Abstract
Drought stress is a major factor limiting symbiotic nitrogen fixation (NF) in soybean crop production. However, the regulatory mechanisms involved in this inhibition are still controversial. Soybean plants were symbiotically grown in a split-root system (SRS), which allowed for half of the root system to be irrigated at field capacity while the other half remained water deprived. NF declined in the water-deprived root system while nitrogenase activity was maintained at control values in the well-watered half. Concomitantly, amino acids and ureides accumulated in the water-deprived belowground organs regardless of transpiration rates. Ureide accumulation was found to be related to the decline in their degradation activities rather than increased biosynthesis. Finally, proteomic analysis suggests that plant carbon metabolism, protein synthesis, amino acid metabolism, and cell growth are among the processes most altered in soybean nodules under drought stress. Results presented here support the hypothesis of a local regulation of NF taking place in soybean and downplay the role of ureides in the inhibition of NF.
Key words: Drought, local regulation, N-feedback inhibition, nitrogen fixation, proteomics, soybean, ureides.
Introduction
Legumes are the second most important crop for humans, accounting for 27% of the world’s primary crop production (Graham and Vance, 2003). One of the main characteristics of legumes is their ability to establish symbiotic relations with N2-fixing soil bacteria and as a result, a new organ is formed, the root nodule, where symbiotic nitrogen fixation (NF) is carried out. Soybean, the third most cultivated crop worldwide (Stacey et al., 2004), is a particularly important source of proteins both for human nutrition and animal feed (Graham and Vance, 2003).
Despite the agronomical and environmental advantages of the cultivation of legumes, their production is limited by environmental constraints, particularly drought (Sprent, 2001). The regulation of NF under drought involves diverse factors, namely, internal oxygen availability, carbon limitation, and N-feedback regulation. Despite recent progress in the field, the interactions of the above factors and the regulation of NF at the molecular level are not yet fully understood. An N-feedback inhibition of NF as the possible cause for the decline of nitrogenase activity has been suggested (Serraj et al., 1999, 2001; Vadez et al., 2000; King and Purcell, 2005). This hypothesis is based on the observed accumulation of ureides in soybean leaves (Serraj and Sinclair, 1996; de Silva et al., 1996; Purcell et al., 1998; Serraj et al., 1999) and nodules (Sinclair and Serraj, 1995; Gordon et al., 1997; Serraj et al., 1999; Vadez et al., 2000; King and Purcell, 2005; Ladrera et al., 2007) under different water-deficit treatments. Together with ureides, other N compounds have been proposed to be the ‘signal’ molecules triggering the inhibition of NF of soybean during drought, such as Asn (Serraj et al., 1999; Vadez et al., 2000) and Asp (King and Purcell, 2005; Purcell et al., 2000).
Previous studies have suggested that a local regulation of NF is likely to occur in soybean under drought stress (King and Purcell, 2005; Ladrera et al., 2007), in agreement with results in pea (Marino et al., 2007) and the model legume Medicago truncatula (Gil-Quintana et al., 2013); however, this hypothesis has not been formally tested in the ureide-exporter soybean.
The current work applied a split-root system (SRS) to specifically differentiate between local and systemic responses of nodulated soybean plants subjected to a progressive water deficit. It monitored the physiological responses to drought, analysed the variations in the content of amino acids and ureides in different plant organs, and measured the levels of ureide metabolism enzymatic activities in nodules. To shed further light into the origin of this regulation, the nodule proteome of partial drought plants has been compared. Results presented here support the hypothesis of a local regulation of NF taking place in soybean and downplay the role of ureides in the inhibition of NF.
Materials and methods
Plant growth conditions, SRS, and drought stress treatments
Glycine max (L.) Merr. cv. oxumi seeds were sterilized as pre viously described (Labhilili et al., 1995). After washing, seeds were germinated on trays with a mixture of perlite/vermiculite (1:1, v/v) for 3 days in darkness at 26 °C and subsequently transferred to a controlled environmental chamber for 7 days under the conditions described below.
To set up the SRS, the root tips of 10-day old plantlets were longitudinally cut to obtain two equal parts of 2–3cm length, similarly to the procedure followed by Marino et al. (2007). Plantlets were subsequently planted in a double pot (2×600ml) with a substrate mixture of perlite/vermiculite (1:2, v/v). For inoculation, Bradyrhizobium japonicum UPM752 was grown for 5 days at 27 °C and 150rpm on an orbital shaker using yeast extract-mannitol medium containing (l−1): 0.5g K2HPO4, 0.2g MgSO4.7H2O, 0.1g NaCl, 10g mannitol, and 0.4g yeast extract. Plantlets were inoculated with 1ml Bradyrhizobium japonicum liquid culture on the day of planting and 3 days after the first inoculation. Plants placed in this SRS were grown for 6 weeks in a controlled environmental chamber under the following conditions: 16/8 light/dark cycle, 450 μmol m–2 s–1 light intensity, 22/18 °C, and 60–70% relative humidity. Plants were watered three times a week with nutrient solution lacking nitrogen (Rigaud and Puppo, 1975).
Six-week-old plants were randomly separated into three sets: controls (C) were daily supplied with nutrient solution to field capacity in both sides of the SRS, whereas drought (D) was achieved by withholding water/nutrients. For partial drought (PD), one half of the root system was kept at field capacity (PDC) while the other half was kept unwatered (PDD) for 2, 4, or 7 days (Fig. 1A). Four types of nodule and root samples (C, PDC, PDD, and D) and three types of leaf samples (C, PD, and D) were collected at each time point.
Fig. 1.
(A) Schematic representation of the split-root experimental system. (B–E) Effect of partial drought on leaf water potential (B), nodule water potential (C), stomatal conductance (D), and evapotranspiration (E). In B, D, and E, PD denotes partial drought and in (C) both root parts of PD are represented independently: PDC denotes the watered side and PDD the unwatered fraction. Asterisks indicate significant differences (Student’s t-test, P ≤ 0.05) between D and C treatments; hash indicates significant differences between PDD and C plants. Values are mean ± SE (n = 3 for B–D; n = 9 for E) (this figure is available in colour at JXB online).
For measurements of total amino acid and ureide metabolism activities, non-SRS experiments were carried out. Plants were grown in single 1-l pots either under symbiotic conditions or supplied with 5mM KNO3 under the conditions described above and samples from C and D plants were collected and physiologically characterized.
In all cases, leaf, root, and nodule samples were harvested, immediately frozen in liquid nitrogen, and stored at −80 °C for analytical determinations. For dry weight (DW) determination, plant tissue was desiccated for 48h at 80 °C.
Physiological characterization
Evapotranspiration was gravimetrically determined on a daily basis. Leaf water potential (Ψleaf) was measured in the first fully expanded leaf 2h after the beginning of the photoperiod using a pressure chamber (Soil Moisture Equipment, Santa Barbara, CA, USA) as earlier described (Scholander et al., 1965). Water potential of detached nodules (Ψnodule) was measured in C52 sample chambers coupled to a Wescor HR-33T Dew Point Microvoltmeter (Wescor, Logan, UT, USA). Approximately four nodules per treatment were collected and confined in each chamber for at least 1h until temperature and vapour equilibration was reached.
Stomatal conductance was measured in the youngest fully expanded leaf with an AP4 porometer (Delta-T Devices, Cambridge, UK).
NF was measured as apparent nitrogenase activity (ANA). H2 evolution from sealed roots systems was measured in an open flow-through system under N2/O2 (79:21, v/v) using an electrochemical H2 sensor (Qubit System, Canada) as previously described (Marino et al., 2007).
Ureide and amino acid determination
For ureide determination, dried and ground leaf, stem, root, and nodule aliquots (5–10mg) were homogenized with 1ml 0.2M NaOH. The homogenates were placed in a boiling water bath for 30min. After cooling on ice, homogenates were centrifuged at 21,000 g for 20min. The supernatants were filtered in PVDF 0.22 μm filters. In this method, allantoin is transformed to allantoate by an alkaline hydrolysis and determined using high-performance capillary electrophoresis (P/ACE 5500; Beckman Coulter Instruments, Fullerton, CA, USA) as described in Sato et al. (1998). A fused-silica capillary (length 60 cm) was employed and 0.1 M Na2B4O7·10H2O (pH 9.2) in 25 mL L-1 OFMAnion BT (Waters) was used as electrolyte.
Amino acid determination was carried out using capillary electrophoresis as previously described (Gil-Quintana et al., 2013).
Determination of ureide metabolism enzyme activities
Frozen plant aliquots were ground to a fine powder under liquid nitrogen and 4ml extraction buffer per g of tissue were added. Urate oxidase activity was determined by the decrease in absorbance at 292nm due to the aerobic oxidation of urate, as described (Pineda et al., 1984). Allantoate-degrading activity was determined by a colorimetric assay based on the determination of glyoxylate as described in Raso et al. (2007) with minor modifications. Plant extracts were obtained by adding 5ml of 50mM triethanolamine-NaOH (pH 7.0), 1mM MnSO4, and 0.15% (w/v) sodium deoxycholate per g of tissue. The reaction was carried out at 35 °C and started by the addition of the enzymic extract. Controls to account for non-enzymic hydrolysis of allantoate were carried out alongside the assays. The reaction mixture was 50mM triethanolamine-NaOH pH 7.0, 6mM potassium allantoate, 1mM MnSO4, 0.7mM phenylhydrazine, and crude extract. One unit of enzymic activity is the amount of enzyme that catalyses the transformation of one μmol of substrate min–1. Results of enzymic activity are given as mU (g DW)–1.
Proteomic analysis
Frozen nodules (0.1g fresh weight) were homogenized in a mortar and pestle with 2ml extraction buffer (50mM HEPES pH 7.5, 1mM EDTA, 1mM KCl, 2mM MgCl2, 2.5% (w/v) PVP, 1mM PMSF). Homogenates were centrifuged at 20,000 g at 4 °C for 30min. Supernatants were collected and soluble proteins were precipitated overnight at –20 °C after adding five volumes of pre-cooled acetone. Pellets recovered by centrifugation at 10,000 g at 4 °C for 10min were air dried and resuspended in 300 μl solubilization buffer (8M urea buffer, 100mM NH4HCO3 pH 8–8.5, 5mM DTT).
Aliquots containing 100 μg protein were digested for 5h at 30 °C with sequencing-grade endoproteinase Lys-C (1:100, v/v, Roche Applied Science, Spain). Samples were then diluted 1:4 in buffer containing 25mM NH4HCO3 (pH 8–8.5), 10% (v/v) acetonitrile, and 5mM CaCl2 and further digested overnight at 37 °C under rotation using Poroszyme immobilized trypsin beads (1:20, v/v, Applied Biosystems, Life Technologies, Spain). After centrifugation for bead removal, the obtained peptide mixtures were desalted using SPEC C18 columns according to the manufacturer’s instructions (Varian, Agilent Technologies). Finally, desalted digest solutions were dried and pellets were stored at –80 °C until use.
Prior to the mass spectrometric measurement, protein digest pellets were dissolved in 0.1% (v/v) formic acid. Protein digests (5 μg) were analysed via shotgun nano-LC-ultra (Eksigent System, Axel Semrau, Germany) using a monolithic reversed-phase column (Chromolith 150×0.1mm, Merck, Darmstadt, Germany) directly coupled to an Orbitrap XL mass spectrometer (Thermo Scientific, Rockford, IL, USA), as described elsewhere (Larrainzar et al., 2007). Peptides were eluted with a 100-min gradient from 5 to 60% acetonitrile. Dynamic exclusion settings were as described in (Hoehenwarter and Wienkoop, 2010).
After mass spectrometric analysis, raw files were searched against the most recent database available using the Sequest algorithm. For protein identification, the Soybean Gene Index release 15 database, downloaded from the Plant Gene Index Project, was employed using the Proteome Discoverer 1.2 software (Thermo Electron, San Jose, CA, USA). Protein and peptide information can be found in the ProMEX DB under the experimental ID ‘Gly max 001’ (Wienkoop et al., 2012).
A decoy database enabled false-positive rate analysis. Only high-confidence peptides (false-positive rate <0.1%) with better than 5 ppm precursor mass accuracy and at least two distinct peptides per protein passed criteria. This led to a data matrix including the spectral count for relative quantification, generated by the Proteome Discoverer. Spectral count is a semi-quantitative measure for tracking changes in protein abundance in complex samples, based on the cumulative sum of ion fragments that are recorded in a MS/MS spectrum (Liu et al., 2004).
Statistical analysis
For physiological measurements the normal distribution of the samples was checked via Shapiro–Wilk tests and the homogeneity of variances via Levene’s test. Significant differences between treatments were determined using one-way ANOVA. If significant di fferences between means were obtained, then comparisons between each treatment and its control were performed using LSD test. Differences were considered to be significant at P ≤ 0.05.
For the proteomic data, relative changes in protein abundance were calculated. Using the average spectral count values from five biological replicates, the log2 ratios (treatment/control) were calculated. Proteins showing more than a 2-fold change in relative abundance and differences (Student’s t-test, P ≤ 0.05) between treatments were considered as significantly changing in the study.
Results
Physiological characterization of drought using SRS
To test whether the drought-induced inhibition of NF in soybean occurred at the local or systemic level, SRS-based experiments were conducted. Changes occurring in the untreated part of the root system would correspond to systemic signalling responses (mediated by signals in shoots), whereas no changes in the untreated fraction of the root system would imply a local regulation mechanism. Plants in this experimental set-up were assigned to the following categories: controls (C), plants watered to field capacity; partial drought (PD), in which one half of the root system was kept well watered (partial-drought control, PDC) while the other one remained unwatered (partial-drought drought, PDD); and drought plants (D). A schematic representation of the experimental set-up is shown in Fig. 1A.
To physiologically characterize the water status of gradually water-deprived SRS soybean plants, diverse parameters were measured (Fig. 1B–E). Leaf water potential (Fig. 1B) values for C and PD leaves remained constant over the course of the experiment (–0.3±0.02MPa), while a significant decrease was measured in D treatment on day 7 (–1.1±0.08MPa). Similarly, Ψnodule (Fig. 1C) presented steady values in C and PDC treatments, whereas a significant decrease was observed in D nodules as early as day 4. PDD showed a reduction of Ψnodule (–30%) compared to C nodules on day 7 of drought.
Stomatal conductance values remained constant (0.48cm s–1) over the time course in C plants, whereas leaves of D plants showed values close to zero 2 days after the onset of drought (Fig. 1D). In PD plants, the reduction in stomatal conduc tance was more progressive, reaching values of 0.25cm s–1.
Evapotranspiration was evaluated daily over the time course of the experiment (Fig. 1E). During the first 3 days, evapotranspiration rates were around 110g H2O plant–1 day–1. Afterwards, C plants increased slightly their evapotranspiration up to 139g H2O plant–1 day–1 on the last day, while evapotranspiration in D declined from day 4, reaching values 69% lower than C plants. Similarly to the trend observed for stomatal conductance, PD plants showed a moderate decline in evapotranspiration starting on day 4 (23% lower compared to C plants).
ANA was measured as an estimation of NF rates (Fig. 2). Drought stress caused a progressive inhibition of NF both in D plants and root systems subjected to drought (PDD). This decline was more dramatic in the former, with an almost complete inhibition at the most severe drought stage. In contrast, the PDC root systems presented similar ANA values to those of C plants over the study period.
Fig. 2.
Effect of partial drought on nitrogen fixation. Legend as for Fig. 1C. NDW, nodule dry weight. Asterisks indicate significant differences (Student’s t-test, P ≤ 0.05) between D and C treatments; hash indicates significant differences between PDD and C plants. Values are mean ± SE (n = 6).
Amino acid distribution under partial drought
Two types of amino acid determinations were performed: total amino acid content (Supplementary Fig. S1, available at JXB online) and amino acid profiling (Fig. 3). Under C conditions, the level of total amino acids remained stable over the study period in all tissues tested (Supplementary Fig. S1). Drought provoked a progressive accumulation of amino acids in nodules, which was mirrored by a delay in roots (Supplementary Fig. S1).
Fig. 3.
Effect of partial drought on nodule single amino-acid content (µmol g-1 DW). Legend as for Fig. 1C. Asterisks indicate significant differences (Student’s t-test, P ≤ 0.05) between D and C treatments; hash indicates significant differences between PDD and C plants. Values represent mean ± SE (n = 3).
Regarding the amino acid profiling, drought induced a generalized accumulation of most amino acids in D nodules, with the exception of Tyr and Asp (Fig. 3). For most amino acids, the increase was already significant on day 4. Pro showed the highest accumulation at day 7, with a 96-fold increase compared to C nodules, followed by Ile, Leu, Trp, and Val with more than 5-fold increases. Asn and Glu, the most abundant amino acids in soybean nodules, accumulated progressively as the drought level increased.
In PDD nodules, the content of certain amino acids significantly increased on day 4, including Phe, Ala, Arg, Thr, and Met. In contrast to D nodules, Asn and Glu did not accumulate significantly. Unlike PDD, amino acid levels in PDC nodules were maintained at C levels over the study period.
Drought-induced changes in ureide distribution
The total content of ureide (allantoin and allantoate) was determined in leaves, stems, roots, and nodules of PD plants (Fig. 4). Both C plants and the well-watered root system fraction (PDC) maintained root and nodule ureide levels relatively constant during the experiment (Fig. 4C, D). However, root systems subjected to D or PDD experienced an accumulation of ureides in root and nodule tissue. It is remarkable that the accumulation observed in PDD roots was significantly larger than observed in D roots (Fig. 4C, D). However, the aerial ti ssues showed different trends. C shoots increased their content of ureides over the time course while D provoked an accumulation of ureides in stems but not in leaves (Fig. 4A, B).
Fig. 4.
Effect of partial drought on ureide content in leaf (A), stem (B), root (C), and nodule (D). Legend as for Fig. 1. Asterisks indicate significant differences (Student’s t-test, P ≤ 0.05) between D and C treatments; hash indicates significant differences between PDD and C plants. Values represent mean ± SE (n = 3).
Additionally, it was investigated whether this drought-induced accumulation of ureides in roots and stems was restricted to soybean plants grown under symbiosis. To that end purpose, a second set of plants grown under 5mM potassium nitrate was subjected to drought and a single time point was measured (Ψleaf for C plants was –0.37±0.01MPa, for D plants –0.75±0.06MPa). The level of total ureides in these plants was in general much lower than those of N2-fixing plants. In agreement with observations in plants grown in symbiosis, nitrate-fed plants did not accumulate ureides in droughted leaves (0.973±0.180 μmol (g DW)–1) compared with C (1.15±0.13 μmol (g DW)–1), but did in stems (in μmol (g DW)–1: 0.40±0.01 for C and 0.78±0.07 for D) and roots (0.13±0.01 for C and 0.59±0.11 for D). The analysis of these plants allowed this study to confirm that drought stress-activated ureide metabolism in some organs was independent of the NF process.
Ureide metabolism under drought
To test whether the observed ureide accumulation in drought-stressed nodules was correlated to changes in ureide metabolism, the activities of urate oxidase and ureide-degrading enzymes including allantoate amidohydrolase were measured (Fig. 5). Urate oxidase catalyses the first step in allantoin biosynthesis in nodules (Triplett et al., 1980; Tajima et al., 2004), while allantoate amidohydrolase is involved in the degradation of allantoate (Todd and Polacco, 2004; Todd et al., 2006; Werner et al., 2010). In both cases, D nodules showed a decline in these enzymatic activities, whereas levels remained relatively constant over the time course in C plants. This decline was more dramatic in the case of the ureide-degrading activity (Fig. 5B), showing a 10-fold reduction compared to C plants from day 4 on.
Fig. 5.
Effect of drought on nodule urate oxidase activity (A) and allantoate-degrading activity (B). Asterisks indicate significant differences (Student’s t-test, P ≤ 0.05) between C and D treatments. Values are mean ± SE (n = 3).
Local and systemic changes in the proteome subjected to partial drought
In order to identify local or systemic changes induced by drought stress in soybean, nodule samples from plants grown under SRS were subjected to liquid chromatography coupled to mass spectrometry protein profiling. Spectral count was used to estimate the relative abundance changes between the different treatments (Liu et al., 2004). Two types of comparisons were made: PDC/C, which represented changes associated with systemic drought responses, and PDD/PDC, which could be correlated to local drought responses. Among the 320 soybean proteins identified, 16 proteins showed more than a 2-fold decline and significant differences between PDC and PDD samples (Table 1). The main functional categories altered in the PDD/PDC comparison included glycolysis/TCA cycle, amino acid metabolism, protein synthesis/degradation and cell wall and cell organization (Table 1). These changes could be interpreted as local responses induced by drought.
Table 1.
G. max nodule proteins significantly changing in the PDD/PDC comparison. Student’s t-test P ≤ 0.05 and fold change ≥ 2. ID, identification code of tentative consensus sequences retrieved from the Soybean Gene Index release 15 database; SC, the average of spectral count value for each treatment (from left to right: C (control), PDC (partial drought control), PDD (partial drought drought), and D (drought); values are mean ± SE for five biological replicates). Protein description corresponds to the closest protein homology found as in the database with Universal Protein Resource knowledgebase (UniProtKB) accession number and protein name.
| ID | SC | Protein description | Biological function |
|---|---|---|---|
| TC379596 |

|
Homologue to O65735 fructose-bisphosphate aldolase | Glycolysis/TCA |
| TC348716 |

|
Q8H928 phosphoenolpyruvate carboxylase | |
| TC358115 |

|
Homologue to Q9SPB8 malate dehydrogenase | |
| TC360116 |

|
P13708 sucrose synthase | |
| TC392227 |

|
Similar to P13708 sucrose synthase | |
| TC352339 |

|
Homologue to P34921 glyceraldehyde-3-phosphate dehydrogenase | |
| TC349305 |

|
Similar to A9PL11 plastid serine hydroxymethyltransferase | Amino acid metabolism |
| TC386106 |

|
Q9FUK4 cytosolic glutamine synthetase | |
| TC388897 |

|
Homologue to Q3LUM5 elongation factor 1-alpha | Protein synthesis/ degradation |
| TC350506 |
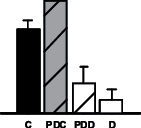
|
Similar to A9QY38 subtilase | |
| TC380021 |

|
Homologue to Q02028 stromal 70kDa heat shock-related protein | Response to stress |
| TC348718 |

|
Homologue to Q84XV9 phosphoribosylformylglycinamidine synthase | Nucleotide metabolism |
| TC394644 |

|
Homologue to P12460 tubulin beta-2 chain | Cell wall and organization |
| TC356600 |

|
Unknown protein | |
| TC407811 |
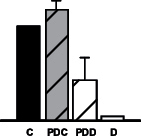
|
Unknown protein | |
| TC351567 |

|
Unknown protein |
Results from the PDC/C comparison led to the identification of five soybean proteins, whose relative abundance was reduced in all the treatments compared to C samples (Table 2). These proteins were one isoform of alanine aminotransferase (TC350292), a 14-3-3-like protein (TC372027), a heat shock protein (TC394883), an ascorbate peroxidase isoform (GD751541), and one component of the cytoske leton, alpha-tubulin (TC393954) (Table 2).
Table 2.
G. max nodule proteins significantly changing in the PDC/C comparison. Student’s t-test P ≤ 0.05 and fold change ≥ 2. Legend as for Table 1.
| ID | SC | Protein description | Biological function |
|---|---|---|---|
| TC350292 |

|
A8IKE1 alanine aminotransferase 1 | Amino acid metabolism |
| TC372027 |

|
Homologue to A5YM78 14–3-3-like protein | Protein synthesis |
| TC394883 |

|
Homologue to Q8GSN2 heat shock cognate protein 70 | Response to stress |
| GD751541 |

|
Homologue to Q41712 cytosolic ascorbate peroxidase | |
| TC393954 |

|
A9PL19 alpha-tubulin | Cell wall and organization |
Discussion
Ureides accumulation in drought-stressed plants: where do they come from?
The fact that NF is rapidly inhibited in nodulated legumes under a water-deficit situation is widely known. However, it remains to be understood why, under drought stress, rather than a reduction in the content of N compounds, an accumulation of reduced N, mainly ureides in the case of soybean and other tropical legumes, is observed in various plant tissues (de Silva et al., 1996; Serraj and Sinclair, 1998; Serraj et al., 1999; King and Purcell, 2005; Ladrera et al., 2007; Alamillo et al., 2010). To explain this apparent paradox, several authors suggested that this ureide accumulation could have a regulatory role and participate in an N-feedback inhibition of the nitrogenase enzymatic complex (Serraj et al., 1999, 2001; Vadez et al., 2000; King and Purcell, 2005).
At least three possible origins have been postulated to explain this drought-induced accumulation of N compounds: (i) reduced transpiration rates; (ii) a decrease in the shoot N demand; and (iii) alterations in the metabolism of ureides (reviewed in Valentine et al., 2011). The first option implies a model in which ureides would accumulate in no dules due to lower xylem translocation rates as a consequence of the decreased transpiration (Pate et al., 1969). The present study employed the SRS technique and provided experimental evidences to show that the accumulation of ureides and reduction of transpiration rates are not correlated under the conditions tested. PD plants presented a reduction of both stomatal conductance and evapotranspiration rates (Fig. 1D, E), while the level of ureides in PDC roots remained close to C values over the course of the experiment (Fig. 4C, D). Interestingly, the drought-stressed side of the root system (PDD roots and nodules) presented a significant accumulation of ureides, even higher than this measured in D roots (Fig. 4C).
The second postulated hypothesis to explain the N-compound accumulation in the underground organs su ggests a decline in shoot N demand (Valentine et al., 2011). However, the decline in N demand that has been shown when inorganic N is applied to N2-fixing legumes (Parsons et al., 1993) does not seem to occur under drought stress. If the shoot N demand were responsible for the accumulation of ureides in the underground tissues, there should not be differences between PDC and PDD roots in terms of ureide content, given that they share the same aerial part. However, this study observed that PDD roots and nodules accumulated ureides while PDC tissues did not (Fig. 4C, D).
Recent works have explored the involvement of ureide metabolism in the accumulation of N compounds under stress. The activity of the enzyme responsible for allantoate breakdown, allantoate amidohydrolase, was initially found to correlate to drought tolerance when cultivars with contrasting sensitivity were compared (Purcell et al., 2000). However, in subsequent studies, differences in drought tolerance were not associated with differential transcript expression of allantoate amidohydrolase, suggesting that other factors may regulate its activity such as the post-translational regulation of the enzyme and/or the availability of Mn2+ (Charlson et al., 2009). Alamillo et al. (2010) studied the ureide metabolism in diverse organs of drought-stressed Phaseolus vulgaris and concluded that the enzymes of ureide synthesis, such as urate oxidase, were more affected by the stress than allantoate amidohydrolase.
In the present study, both urate oxidase and allantoate-degrading activities were negatively affected in drought-stressed soybean nodules (Fig. 5), even at early stages where Ψnodule had not yet significantly declined (Fig. 1C). It is worth mentioning that the relative reduction of nodule allantoate-degrading activity was greater than that of urate oxidase, su ggesting that ureide catabolism was more affected than the de novo synthesis under stress. Furthermore, results presented here suggest that the observed accumulation of ureides in nodules may be more attributable to a decline in degradation activity rather than to increased biosynthesis.
Local regulation of NF in soybean plants under drought stress: is it N-mediated?
Several lines of evidence suggest that the regulation of NF in drought-stress soybean plants follows the pattern of a local regulatory mechanism. Not only were the above-mentioned changes in ureide concentration locally driven but also the study of ANA in the SRS plants provided support for this type of regulation. For instance, regulation at the systemic level would be translated into inhibition of ANA in PDC roots. However, ANA rates showed a reduction both in D and PDD treatments, while ANA values for PDC root systems remained close to C values over the drought experiment (Fig. 2). The local inhibition of NF operating in drought-stressed soybean plants is in agreement with studies in the amide-exporting legumes, pea (Marino et al., 2007) and the model legume M. truncatula (Gil-Quintana et al., 2013).
To test the contribution of amino acids and ureides as po ssible N-feedback regulatory signals, the amino acid profile and ureide content were analysed. The results showed local accumulation of both Asn and ureides in nodules (Figs. 3 and 4) concomitant with the reduction of ANA rates (Fig. 2). Although the particular compound involved could not be pinpointed, this study showed that ureide accumulation occurred not only in nodules but also in roots. Additionally, in PDD nodules, ureides increased only at the most severe drought stage, 3 days after a significant reduction of ANA rates was measured. This delayed accumulation of ureides would downplay the role of these N compounds as the primary cause of NF inhibition in soybean.
On the other hand, ureides were found to accumulate not only when grown under symbiosis but also in nitrate-fed soybean plants when exposed to drought, even though the ureide levels were much lower compared to nodulated plants. Thus, ureide accumulation may be a more widespread response to water deficit not necessarily related to the regulation of NF (Alamillo et al., 2010). In this regard, the level of ureides has been recently shown to depend on the plant developmental stage rather than on the growth conditions (nitrogen-fixing versus nitrate-fed) (Diaz-Leal et al., 2012).
Asn (Serraj et al., 1999; Vadez et al., 2000; Sulieman et al., 2010) and Asp (King and Purcell, 2005) have been also proposed as candidate molecules for the N-feedback regulation of NF. The present study did not observe significant changes in the content of Asp under drought conditions, and the accumulation of Asn in drought-stressed tissues occurred early but simultaneously with most amino acids. For instance, amino acids that showed an accumulation before any significant decline in ANA rates include Glu, Tyr, Phe, Val, Ala, Leu, Gaba, Arg, His, Thr, Met, Ile, and Asn (Fig. 3). However, this study cannot conclude that this accumulation was directly related to the regulation of NF since it has also been reported in different plant species under various stresses (Kramer et al., 1996; Nambara et al., 1998; Nikiforova et al., 2006; Sharma and Dietz, 2006; Lea et al., 2007). One alternative possibility is that increased free amino acid levels may represent changes in cell protein metabolism occurring under drought stress (e.g. protein biosynthesis, Good and Zaplachinski, 1994). Indeed, abundance of the proteins identified in nodules directly subjected to drought (PDD) showed a significant decline compared to PDC (Table 1). This is the case of elongation factor 1-alpha, which promotes the binding of aminoacyl-tRNA to the ribosome during protein biosynthesis and whose expression has been correlated to high rates of protein synthesis in developing plant tissues (Xu et al., 2007).
Response of the nodule proteome when subjected to drought stress
In recent years, soybean has been the subject of various proteomic studies particularly focusing on leaf (Xu et al., 2006), root (Brechenmacher et al., 2009), root hairs (Wan et al., 2005), nodule cytosol (Oehrle et al., 2008), mitochondria (Hoa et al., 2004), and peribacteroid membrane (Panter et al., 2000). Nevertheless, as far as it is known, the soybean proteome responses to drought stress have not yet been investigated. The current work initiated the proteomic characterization of this response with special emphasis on the differential local and systemic responses of the plant nodule proteome.
One of the main features of the SRS experimental set-up is that it permits the identification of local responses when comparing protein changes between PDD and PDC nodule samples (Table 1). The largest functional group showing significant changes was glycolysis/TCA cycle and it included enzymes such as sucrose synthase, fructose-bisphosphate aldolase, phosphoenolpyruvate carboxylase, and malate dehydrogenase. These results are in agreement with previous works in which the hypothesis of carbon shortage to fuel bacteroid nitrogenase activity during drought stress has been suggested in soybean (Gonzalez et al., 1995; Gordon et al., 1997) and other legume plants (Gonzalez et al., 1998, 2001; Galvez et al., 2005; Marino et al., 2007).
Several other metabolic pathways appeared to be altered such as amino acid and protein metabolism (Table 1). The le vels of one of the main N assimilatory enzyme in nodules, glutamine synthetase (TC386106), declined in drought-stressed samples, as similarly observed for M. truncatula (Larrainzar et al., 2009) and coincident with the decline in NF rates (Fig. 2). Other stress-related proteins indentified included a subtilase (TC350506), a Ser peptidase implicated in protein turnover, programmed cell death, and plant responses to biotic and abiotic stresses (Schaller et al., 2012), and a homologue to elongation factor 1-alpha (TC388897). Interestingly, elongation factor 1-alpha has also been involved in heat tolerance (Moriarty et al., 2002; Bukovnik et al., 2009), resistance to salt stress (Shin et al., 2009), and drought-stress response in rice (Li and Chen, 1999). Additionally, the relative content of a beta-tubulin homologue (TC394644) was observed to decline in PDD (Table 1), suggesting that normal nodule cell growth and development was affected at these drought stages.
The C/PDC comparison provided insights into systemic responses triggered by drought (Table 2). This comparison led to the identification of proteins involved in antioxidant defence, such as a homologue of ascorbate peroxidase (GD751541) as well as other proteins involved in stress responses. Ascorbate peroxidase is considered a key antioxidant enzyme in plants (Orvar and Ellis, 1997) and it is very abundant in legume nodules, accounting for around the 1% of the total soluble protein (Becana et al., 2000). However, reports on its role in stress response are contradictory and it seems that responses vary depending on how the stress is applied (D’Arcy-Lameta et al., 2006; Shi et al., 2008).
In summary, results presented here support the hypothesis of a local regulation of NF taking place in soybean and downplay the role of ureides in the inhibition of NF. Proteomic analyses suggest that alterations in carbon metabolism, nitrogen assimilation, and protein biosynthesis occur in drought-stressed soybean nodules. This proteomic study paves the way for more detailed work on the soybean proteome responses to progressive water-deficit stress.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Fig. S1. Effect of drought on total amino acid content in leaf, root, and nodule tissue.
Acknowledgements
The authors would like to thank Prof Tomás Ruiz-Argüeso for providing B. japonicum UPM752, Gustavo Garijo and Xabier Sanz-Corres for technical assistance, and Dr Frank R Minchin for critical reading and helpful comments on the manuscript. This work was financed by the Spanish Ministry of Economy and Competitiveness (AGL 2011–23738 and AGL 2011-30386-C02-01). EG-Q and AS are holders of PhD fellowships from the Public University of Navarre (735/2008 and 134/2012). EL is a recipient of a Marie Curie International Outgoing Fellowship for Career Development (PIOF-GA-2009–253141).
References
- Alamillo JM, Diaz-Leal JL, Sanchez-Moran MV, Pineda M. 2010. Molecular analysis of ureide accumulation under drought stress in Phaseolus vulgaris L. Plant, Cell and Environment 33, 1828–1837. [DOI] [PubMed] [Google Scholar]
- Becana M, Dalton DA, Moran JF, Iturbe-Ormaetxe I, Matamoros MA, Rubio MC. 2000. Reactive oxygen species and antioxidants in legume nodules. Physiologia Plantarum 109, 372–381. [Google Scholar]
- Brechenmacher L, Lee J, Sachdev S, et al. 2009. Establishment of a protein reference map for soybean root hair cells. Plant Physiology 149, 670–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukovnik U, Fu J, Bennett M, Prasad PVV, Ristic Z. 2009. Heat tolerance and expression of protein synthesis elongation factors, EF-Tu and EF-1 alpha, in spring wheat. Functional Plant Biology 36, 234–241. [DOI] [PubMed] [Google Scholar]
- Charlson DV, Korth KL, Purcell LC. 2009. Allantoate amidohydrolase transcript expression is independent of drought tolerance in soybean. Journal of Experimental Botany 60, 847–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcy-Lameta A, Ferrari-Iliou R, Contour-Ansel D, Pham-Thi AT, Zuily-Fodil Y. 2006. Isolation and characterization of four ascorbate peroxidase cDNAs responsive to water deficit in cowpea leaves. Annals of Botany 97, 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva M, Purcell LC, King CA. 1996. Soybean petiole ureide response to water deficits and decreased transpiration. Crop Science 36, 611–616. [Google Scholar]
- Diaz-Leal JL, Galvez-Valdivieso G, Fernandez J, Pineda M, Alamillo JM. 2012. Developmental effects on ureide levels are mediated by tissue-specific regulation of allantoinase in Phaseolus vulgaris L. Journal of Experimental Botany 63, 4095–4106. [DOI] [PubMed] [Google Scholar]
- Galvez L, Gonzalez EM, Arrese-Igor C. 2005. Evidence for carbon flux shortage and strong carbon/nitrogen interactions in pea nodules at early stages of water stress. Journal of Experimental Botany 56, 2551–2561. [DOI] [PubMed] [Google Scholar]
- Gil-Quintana E, Larrainzar E, Arrese-Igor C, González EM. 2013. Is N-feedback involved in the inhibition of nitrogen fixation in drought-stressed Medicago truncatula? Journal of Experimental Botany 64, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EM, Aparicio-Tejo PM, Gordon AJ, Minchin FR, Royuela M, Arrese-Igor C. 1998. Water-deficit effects on carbon and nitrogen metabolism of pea nodules. Journal of Experimental Botany 49, 1705–1714. [Google Scholar]
- Gonzalez EM, Galvez L, Royuela M, Aparicio-Tejo PM, Arrese-Igor C. 2001. Insights into the regulation of nitrogen fixation in pea nodules: lessons from drought, abscisic acid and increased photoassimilate availability. Agronomie 21, 607–613. [Google Scholar]
- Gonzalez EM, Gordon AJ, James C, Arrese-Igor C. 1995. The role of sucrose synthase in the response of soybean nodules to drought. Journal of Experimental Botany 46, 1515–1523. [Google Scholar]
- Good AG, Zaplachinski ST. 1994. The effects of drought stress on free amino acid accumulation and protein synthesis in Brassica napus . Physiologia Plantarum 90, 9–14. [Google Scholar]
- Gordon AJ, Minchin FR, Skot L, James CL. 1997. Stress-induced declines in soybean N2 fixation are related to nodule sucrose synthase activity. Plant Physiology 114, 937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham PH, Vance CP. 2003. Legumes: importance and constraints to greater use. Plant Physiology 131, 872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoa LTP, Nomura M, Kajiwara H, Day DA, Tajima S. 2004. Proteomic analysis on symbiotic differentiation of mitochondria in soybean nodules. Plant and Cell Physiology 45, 300–308. [DOI] [PubMed] [Google Scholar]
- Hoehenwarter W, Wienkoop S. 2010. Spectral counting robust on high mass accuracy mass spectrometers. Rapid Communications in Mass Spectrometry 24, 3609–3614. [DOI] [PubMed] [Google Scholar]
- King CA, Purcell LC. 2005. Inhibition of N2 fixation in soybean is associated with elevated ureides and amino acids. Plant Physiology 137, 1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer U, CotterHowells JD, Charnock JM, Baker AJM, Smith JAC. 1996. Free histidine as a metal chelator in plants that accumulate nickel. Nature 379, 635–638. [Google Scholar]
- Labhilili M, Joudrier P, Gautier MF. 1995. Characterization of cDNAs encoding Triticum durum dehydrins and their expression patterns in cultivars that differ in drought tolerance. Plant Science 112, 219–230. [Google Scholar]
- Ladrera R, Marino D, Larrainzar E, Gonzalez EM, Arrese-Igor C. 2007. Reduced carbon availability to bacteroids and elevated ureides in nodules, but not in shoots, are involved in the nitrogen fixation response to early drought in soybean. Plant Physiology 145, 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrainzar E, Wienkoop S, Scherling C, Kempa S, Ladrera R, Arrese-Igor C, Weckwerth W, Gonzalez EM. 2009. Carbon metabolism and bacteroid functioning are involved in the regulation of nitrogen fixation in Medicago truncatula under drought and recovery. Molecular Plant–Microbe Interactions 22, 1565–1576. [DOI] [PubMed] [Google Scholar]
- Larrainzar E, Wienkoop S, Weckwerth W, Ladrera R, Arrese-Igor C, Gonzalez EM. 2007. Medicago truncatula root nodule proteome analysis reveals differential plant and bacteroid responses to drought stress. Plant Physiology 144, 1495–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea PJ, Sodek L, Parry MAJ, Shewry R, Halford NG. 2007. Asparagine in plants. Annals of Applied Biology 150, 1–26. [Google Scholar]
- Li ZY, Chen SY. 1999. Inducible expression of translation elongation factor 1A gene in rice seedlings in response to environmental stresses. Acta Botanica Sinica 41, 800–806. [Google Scholar]
- Liu H, Sadygov RG, Yates JR., 3rd 2004. A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Analytical Chemistry 76, 4193–4201. [DOI] [PubMed] [Google Scholar]
- Marino D, Frendo P, Ladrera R, Zabalza A, Puppo A, Arrese-Igor C, Gonzalez EM. 2007. Nitrogen fixation control under drought stress. Localized or systemic? Plant Physiology 143, 1968–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty T, West R, Small G, Rao D, Ristic Z. 2002. Heterologous expression of maize chloroplast protein synthesis elongation factor (EF-Tu) enhances Escherichia coli viability under heat stress. Plant Science 163, 1075–1082. [Google Scholar]
- Nambara E, Kawaide H, Kamiya Y, Naito S. 1998. Characterization of an Arabidopsis thaliana mutant that has a defect in ABA accumulation: ABA-dependent and ABA-independent accumulation of free amino acids during dehydration. Plant and Cell Physiology 39, 853–858. [DOI] [PubMed] [Google Scholar]
- Nikiforova VJ, Bielecka M, Gakiere B, Krueger S, Rinder J, Kempa S, Morcuende R, Scheible WR, Hesse H, Hoefgen R. 2006. Effect of sulfur availability on the integrity of amino acid biosynthesis in plants. Amino Acids 30, 173–183. [DOI] [PubMed] [Google Scholar]
- Oehrle NW, Sarma AD, Waters JK, Emerich DW. 2008. Proteomic analysis of soybean nodule cytosol. Phytochemistry 69, 2426–2438. [DOI] [PubMed] [Google Scholar]
- Orvar BL, Ellis BE. 1997. Transgenic tobacco plants expressing antisense RNA for cytosolic ascorbate peroxidase show increased susceptibility to ozone injury. The Plant Journal 11, 1297–1305. [Google Scholar]
- Panter S, Thomson R, de Bruxelles G, Laver D, Trevaskis B, Udvardi M. 2000. Identification with proteomics of novel proteins associated with the peribacteroid membrane of soybean root nodules. Molecular Plant–Microbe Interactions 13, 325–333. [DOI] [PubMed] [Google Scholar]
- Parsons R, Stanforth A, Raven JA, Sprent JI. 1993. Nodule growth and activity may be regulated by a feedback mechanism involving phloem nitrogen. Plant, Cell and Environment 16, 125–136. [Google Scholar]
- Pate JS, Gunning BES, Briarty LG. 1969. Ultrastructure and functioning of transport system of leguminous root nodule. Planta 85, 8–34. [DOI] [PubMed] [Google Scholar]
- Pineda M, Fernandez E, Cardenas J. 1984. Urate oxidase of Chlamydomonas-reinhardii . Physiologia Plantarum 62, 453–457. [Google Scholar]
- Purcell LC, King CA, Ball RA. 2000. Soybean cultivar differences in ureides and the relationship to drought tolerant nitrogen fixation and manganese nutrition. Crop Science 40, 1062–1070. [Google Scholar]
- Purcell LC, Serraj R, de Silva M, Sinclair TR, Bona S. 1998. Ureide concentration of field-grown soybean in response to drought and the relationship to nitrogen fixation. Journal of Plant Nutrition 21, 949–966. [Google Scholar]
- Raso MJ, Munoz A, Pineda M, Piedras P. 2007. Biochemical characterisation of an allantoate-degrading enzyme from French bean (Phaseolus vulgaris): the requirement of phenylhydrazine. Planta 226, 1333–1342. [DOI] [PubMed] [Google Scholar]
- Rigaud J, Puppo A. 1975. Indole-3-acetic-acid catabolism by soybean bacteroids. Journal of General Microbiology 88, 223–228. [Google Scholar]
- Sato T, Yashima H, Ohtake N, Sueyoshi K, Akao S, Harper JE, Ohyama T. 1998. Determination of leghemoglobin components and xylem sap composition by capillary electrophoresis in hypernodulation soybean mutants cultivated in the field. Soil Science and Plant Nutrition 44, 635–645. [Google Scholar]
- Schaller A, Stintzi A, Graff L. 2012. Subtilases – versatile tools for protein turnover, plant development, and interactions with the environment. Physiologia Plantarum 145, 52–66. [DOI] [PubMed] [Google Scholar]
- Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA. 1965. Letters to the Editor. Science 149, 920–922. [DOI] [PubMed] [Google Scholar]
- Serraj R, Sinclair TR. 1996. Processes contributing to N2-fixation insensitivity to drought in the soybean cultivar Jackson. Crop Science 36, 961–968. [Google Scholar]
- Serraj R, Sinclair TR. 1998. N2 fixation response to drought in common bean (Phaseolus vulgaris L.). Annals of Botany 82, 229–234. [Google Scholar]
- Serraj R, Vadez V, Denison RF, Sinclair TR. 1999. Involvement of ureides in nitrogen fixation inhibition in soybean. Plant Physiology 119, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serraj R, Vadez V, Sinclair TR. 2001. Feedback regulation of symbiotic N2 fixation under drought stress. Agronomie 21, 621–626. [Google Scholar]
- Sharma SS, Dietz KJ. 2006. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. Journal of Experimental Botany 57, 711–726. [DOI] [PubMed] [Google Scholar]
- Shi F, Yamamoto R, Shimamura S, Hiraga S, Nakayama N, Nakamura T, Yukawa K, Hachinohe M, Matsumoto H, Komatsu S. 2008. Cytosolic ascorbate peroxidase 2 (cAPX 2) is involved in the soybean response to flooding. Phytochemistry 69, 1295–1303. [DOI] [PubMed] [Google Scholar]
- Shin D, Moon SJ, Park SR, Kim BG, Byun MO. 2009. Elongation factor 1 alpha from Arabidopsis thaliana functions as molecular chaperone and confers resistance to salt stress in yeast and plants. Plant Science 177, 156–160. [Google Scholar]
- Sinclair TR, Serraj R. 1995. Legume nitrogen fixation and drought. Nature 378, 344–344. [Google Scholar]
- Sprent JI. 2001. Nodulation in legumes. Kew: Royal Botanic Gardens. [Google Scholar]
- Stacey G, Vodkin L, Parrott WA, Shoemaker RC. 2004. National science foundation-sponsored workshop report. Draft plan for soybean genomics. Plant Physiology 135, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulieman S, Fischinger SA, Gresshoff PM, Schulze J. 2010. Asparagine as a major factor in the N-feedback regulation of N2 fixation in Medicago truncatula . Physiologia Plantarum 140, 21–31. [DOI] [PubMed] [Google Scholar]
- Tajima S, Nomura M, Kouchi H. 2004. Ureide biosynthesis in legume nodules. Frontiers in Bioscience 9, 1374–1381. [DOI] [PubMed] [Google Scholar]
- Todd CD, Polacco JC. 2004. Soybean cultivars ‘Williams 82’ and ‘Maple Arrow’ produce both urea and ammonia during ureide degradation. Journal of Experimental Botany 55, 867–877. [DOI] [PubMed] [Google Scholar]
- Todd CD, Tipton PA, Blevins DG, Piedras P, Pineda M, Polacco JC. 2006. Update on ureide degradation in legumes. Journal of Experimental Botany 57, 5–12. [DOI] [PubMed] [Google Scholar]
- Triplett EW, Blevins DG, Randall DD. 1980. Allantoic acid synthesis in soybean root nodule cytosol via xanthine dehydrogenase. Plant Physiology 65, 1203–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadez V, Sinclair T, Serraj R. 2000. Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean. Physiologia Plantarum 110, 215–223. [Google Scholar]
- Valentine AJ, Benedito VA, Kang Y. 2011. Legume nitrogen fixation and soil abiotic stress: from physiology to genomics and beyond. Annual Plant Reviews 42, 207–248. [Google Scholar]
- Wan JR, Torres M, Ganapathy A, Thelen J, DaGue BB, Mooney B, Xu D, Stacey G. 2005. Proteomic analysis of soybean root hairs after infection by Bradyrhizobium japonicum . Molecular Plant–Microbe Interactions 18, 458–467. [DOI] [PubMed] [Google Scholar]
- Werner AK, Romeis T, Witte CP. 2010. Ureide catabolism in Arabidopsis thaliana and Escherichia coli . Nature Chemical Biology 6, 19–21. [DOI] [PubMed] [Google Scholar]
- Wienkoop S, Staudinger C, Hoehenwarter W, Weckwerth W, Egelhofer V. 2012. ProMEX – a mass spectral reference database for plant proteomics. Frontiers in Plant Science 3, 125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Garrett WM, Sullivan J, Caperna TJ, Natarajan S. 2006. Separation and identification of soybean leaf proteins by two-dimensional gel electrophoresis and mass spectrometry. Phytochemistry 67, 2431–2440. [DOI] [PubMed] [Google Scholar]
- Xu WL, Wang XL, Wang H, Li XB. 2007. Molecular characterization and expression analysis of nine cotton GhEF1A genes encoding translation elongation factor 1A. Gene 389, 27–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







