar]
- 32.Birnbaumer G, et al. Rapid liposome quality assessment using a lab-on-a-chip. Lab Chip. 2011;11:2753–2762. doi: 10.1039/c0lc00589d. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, et al. A rapid pathway toward a superb gene delivery system: programming structural and functional diversity into a supramolecular nanoparticle library. ACS Nano. 2010;4:6235–6243. doi: 10.1021/nn101908e. This article is one of the first examples that exploit microfluidic systems for rapid combinatorial synthesis of nanoparticles with a variety of physical and chemical properties.
- 34.Chen D, et al. Rapid discovery of potent siRNA-lipid-nanoparticles enabled by controlled microfluidic formulation. J. Am. Chem. Soc. 2012;134:6948–6951. doi: 10.1021/ja301621z. [DOI] [PubMed] [Google Scholar]
- 35.Kim Y, et al. Mass production and size control of lipid-polymer hybrid nanoparticles through controlled microvortices. Nano Lett. 2012;12:3587–3591. doi: 10.1021/nl301253v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobrovolskaia MA, Germolec DR, Weaver JL. Evaluation of nanoparticle immunotoxicity. Nature Nanotech. 2009;4:411–414. doi: 10.1038/nnano.2009.175. [DOI] [PubMed] [Google Scholar]
- 37.Cho EC, Zhang Q, Xia Y. The effect of sedimentation and diffusion on cellular uptake of gold nanoparticles. Nature Nanotech. 2011;6:385–391. doi: 10.1038/nnano.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziolkowska K, Kwapiszewski R, Brzozka Z. Microfluidic devices as tools for mimicking the in vivo environment. New J. Chem. 2011;35:979–990. [Google Scholar]
- 39.Mahto SK, Yoon TH, Rhee SW. A new perspective on in vitro assessment method for evaluating quantum dot toxicity by using microfluidics technology. Biomicrofluidics. 2010;4:034111. doi: 10.1063/1.3486610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE. Microengineered physiological biomimicry: organs-on-chips. Lab Chip. 2012;12:2156–2164. doi: 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- 41.Huh D, et al. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. This article describes the design and assembly of a microfluidic system that recreates the alveolar-endothelial interface in lungs.
- 42.Kim HJ, Huh D, Hamilton G, Ingber DE. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12:2165–2174. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- 43.Toh YC, et al. A microfluidic 3D hepatocyte chip for drug toxicity testing. Lab Chip. 2009;9:2026–2035. doi: 10.1039/b900912d. [DOI] [PubMed] [Google Scholar]
- 44.Crane MM, Chung K, Stirman J, Lu H. Microfluidics-enabled phenotyping, imaging, and screening of multicellular organisms. Lab Chip. 2010;10:1509–1517. doi: 10.1039/b927258e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.George S, et al. Use of a high-throughput screening approach coupled with in vivo zebrafish embryo screening to develop hazard ranking for engineered nanomaterials. ACS Nano. 2011;5:1805–1817. doi: 10.1021/nn102734s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi W, Wen H, Lin B, Qin J. Microfluidic platform for the study of Caenorhabditis elegans. Top. Curr. Chem. 2011;304:323–338. doi: 10.1007/128_2011_145. [DOI] [PubMed] [Google Scholar]
- 47.Baker M. Screening: the age of fishes. Nature Meth. 2011;8:47–51. doi: 10.1038/nmeth0111-47. [DOI] [PubMed] [Google Scholar]
- 48.Samara C, et al. Large-scale in vivo femtosecond laser neurosurgery screen reveals small-molecule enhancer of regeneration. Proc. Natl Acad. Sci. USA. 2010;107:18342–18347. doi: 10.1073/pnas.1005372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dendukuri D, Doyle PS. The synthesis and assembly of polymeric microparticles using microfluidics. Adv. Mater. 2009;21:4071–4086. [Google Scholar]
- 50.Zhao J, Grant SF. Advances in whole genome sequencing technology. Curr. Pharm. Biotechnol. 2011;12:293–305. doi: 10.2174/138920111794295729. [DOI] [PubMed] [Google Scholar]