Abstract
Despite numerous reports implicating salicylic acid (SA) in plant salinity responses, the specific ionic mechanisms of SA-mediated adaptation to salt stress remain elusive. To address this issue, a non-invasive microelectrode ion flux estimation technique was used to study kinetics of NaCl-induced net ion fluxes in Arabidopsis thaliana in response to various SA concentrations and incubation times. NaCl-induced K+ efflux and H+ influx from the mature root zone were both significantly decreased in roots pretreated with 10–500 μM SA, with strongest effect being observed in the 10–50 μM SA range. Considering temporal dynamics (0–8-h SA pretreatment), the 1-h pretreatment was most effective in enhancing K+ retention in the cytosol. The pharmacological, membrane potential, and shoot K+ and Na+ accumulation data were all consistent with the model in which the SA pretreatment enhanced activity of H+-ATPase, decreased NaCl-induced membrane depolarization, and minimized NaCl-induced K+ leakage from the cell within the first hour of salt stress. In long-term treatments, SA increased shoot K+ and decreased shoot Na+ accumulation. The short-term NaCl-induced K+ efflux was smallest in the gork1-1 mutant, followed by the rbohD mutant, and was highest in the wild type. Most significantly, the SA pretreatment decreased the NaCl-induced K+ efflux from rbohD and the wild type to the level of gork1-1, whereas no effect was observed in gork1-1. These data provide the first direct evidence that the SA pretreatment ameliorates salinity stress by counteracting NaCl-induced membrane depolarization and by decreasing K+ efflux via GORK channels.
Key words: H+-ATPase, H+ flux, depolarization, K+ flux, membrane potential, outward-rectifying K+ channel, potassium homeostasis.
Introduction
Salicylic acid (SA) is known as a signalling molecule that modifies plant responses to pathogen infection. The SA concentrations increase after a pathogen attack, inducing the expression of pathogen-related genes and initiating the development of systemic acquired resistance and hypersensitive response (Dempsey et al., 1999; Durrant and Dong, 2004). Necrotic damage occurs at a site of the pathogen entry during the hypersensitive response, which is usually accompanied by production of reactive oxygen species (ROS). In addition to being an important component of biotic stress tolerance mechanism, SA also regulates various aspects of plant responses to abiotic stresses through extensive signalling cross-talk with other growth hormones (Horváth et al., 2007; Tuteja and Sopory, 2008; Asensi-Fabado and Munné-Bosch, 2011). In particular, SA plays a key role in plant adaptive responses to osmotic stress, high salinity, oxidative stress, heavy metals, ozone, UV radiation, high temperatures, and chilling and drought stresses (reviewed in Horváth et al., 2007; Ashraf et al., 2010; Hayat et al., 2010). However, exact mechanisms by which SA protects plants during abiotic stresses remain obscure.
Exogenous addition of SA can ameliorate toxicity symptoms induced by salinity stress in many plant species (reviewed in Horváth et al., 2007; Ashraf et al., 2010; Hayat et al., 2010). The ability of exogenously applied SA to improve photosynthetic capacity, enhance antioxidant protection by modifying the activities of antioxidant enzymes, increase accumulation of soluble carbohydrates, increase ATP content, and maintain optimum K+/Na+ ratio under saline conditions has been suggested as potential mechanisms of salt tolerance in plants (reviewed in Horváth et al., 2007; Ashraf et al., 2010; Hayat et al., 2010). However, most of these results are from long-term (days to months) salt-exposure experiments. Hence, the above effects are likely to be indirect and strongly dependent on doses of SA used, plant species studied, and intensity and duration of salt stress (reviewed in Horváth et al., 2007). Moreover, the critical role of SA in modulation of ion transport processes (e.g. Na+, K+, and H+ fluxes) at the cellular level has been overlooked during salt stress. Thus, direct measurements of transport processes in short-term salt exposure studies (few minutes to hours) are critical to characterize how the specific ion transporters are modulated by exogenous SA.
The intracellular K+ decreases significantly under salinity (Carden et al., 2003). The problem is intensified when K+ loss is accompanied by accumulation of Na+ inside the cytosol in the 50–200mM range (Maathuis and Amtmann, 1999; Flowers and Hajibagheri, 2001) whereby it replaces K+ in metabolic reactions, thus impairing normal enzymatic activity and metabolism and finally causing cell death (Maathuis and Amtmann, 1999; Shabala and Cuin, 2008; Demidchik et al., 2010). In this context, reports on the effects of exogenous SA on ionic relations and membrane-transport processes in plants are rare and highly controversial. Using radioisotope 86Rb+, it has been shown that SA application alone induced plasma-membrane depolarization (Glass, 1974a) and inhibited K+ uptake in barley seedlings (Glass, 1974a, b; Harper and Balke, 1981). These results are very difficult to explain given the importance of K+ retention to plant metabolism (Marschner, 1995) and the reported ameliorative effects of SA on salinity stress via increasing K+ concentration at the whole-plant level (He and Zhu, 2008; Kováčik et al., 2009). Hence, the role of K+ dynamics in the amelioration of salt stress by SA remains to be elucidated.
Under salt conditions, a passive entry of Na+ ions through the plasma membrane causes a strong membrane depolarization that favours K+ leakage via depolarization-activated K+ outward-rectifying (KOR) channels (reviewed in Shabala and Cuin, 2008). The K+ can also leak from the cytosol via non-selective cation channels (NSCC), following their activation by ROS. Indeed, ROS concentrations increase significantly under saline conditions (reviewed by Miller et al., 2009), and ROS-induced activation of NSCC channels is also widely reported (Demidchik et al., 2003, 2010; Mittler et al., 2004; Demidchik and Maathuis, 2007; Miller et al., 2008). Hence, prevention of K+ loss through above channels during salt stress is critical for salt tolerance in plants. Indeed, divalent cations (Shabala et al., 2003, 2006), polyamines (Pandolfi et al., 2010) and compatible solutes (Cuin and Shabala, 2005; Cuin and Shabala, 2007) are efficient in preventing NaCl-induced K+ leakage and improving plant growth during salt stress. However, it is unknown whether SA plays the same role in preventing NaCl-induced K+ leakage.
The activation of proton pumps by salt stress (Kerkeb et al., 2001) is positively correlated with salinity tolerance, and this effect is stronger in salt-tolerant than salt-sensitive species (Niu et al., 1993; Chen et al., 2007b; Sahu and Shaw, 2009). Such an increase in H+ pumping could act in two parallel pathways. First, enhanced activity of H+-ATPase would regulate voltage-dependent outward-rectifying K+ channels and prevent K+ leakage via KOR channels (Chen et al., 2007b). Secondly, H+ pumping would provide a driving force for the plasma-membrane Na+/H+ exchanger (SOS1-Salt overly sensitive 1) to remove Na+ from the cytoplasm to the apoplast (Shi et al., 2000; Apse and Blumwald, 2007), thus reducing Na+ load in the cytoplasm. Interestingly, the recent studies on temperature stress have demonstrated that SA pretreatment increased the activity of the plasma-membrane H+-ATPase in grape and peas (Liu et al., 2008, 2009) and, hence, each of the two pathways mentioned above may be potentially affected by SA.
Thus, the working hypothesis for this study was that beneficial effects of SA during salt stress may be related to upregulation of the plasma-membrane H+-ATPase activity and consequent effects on intracellular ionic homeostasis of Na+ and K+. Consequently, the aim of this work was characterization of the downstream targets of SA signalling. This was achieved by applying the non-invasive microelectrode ion flux estimation (MIFE) technique (Shabala et al., 1997; Newman, 2001) to characterize the effects of exogenous SA application on the functioning of the plasma-membrane transporters in epidermal cells of Arabidopsis roots under salt stress. This study shows that root pre-incubation with micromolar concentrations of SA leads to a significant mitigation of salt stress due to enhanced K+ retention in SA-treated roots, resulting from enhanced H+-ATPase activity and reduced salt-induced K+ loss via a GORK channel.
Materials and methods
Plant materials
Arabidopsis thaliana L. Heynch wild-type ecotype Columbia (Col-0) and rbohD (SALK_021661, Col-0) mutant seeds were obtained from the Arabidopsis Biological Resource Center (The Ohio State University, Columbus, OH, USA); gork1-1 (SALK_092448, Col-0) mutant seeds were a generous gift from Prof. Hervé Sentenac (ENSAM, Montpellier, France).
Growth experiments
Whole-plant responses to SA under salinity stress were studied in hydroponic and soil culture experiments.
Hydroponic culture
Arabidopsis seeds were surface sterilized with 1% (v/v) sodium hypochlorite (commercial bleach) plus 0.01 % Triton for 10min and washed thoroughly with sterilized deionized water. Seeds were then sown on 0.8% (w/v) agar containing nutrient solution (1.25mM KNO3, 0.625mM KH2PO4, 0.5mM MgSO4, 0.5mM Ca(NO3)2, 0.045mM FeNaEDTA, 0.16 μM CuSO4, 0.38 μM ZnSO4, 1.8 μM MnSO4, 45 μM H3BO3, 0.015 μM (NH4)6Mo7O24, and 0.01 μM CoCl2) in 1.5-ml centrifuge tubes and vernalized at 4 °C for 48h. The bottom of the tubes was cut off and the tubes were inserted in a floater with holes and suspended over aerated nutrient solution. The seedlings were grown in a controlled growth room under a 16/8 light/dark cycle at 23 °C with an irradiance of 150 μmol m−2 s−1. After 1 week, seedlings were thinned to one healthy seedling in each centrifuge tube. After 3 weeks, seedlings were kept in nutrient solution supplemented with 50 μM SA for 1h. Then, the SA-pretreated seedlings were transferred to nutrient solution containing 100mM NaCl. The salt treatment continued for 2 weeks. A randomized complete block design was used with four replications (four pots, each pot containing four centrifuge tubes) for each treatment. Nutrient solutions were replaced every 2 days to ensure a relatively constant ion composition.
Soil culture
The pots containing a peat/perlite/vermiculate soil mixture (1:1:1, v/v) was drenched overnight with the same growth nutrient solution used for hydroponic culture before the surface-sterilized and vernalized Arabidopsis seeds were placed on top. Plants were grown under a 16/8 light/dark cycle at 23 °C with an irradiance of 150 μmol m−2 s−1. After 1 week, each pot was thinned to four uniform and healthy seedlings. After 3 weeks, pots were irrigated with nutrient solution supplemented with 50 μM SA for an hour and then exposed to salt stress by adding nutrient solution containing 100mM NaCl. The salt treatment was maintained for 2 weeks by watering with nutrient solution containing 100mM NaCl every 3 days. Control plants were irrigated with the nutrient solution without NaCl. A randomized complete block design was used, with four replicate pots for each treatment.
Biomass
Plants in both hydroponic and soil experiments were harvested after 2 weeks of NaCl treatment (at the age of 5 weeks). Shoots were thoroughly rinsed with ice-cold 0.5mM CaSO4 solution, excess water was removed by blotting shoots with paper towels, and freshweight was measured immediately. Plants were then dried at 65 °C for 2 d in a Unitherm Dryer (Birmingham, UK) and weighed. Shoot water content (%) was calculated as the difference between fresh and dry weight of the biomass.
Shoot Na+ and K+ concentrations
Dry Arabidopsis shoots were weighed and digested in 6ml of 98% (v/v) H2SO4 and 3ml 30% (v/v) H2O2 for 5h as described by Skoog et al. (2000). The shoot Na+ and K+ concentrations were determined by a flame photometer.
Electrophysiology
For the MIFE and membrane potential experiments, surface-sterilized Arabidopsis seeds were placed in 90-mm Petri dishes containing agar (0.8% w/w) media with 1mM KCl plus 0.1mM CaCl2, pH 5.5 (Guo et al., 2009; Jayakannan et al., 2011). Seeds were vernalized at 4 °C for 2 days in the dark and then transferred into a growth chamber under a 16/8 day/night cycle at 23 °C with the irradiance of 150 μmol photons m−2 s−1 during the day. Petri dishes were positioned vertically to prevent roots penetrating into the agar. Four- to five-day-old seedlings were used for ion flux and membrane potential measurements. All the measurements were made at the mature root zone (>2mm from the root tip).
Ion-flux measurements
Net fluxes of H+, K+, and Na+ were measured non-invasively using ion-selective vibrating microelectrodes (University of Tasmania, Hobart, Australia) as described previously (Shabala et al., 1997, 2003). Briefly, microelectrodes were pulled from borosilicate glass capillaries (GC 150-10; Harvard Apparatus, Kent, UK), oven-dried at 230 °C overnight and silanized using tributylchlorosilane (no. 90796; Fluka). Electrode tips were broken to obtain external tip diameters of 2–3 μm. The tips of blank electrodes were back-filled with appropriate solutions (0.15mM NaCl + 0.4mM KH2PO4, adjusted to pH 6.0 using NaOH for the H+ electrodes; 0.5M KCl for the K+ electrodes; and 0.5M NaCl for the Na+ electrodes). The electrode tips were then front-filled with commercially available ion-selective cocktails (H+ 95297; K+ 60031; both from Sigma-Aldrich). For Na+ measurements, an improved calixarene-based Na+ cocktail was used (see Jayakannan et al., 2011 for details). Prepared electrodes were calibrated in a set of standards (pH 4.76–7.10; K+ 0.25–1.5mM; Na+ 5–150mM). Electrodes with the Nernst slope responses of less than 50 mV per decade were discarded. Electrodes were mounted on a manually operated 3D-micromanipulator (MMT-5; Narishige, Toyko, Japan) and their tips were aligned and positioned 40 μm away from the root surface. During the measurements, a computer-controlled stepper motor moved electrodes in a slow (10-s cycle, 40-μm amplitude) square-wave between the two positions, close to (40 μm) and away from (80 μm) the root surface. CHART software (Shabala et al., 1997; Newman, 2001) recorded the potential difference between two positions and converted it to electrochemical potential difference using the calibrated Nernst slope of the electrode. Net ion fluxes were calculated using the MIFEFLUX software for cylindrical diffusion geometry (Newman, 2001).
Ion-flux measuring protocols
A seedling was placed on a glass slide; the root was immobilized horizontally by thin parafilm strips and then placed in a Petri dish with 30ml basal salt medium (1mM KCl, 0.1mM CaCl2, pH 5.5) (Guo et al., 2009; Jayakannan et al., 2011) for 60min before commencing MIFE measurements. For the SA treatments, plants were pretreated with different SA concentrations for specified times (1, 4, 6, or 8h). After the specified pretreatment duration, the pretreatment solution was withdrawn and the measuring solution was introduced. Net ion fluxes were measured in the basal salt medium for 5 minutes to ensure steady initial values, then 4M stock solution of NaCl was applied to reach a final NaCl concentration of 100mM. Transient recordings of the flux kinetics of K+, H+, and Na+ were measured for specified times. The time required for the stock addition and the establishment of the diffusion gradients is reported to be about 40 s (Shabala and Hariadi, 2005). Accordingly, the data for the first 60 s after the solution change were discarded from the analysis, creating a gap in all figures.
Pharmacology experiment
Prior to flux measurements, Arabidopsis seedlings were pretreated with 1mM sodium orthovanadate (Na2VO4; P-type H+-ATPase inhibitor) or 0.1mM N,N’-dicyclohexylcarbodiimide (DCCD; H+-ATPase inhibitor) for 1h. Both chemicals were purchased from Sigma-Aldrich.
Membrane-potential measurements
The root of an Arabidopsis seedling was immobilized and preconditioned as described above. The membrane potential measurements were made as described by Bose et al. (2010a,b). Conventional microelectrode (Harvard Apparatus) with a tip diameter of ~0.5 μm was filled with 1mM KCl and connected to a MIFE electrometer (Newman, 2001) via an Ag-AgCl half-cell. Then, the mounted electrode was impaled into the epidermal cells of mature root zone with a manually operated 3D-micromanipulator. The membrane potential was monitored continually using CHART software (Newman, 2001). Once a stable membrane potential value was obtained for 1 minute, salt treatment (100mM NaCl) was imposed. The transient membrane potential kinetics was recorded up to 30 minutes after treatment commencement. The membrane potential values of eight individual seedlings were averaged for every treatment combination.
Statistical analysis
Statistical significance of mean and standard error values was determined using the standard least significant difference test at P ≤ 0.05.
Results
SA pretreatment improved shoot growth and water content of Arabidopsis during salt stress
The 50 μM SA root pretreatment for 1h in both soil and hydroponic experiments did not affect shoot growth, biomass, or water content under control conditions (Fig. 1 and Table 1). In contrast, salt stress reduced shoot growth (Fig. 1), biomass, and water content (Table 1) in both SA-untreated and -pretreated plants. However, salt-induced negative effects were significantly diminished in the SA-pretreated plants (Fig. 1 and Table 1).
Fig. 1.
Effect of salicylic acid (SA) pretreatment on growth of Arabidopsis thaliana wild-type (Col-0) plants under control or saline (100mM NaCl for 2 weeks) conditions. Plants were grown in either soil (top panel) or a hydroponic system (bottom panel) for 3 weeks before pretreatment and treatment. The treatments lasted for 2 weeks. (A) Control, (B) 50 μM SA pretreatment (1h) without salt stress, (C) 100mM NaCl, and (D) 50 μM SA pretreatment (1h) plus 100mM NaCl. For SA pretreatment, the pots were irrigated with 50 μM SA for 1h.
Table 1.
Effect of salicylic acid on shoot growth and water content of Arabidopsis thaliana grown under saline conditions (100mM NaCl) for 2 weeks. Wild-type (Col-0) plants were grown either in hydroponic or soil culture until 3 weeks old before the treatment was applied. For salicylic acid (SA) pretreatment, seedlings in centrifuge tubes were kept in nutrient solution supplemented with 50 μM SA (in hydroponic culture) or the pots were irrigated with 50 μM SA (for soil culture) for 1h. Values are mean ± SE (n = 4). Data with different letters are significantly different (P ≤ 0.05).
| Treatment | Freshweight (mg/pot) | Dryweight (mg/pot) | Water content (%, w/w) |
|---|---|---|---|
| Control | 620±40a | 123±9a | 84.3±1.1a |
| SA | 618±62a | 122±4a | 83.3±1.3a |
| NaCl | 186±15c | 61±4c | 69.7±2.2c |
| NaCl + SA | 319±36b | 93±10b | 74.2±0.9b |
SA pretreatment minimized salt-induced K+ efflux from Arabidopsis roots
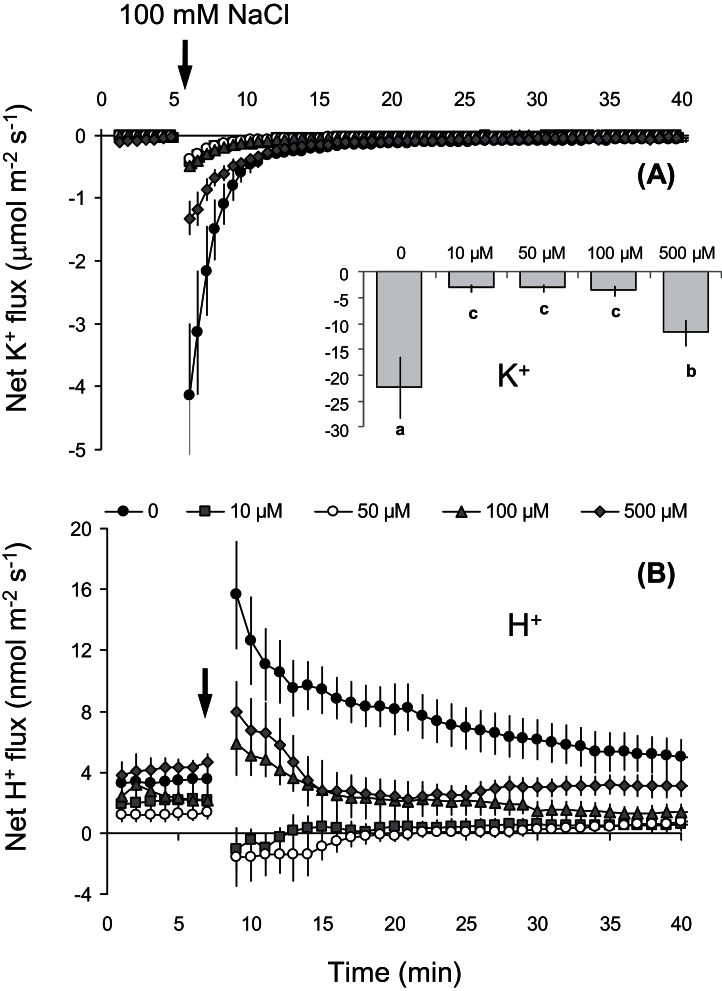
Similarly to previous reports (e.g Shabala et al., 2005, 2006), acute NaCl stress resulted in a large K+ efflux (Fig. 2A) from the mature root zone of Arabidopsis. A 1-h pretreatment of Arabidopsis roots in various concentrations of SA (10, 50, 100, or 500 μM) reduced the NaCl-induced K+ efflux in a dose-dependent manner (Fig. 2A). The most beneficial SA concentrations were 10, 50, and 100 μM (Fig. 2A inset), resulting in more than a 5-fold decrease in the magnitude of NaCl-induced K+ efflux compared with controls not pretreated with SA.
Fig. 2.
Effect of different salicylic acid concentrations (0 to 500 μM) in the 1-h pretreatment on net K+ (A) and H+ (B) fluxes measured at the mature root zone of 4–5-day-old Arabidopsis thaliana wild-type (Col-0) seedlings in response to 100mM NaCl. Insets shows total K+ (nmol m–2) leaked out (negative flux) during 1h of salt treatment. Values are mean ± SE (n = 7 seedlings). Different letters in the inset indicate significant differences.
SA pretreatment modified NaCl-induced H+ fluxes from Arabidopsis roots
Acute salt stress (100mM NaCl) induced H+ influx in SA-untreated plants. In comparison, SA-pretreated plants showed either lower H+ influx (pretreatment with 100 or 500 μM SA) or had net H+ flux around zero (pretreatment with 10 or 50 μM SA) during salt stress (Fig. 2B).
SA effects are time dependent
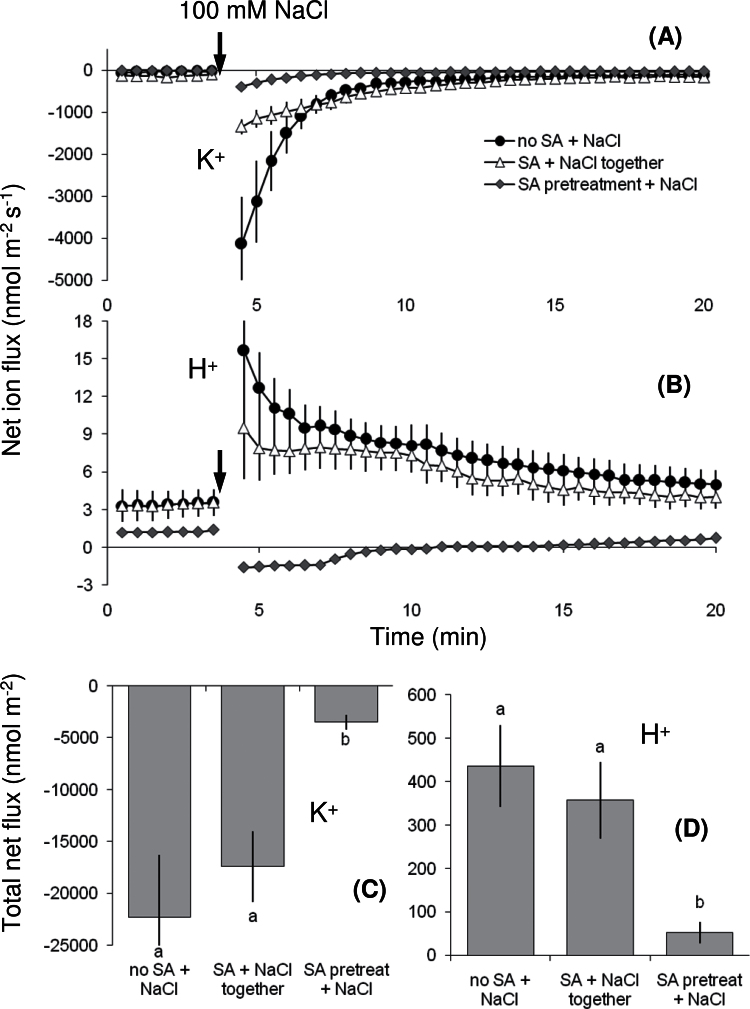
To assess the time dependency of the SA action on K+ and H+ transport systems, the following treatments were compared: (i) salt stress applied in the absence of SA; (ii) 100mM NaCl was applied together with 50 μM SA (i.e. no pretreatment); and (iii) 100mM NaCl applied after 1-h pretreatment with 50 μM SA. Simultaneous application of SA and NaCl slightly reduced K+ loss during the first 3 minutes of salt stress (Fig. 3A), but the total K+ loss during 1h of salt stress was not significantly different from SA-untreated plants (Fig. 3C). Regarding H+ fluxes, simultaneous application of SA and NaCl in comparison with SA-untreated plants did not change the H+ flux kinetics (Fig. 3B) or total H+ flux (Fig. 3D) during salt stress.
Fig. 3.
(A, B) Net fluxes of K+ (A) and H+ (B) were compared for the mature root zones of 4–5-day-old Arabidopsis thaliana wild-type (Col-0) seedlings not pretreated with salicylic acid (SA) or pretreated with 50 μM SA for 1h prior to the addition of 100mM NaCl (salt stress) or simultaneously treated with 50 μM SA and 100mM NaCl. (C, D) Total fluxes of K+ (C) and H+ (D) during 1h of salt treatment. Values are mean ± SE (n = 7 seedlings). Different letters in (C) and (D) indicate significant differences.
Compared to treatments with no SA addition or simultaneous SA and NaCl addition, the 1-h pretreatment with SA resulted in the lowest K+ efflux (Fig. 3A, C), the lowest net H+ fluxes (Fig. 3B), and the lowest total H+ influx during salt stress (Fig. 3D). Given that the SA action is dependent on the plant exposure to SA prior to exposure to NaCl, the results suggest that SA needs to be taken up across the plasma membrane to exert control over K+ and H+ transport systems from the cytoplasmic side.
Effect of the SA pretreatment duration on NaCl-induced K+ efflux
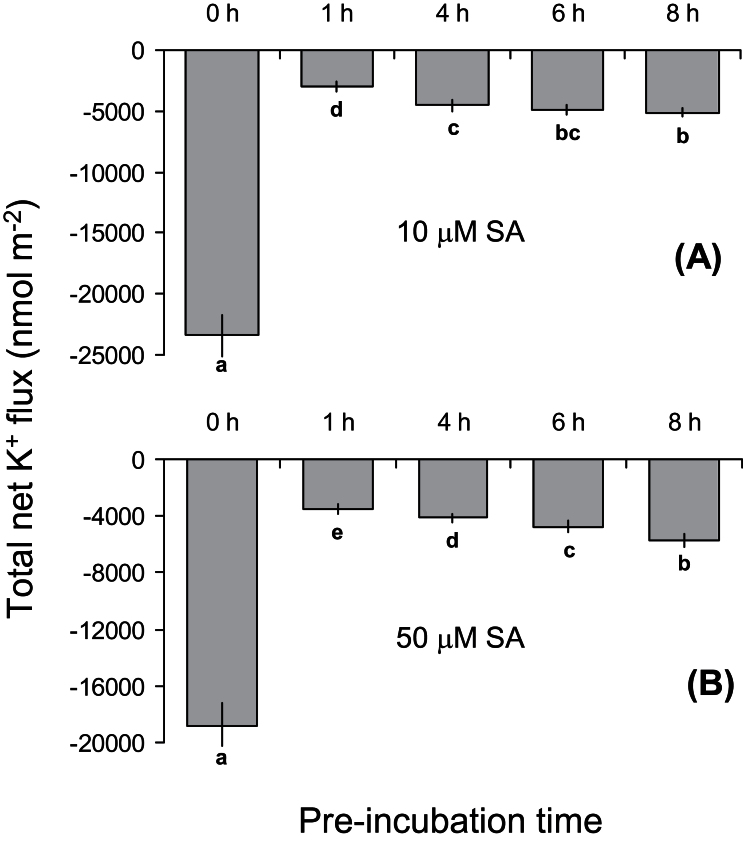
To determine the optimal duration of SA pretreatment, Arabidopsis seedlings were pretreated with two SA concentrations found to be effective (10 and 50 μM; Fig. 2) for four different times (1, 4, 6, or 8h). Although all the pretreatment durations significantly reduced the salt-induced K+ efflux, the 1-h pretreatment resulted in the lowest K+ efflux (Fig. 4A, B).
Fig. 4.
Total amount of K+ leaked from the mature root zone of 4–5-day-old Arabidopsis thaliana wild-type (Col-0) seedlings exposed to 100mM NaCl for 1h. Seedlings were pre-incubated in either 10 (A) or 50 (B) μM salicylic acid (SA) for various periods of time (0, 1, 4, 6, or 8h). Values are mean ± SE (n = 8 seedlings). Different letters indicate significant differences.
SA pretreatment upregulated H+-ATPase during salt stress
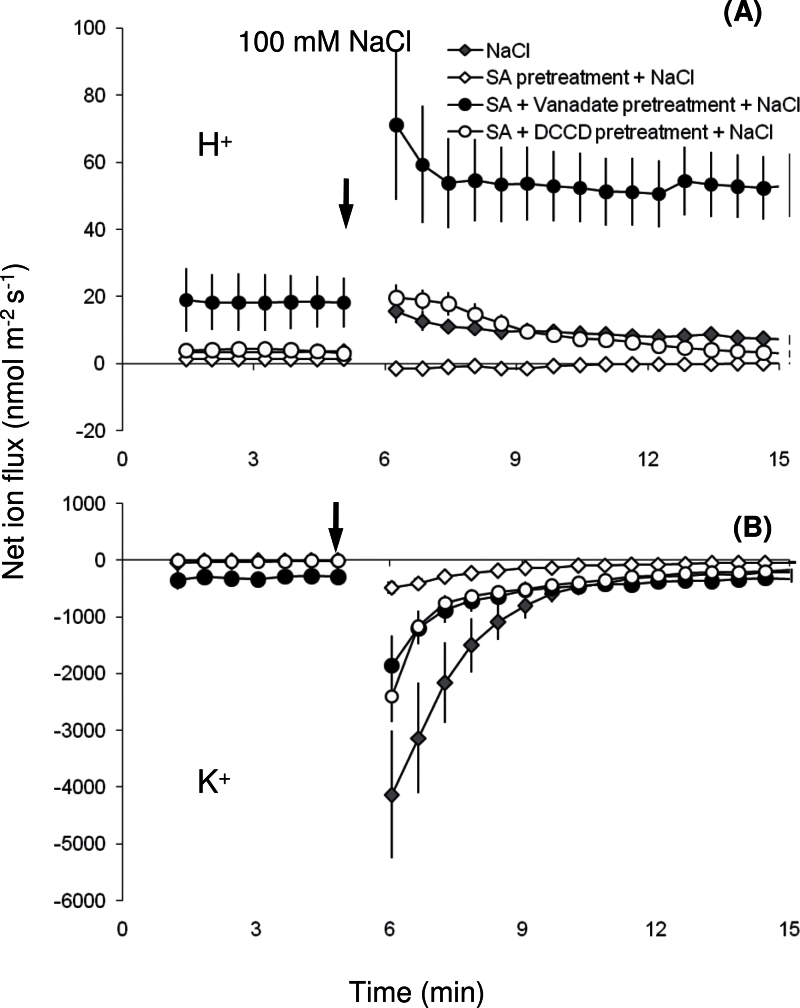
To ascertain whether NaCl-induced H+ efflux (Fig. 2B) was due to enhanced H+-ATPase activity, the Arabidopsis seedlings were pre-incubated with 50 μM SA in the presence of known inhibitors of H+-ATPase (1mM vanadate or 0.1mM DCCD). The 1-h pretreatment with SA and either vanadate or DCCD shifted H+ efflux (pretreatment with SA alone) to net H+ influx, which was particularly high with the SA + vanadate pretreatment (Fig. 5A). These results suggest that the partial reversal of the NaCl-induced influx (Fig. 3B, D) by SA pretreatment is due to SA enhancing the activity of H+-ATPase under salt stress.
Fig. 5.
Beneficial effects of salicylic acid (SA) during 100mM NaCl stress are diminished by root exposure to H+-ATPase pump inhibitors. NaCl-induced net H+ (A) and K+ fluxes (B) were measured at the mature root zone of 4- to 5-day-old Arabidopsis thaliana wild-type (Col-0) pretreated with 50 μM SA for 1h in the presence of either 1mM vanadate or 0.1mM N,N’-dicyclohexylcarbodiimide (DCCD). Values are mean ± SE (n = 7 seedlings).
The SA pretreatment combined with either vanadate or DCCD partially reversed improved K+ retention by SA-pretreated Arabidopsis seedlings during salt stress. Hence, a part of SA-related improvement in K+ retention under salt stress is attributed to increased plasma-membrane H+-ATPase activity.
SA pretreatment did not decrease Na+ entry into roots but reduced Na+ accumulation in the shoot
The acute salt stress (either 50 or 100mM NaCl) resulted in massive Na+ influx into the Arabidopsis roots (Table 2) during the first hour. Pretreatment of seedlings with 50 μM SA had no significant effect (P ≤ 0.05) on root Na+ uptake regardless of NaCl concentration (50 or 100mM) in the short-term MIFE experiments. In contrast, SA was effective in reducing shoot Na+ concentration in the long-term experiments (100mM NaCl for 2 weeks) (Table 3). Moreover, the SA pretreatment had a beneficial effect on shoot K+ concentration (Table 3). Hence, net root Na+ uptake appeared unaffected by SA, whereas either xylem Na+ loading and/or Na+ retrieval from the shoot were decreased by SA. This issue needs to be followed up in a separate study.
Table 2.
Effect of 1h pretreatment with 50 μM salicylic acid (SA) on net root Na+ uptake in 4-day-old Arabidopsis thaliana wild-type (Col-0) seedlings exposed to various salinities. Values are mean ± SE (n = 7). Data with different letters are significantly different (P ≤ 0.05).
| Treatment | Net root uptake (μmol Na+ m–2) |
|---|---|
| 50mM NaCl | 73.8±1.5a |
| 50mM NaCl + SA | 74.2±1.6a |
| 100mM NaCl | 137.3±1.8b |
| 100mM NaCl + SA | 136.2±1.9b |
Table 3.
Effect of salicylic acid (SA) pretreatment on shoot ion content of Arabidopsis thaliana grown under saline (100mM NaCl) conditions for 2 weeks. Wild-type (Col-0) plants were grown either in hydroponic or soil culture until 3 weeks old before the treatment was applied. For salicylic acid (SA) pretreatment, seedlings in centrifuge tubes were kept in nutrient solution supplemented with 50 μM SA (for hydroponic culture) or pots were irrigated with 50 μM SA (for soil culture) for 1h. Values are mean ± SE (n = 4). Data with different letters are significantly different (P ≤ 0.05).
| Treatment | Shoot Na (g (kg DW)–1) | Shoot K (g (kg DW)–1) |
|---|---|---|
| Control | 0.83±0.05a | 19.2±0.05a |
| SA | 0.91±0.09b | 19.6±1.42a |
| NaCl | 37.1±2.2d | 9.4±0.48b |
| NaCl + SA | 24.2±1.8c | 13.1±0.69c |
SA pretreatment decreased the extent of plasma-membrane depolarization during salt stress
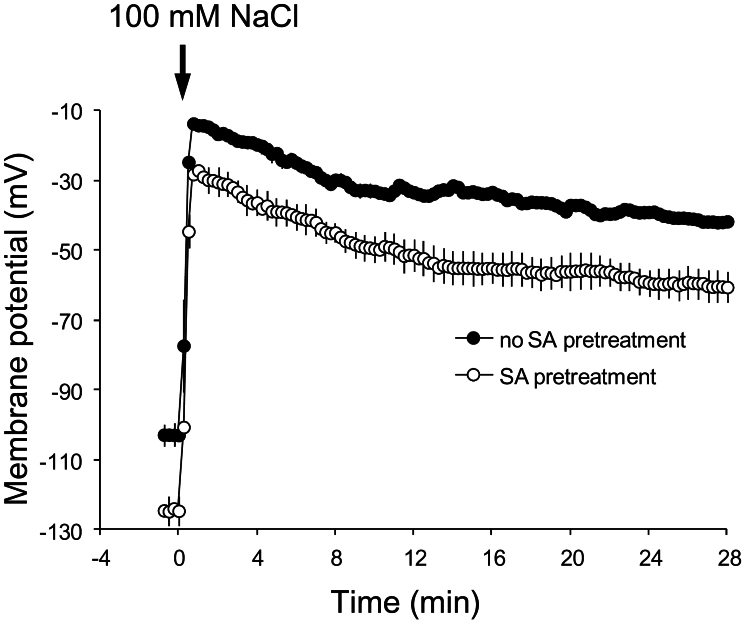
Keeping in mind that many plasma-membrane K+ channels are voltage dependent, effects of SA pretreatment on membrane potential kinetics during NaCl stress in root epidermal cells of Arabidopsis were tested. Pretreatment of roots in 50 μM SA for 1h shifted the resting potential towards more negative values compared with SA-untreated plants (–127±3 and –105±2 mV, respectively) under control conditions. Adding 100mM NaCl to the bathing medium resulted in a highly significant membrane depolarization (Fig. 6); however, a 20 mV difference between SA-untreated and -pretreated plants was maintained throughout the measurement period (Fig. 6).
Fig. 6.
Effect of 1-h pretreatment with 50 μM salicylic acid (SA) on transient plasma-membrane potential changes in the mature root zone of 4–5-day-old Arabidopsis thaliana wild-type (Col-0) seedlings during acute 100mM NaCl treatment. Values are means ± SE (n = 8 seedlings).
SA pretreatment decreased NaCl-induced K+ efflux by modulating K+ conductance via depolarization-activated KOR channels
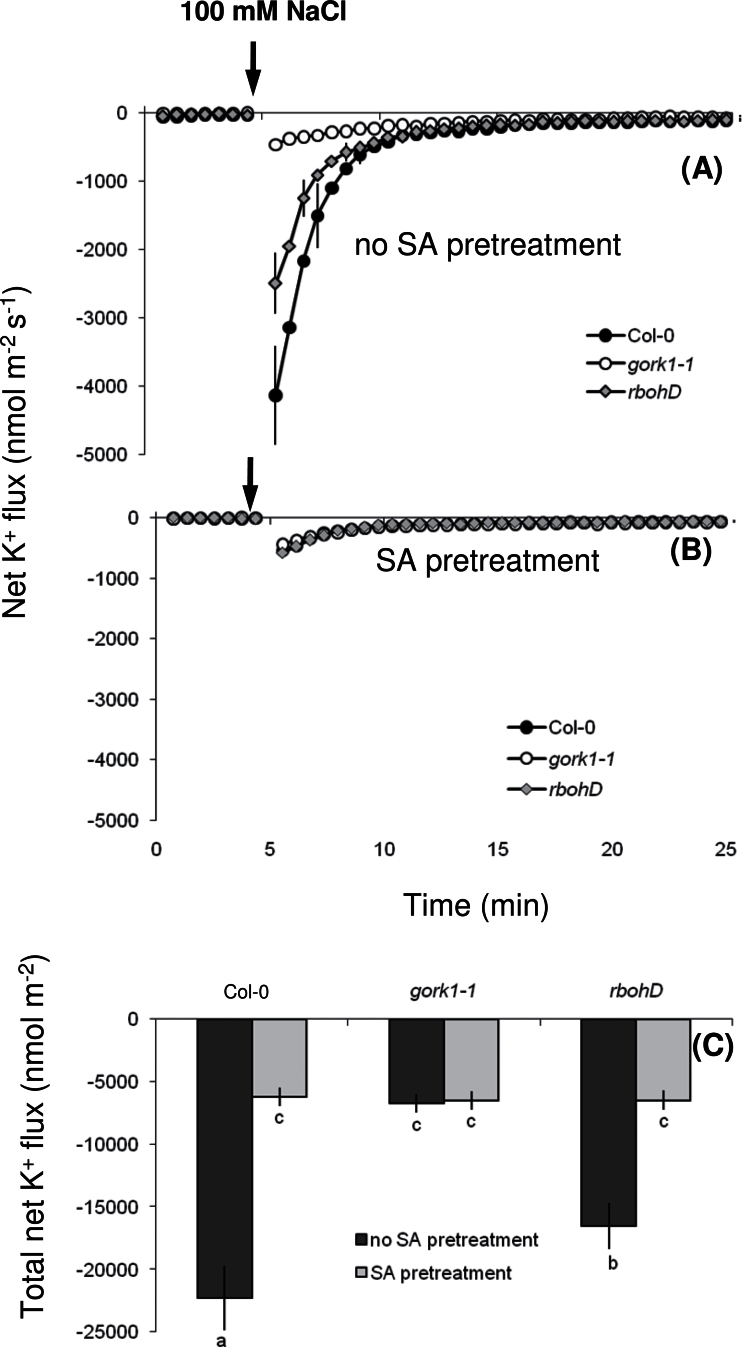
The SA pretreatment might have prevented K+ loss during salt stress by modulating K+ conductance via either depolarization-activated KOR and/or ROS-activated NSCC channels. To delineate between those channels, NaCl-induced K+ fluxes were measured in two Arabidopsis mutants, viz. (i) gork1-1 mutant that lacks depolarization-activated KOR channels in the root epidermal cells (Ivashikina et al., 2001; Hosy et al., 2003; Demidchik et al., 2010) and (ii) rbohD mutant that cannot produce ROS through NADPH oxidase during salt stress (Xie et al., 2011; Ma et al., 2012), but has a fully functional GORK channel.
Without the SA pretreatment, NaCl-induced K+ efflux was smallest in gork1-1, followed by rbohD, and was highest in the wild type (Fig. 7A, C). The SA pretreatment significantly decreased NaCl-induced K+ efflux in the wild type and the rbohD mutant, but not in the gork1-1 mutant (Fig. 7B, C). Thus, these results suggest the SA pretreatment decreased K+ loss by modulating K+ conductance through KOR (GORK) channels.
Fig. 7.
Salinity (100mM NaCl)-induced net K+ fluxes at the mature root zone of 4–5-day-old seedlings of Arabidopsis thaliana wild type (Col-0), gork1-1, and rbohD mutants without (A) and with (B) 1-h 50 μM salicylic acid (SA) pretreatment. (C) The total amount of K+ leaked during 1-h NaCl stress. Values are means ± SE (n = 8 seedlings). Different letters in (C) indicate significant differences.
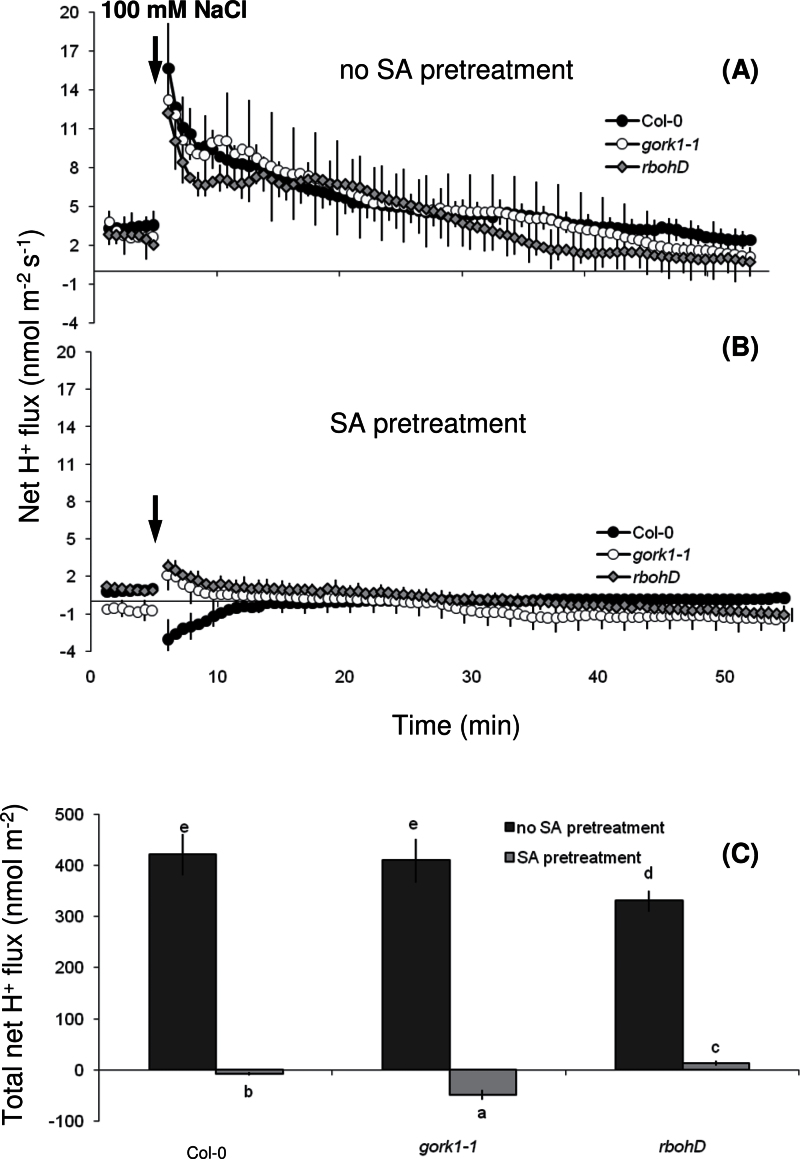
Consistent with previous results (Figs. 2B and 3B), the SA pretreatment either decreased net H+ influx or even induced H+ efflux in all three genotypes during salt stress (Fig. 8). Thus, the SA pretreatment enhanced H+-ATPase activity in all genotypes (Fig. 8), but the beneficial effects on retention of K+ in the cells were not observed in the gork1-1 mutant (Fig. 7C) because it lacks voltage-dependent (depolarization-activated) KOR channels.
Fig. 8.
Salinity (100mM NaCl)-induced net H+ fluxes at the mature root zone of 4–5-day-old seedlings of Arabidopsis thaliana wild type (Col-0), gork1-1, and rbohD mutants without (A) and with (B) 1-h 50 μM salicylic acid (SA) pretreatment. (C) The total H+ flux during 1-h NaCl stress. Values are means ± SE (n = 8 seedlings). Different letters in (C) indicate significant differences.
Discussion
SA pretreatment decreased NaCl-induced K+ efflux from Arabidopsis roots
Ion transport processes, particularly K+ homeostasis maintenance during salt stress, has emerged as a fundamental component of salt tolerance mechanism (Maathuis and Amtmann, 1999; Shabala and Cuin, 2008; Demidchik et al., 2010). Indeed, a strong positive correlation between the ability of roots to retain K+ and salt tolerance was previously reported in barley (Chen et al., 2005, 2007a,b), wheat (Cuin et al., 2008), lucerne (Smethurst et al., 2008), and Arabidopsis (Shabala et al., 2005, 2006). Although exogenous application of SA ameliorated detrimental effects of salinity in many plant species (see Horváth et al., 2007; Ashraf et al., 2010; Hayat et al., 2010) and resulted in increased K+ concentration in roots (e.g He and Zhu, 2008; Kováčik et al., 2009), it is unclear whether enhanced K+ uptake or prevention of K+ loss played a major role in this ameliorative effect.
The entry of positively charged Na+ ions through the plasma membrane (Table 2) resulted in 70–90-mV depolarization during 100mM NaCl stress (Fig. 9). During this depolarization, K+ uptake through K+ inward rectifying (KIR) channels is thermodynamically impossible; at the same time, depolarization would favour increased K+ leakage through depolarization-activated KOR channels (Shabala and Cuin, 2008). Indeed, the SA-untreated plants exhibited relatively large K+ efflux upon exposure to acute salt stress (Fig. 2A). The 1-h pretreatment of Arabidopsis in physiologically relevant concentrations (10–500 μM) of SA decreased NaCl-induced K+ efflux from roots (resulting in enhanced K+ retention). Moreover, SA-pretreated plants decreased the extent of depolarization by about 20 mV during salt stress (Fig. 9). Hence, these results suggest that SA-pretreated roots maintained a lower depolarized membrane potential under salt stress, thereby decreasing NaCl-induced depolarization-activated K+ efflux through KOR channels.
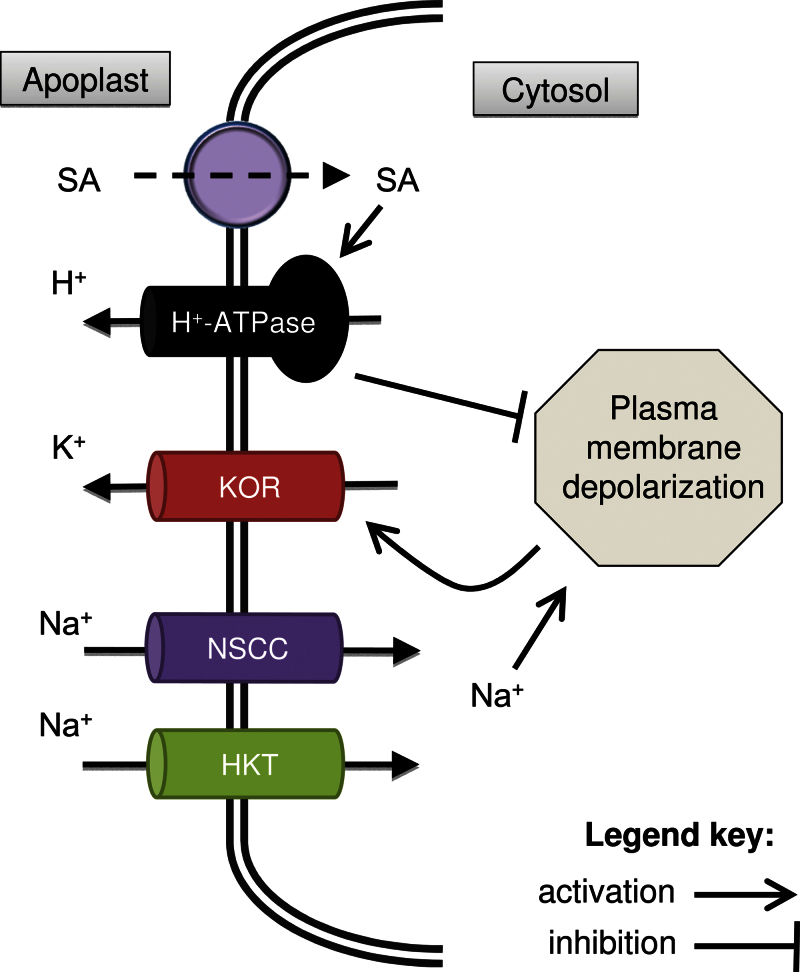
Fig. 9.
Schematic diagram depicting the beneficial effects of salicylic acid to cytosolic K+ homeostasis in plant roots. HKT, high-affinity K+ transporter; KOR, K+ outward-rectifying channels; NSCC- non-selective cation channels; SA, salicylic acid.
GORK channels are downstream targets of salicylic acid
To prove the notion of the KOR channels being downstream targets of SA action, additional experiments were conducted involving two Arabidopsis mutants, namely gork1-1, lacking depolarization-activated KOR (Ivashikina et al., 2001; Hosy et al., 2003; Demidchik et al., 2010), and rbohD, lacking the capacity to produce ROS via NADPH oxidase and thus without the capacity to activate ROS-dependent channels (Xie et al., 2011; Ma et al., 2012). Comparison of NaCl-induced K+ efflux between wild type, gork1-1, and rbohD (Fig. 7) suggested that about 25% of the K+ efflux was mediated by ROS-activated channels and remaining 75% was mediated by depolarization-activated KOR during salt stress (Fig. 7A, C). Pretreatment with SA did not provide any additional decrease in NaCl-induced K+ efflux from the gork1-1 mutant (Fig. 7B, C). In contrast, the SA pretreatment decreased NaCl-induced K+ efflux in the wild type and the rbohD mutant to make it similar to the gork1-1 mutant (Fig. 7B, C). Thus, these results provide explicit evidence that a decrease in K+ efflux through depolarization-activated GORK channels is the key mechanism in SA-mediated salt tolerance in Arabidopsis.
SA action is dependent on SA dose and pretreatment duration
The diminishing effect of exogenously applied SA on both NaCl-induced K+ efflux and H+ influx was not obvious when 50 μM SA was supplied simultaneously with 100mM NaCl, but reached maximum when SA was applied as 1-h pretreatment before the salt addition (Fig. 3). This suggests transport of SA and build up of optimal SA concentration inside the root tissue is critical during salt stress. Reaching the threshold SA concentration inside the root tissue is dependent on several factors, such as external concentration of SA, the mode of plasma-membrane transport, and duration of the pretreatment. The experiments with various concentrations of exogenous SA showed that SA at ≤100 μM was effective in decreasing K+ leakage from cells, whereas 500 μM SA (Fig. 2) and higher concentrations (1mM and above in experiments reported in the literature, Norman et al., 2004) are not as effective and can even be detrimental. Moreover, Norman et al. (2004) showed that a 6–10-fold increase in cytosolic SA concentration is achieved within 0.5–1h of SA pretreatment, and that elevated cytosolic SA concentrations dropped sharply after 4h of pretreatment. Hence, it is suggested here that 1h pretreatment is the optimum duration to reach the desired cytosolic SA concentrations and get the most beneficial effects during salt stress (Fig. 4). The slightly diminished SA effects in longer pretreatment durations (4, 6, and 8h; Fig. 4) can be explained by the metabolic conversion of free SA into inactive SA forms and subsequent transport and storage in the vacuole (Dean et al., 2005; Dempsey et al., 2011).
SA pretreatment enhanced H+-ATPAse activity during salt stress
The SA pretreatment shifted NaCl-induced H+ influx towards zero net H+ flux (Figs. 2B, 3B, 5A, and 8). This effect was diminished in roots pretreated with SA and vanadate or DCCD, two known metabolic inhibitors of H+-ATPase activity (Fig. 5A), giving a strong evidence for the ameliorating effect of SA on the H+-ATPase pump during salt stress. Similarly, the SA pretreatment shifted the resting membrane potential towards more negative values by about –20 mV; the same potential difference between SA-pretreated and SA-untreated roots was maintained during salt stress (Fig. 6). Hence, these results demonstrate that SA pretreatment enhanced H+-ATPase activity under salt stress, which in turn minimized the extent of plasma-membrane depolarization and thus decreased NaCl-induced K+ efflux via depolarization-activated KOR channels. Even though SA was found to enhance the H+-ATPase activity in pea and grape leaves under temperature stress (Liu et al., 2008, 2009), the present paper is the first report, as far as is known, on the role of enhanced H+-ATPase activity in the SA-mediated salinity tolerance in plants.
SA decreased root-to-shoot transport of Na+ during salt stress
Electrophysiological and molecular studies (reviewed in Apse and Blumwald, 2007) suggest that Na+ uptake into the root tissue occurs predominantly through NSCC and high-affinity K+ transporter-like proteins (Fig. 9). In this study, SA had no impact on net Na+ uptake by roots during 1-h-long salt stress (Table 2), suggesting SA would not modulate Na+ entry pathways. On the other hand, shoot Na+ content was significantly lower in SA-pretreated in comparison with SA-untreated plants (Table 3), implying SA decreased xylem loading of Na+ in roots and/or enhanced Na+ retrieval from the shoot during long-term salt stress. The decreased shoot Na+ concentration was also accompanied by higher K+ concentration (Table 3), suggesting that shoots of SA-pretreated plants were better protected from prolonged salt stress. Indeed, the SA-pretreated plants showed marked improvement over SA-untreated plants in shoot growth and water content during long-term salt exposure (Table 1).
In summary, these whole-plant ion flux, membrane potential, and pharmacological experiments suggest the following mechanism (Fig. 9). Under saline conditions, Na+ enters into the cytosol (via either NSCC or high-affinity K+ transporter, or both), causing significant depolarization of the plasma membrane. As a result, K+ leaks through depolarization-activated KOR channels. Pretreatment with SA enhances the H+-ATPase activity under salt stress in a time- and concentration-dependent manner. Enhanced H+-ATPase activity assists in maintaining membrane potential at more negative values, decreasing K+ leakage through depolarization-activated KOR channels during acute salt stress, resulting in better plant growth under long-term stress. In addition, at the whole plant level, SA also exerts beneficial effects by decreasing Na+ transport to the shoot. Hence, compared with SA-untreated plants, the SA-pretreated plants have the higher cytosolic K+/Na+ ratio required for normal cell functioning under salt stress.
Acknowledgements
MJ is a recipient of a Australian Postgraduate Award and a University of Western Australia Postgraduate Award. This work was supported by an ARC grant to SS and ZR.
References
- Apse MP, Blumwald E. 2007. Na+ transport in plants. FEBS Letters 581, 2247–2254. [DOI] [PubMed] [Google Scholar]
- Asensi-Fabado M, Munné-Bosch S. 2011. The aba3-1 mutant of Arabidopsis thaliana withstands moderate doses of salt stress by modulating leaf growth and salicylic acid levels. Journal of Plant Growth Regulation 30, 456–466. [Google Scholar]
- Ashraf M, Akram NA, Arteca RN, Foolad MR. 2010. The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Critical Reviews in Plant Sciences 29, 162–190. [Google Scholar]
- Bose J, Babourina O, Shabala S, Rengel Z. 2010a. Aluminium-induced ion transport in Arabidopsis: the relationship between Al tolerance and root ion flux. Journal of Experimental Botany 61, 3163–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J, Babourina O, Shabala S, Rengel Z. 2010b. Aluminum-dependent dynamics of ion transport in Arabidopsis: specificity of low pH and aluminum responses. Physiologia Plantarum 139, 401–412. [DOI] [PubMed] [Google Scholar]
- Carden DE, Walker DJ, Flowers TJ, Miller AJ. 2003. Single-cell measurements of the contributions of cytosolic Na+ and K+ to salt tolerance. Plant Physiology 131, 676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Cuin TA, Zhou M, Twomey A, Naidu BP, Shabala S. 2007a. Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. Journal of Experimental Botany 58, 4245–4255. [DOI] [PubMed] [Google Scholar]
- Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S. 2005. Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant, Cell and Environment 28, 1230–1246. [Google Scholar]
- Chen Z, Pottosin II, Cuin TA, et al. 2007b. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiology 145, 1714–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuin T, Shabala S. 2007. Amino acids regulate salinity-induced potassium efflux in barley root epidermis. Planta 225, 753–761. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Betts SA, Chalmandrier R, Shabala S. 2008. A root’s ability to retain K+ correlates with salt tolerance in wheat. Journal of Experimental Botany 59, 2697–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuin TA, Shabala S. 2005. Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots. Plant and Cell Physiology 46, 1924–1933. [DOI] [PubMed] [Google Scholar]
- Dean JV, Mohammed LA, Fitzpatrick T. 2005. The formation, vacuolar localization, and tonoplast transport of salicylic acid glucose conjugates in tobacco cell suspension cultures. Planta 221, 287–296. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, Sokolik A, Yurin V. 2010. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. Journal of Cell Science 123, 1468–1479. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Maathuis FJM. 2007. Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytologist 175, 387–404. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM. 2003. Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. Journal of Cell Science 116, 81–88. [DOI] [PubMed] [Google Scholar]
- Dempsey DA, Shah J, Klessig DF. 1999. Salicylic acid and disease resistance in plants. Critical Reviews in Plant Sciences 18, 547–575. [Google Scholar]
- Dempsey DA, Vlot AC, Wildermuth MC, Klessig DF. 2011. Salicylic acid biosynthesis and metabolism. The Arabidopsis book 9, e0156. 0110.1199/tab.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant WE, Dong X. 2004. Systemic acquired resistance. Annual Review of Phytopathology 42, 185–209. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Hajibagheri MA. 2001. Salinity tolerance in Hordeum vulgare: ion concentrations in root cells of cultivars differing in salt tolerance. Plant and Soil 231, 1–9. [Google Scholar]
- Glass ADM. 1974a. Influence of phenolic acids on ion uptake. IV Depolarization of membrane potentials. Plant Physiology 54, 855–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass ADM. 1974b. Influence of phenolic acids upon ion uptake. III Inhibition of potassium absorption. Journal of Experimental Botany 25, 1104–1113. [Google Scholar]
- Guo K-M, Babourina O, Rengel Z. 2009. Na+/H+ antiporter activity of the SOS1 gene: lifetime imaging analysis and electrophysiological studies on Arabidopsis seedlings. Physiologia Plantarum 137, 155–165. [DOI] [PubMed] [Google Scholar]
- Harper JR, Balke NE. 1981. Characetrization of the inhibition of K+ absorption in oat roots by salicylic acid. Plant Physiology 68, 1349–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat Q, Hayat S, Irfan M, Ahmad A. 2010. Effect of exogenous salicylic acid under changing environment: a review. Environmental and Experimental Botany 68, 14–25. [Google Scholar]
- He Y, Zhu Z. 2008. Exogenous salicylic acid alleviates NaCl toxicity and increases antioxidative enzyme activity in Lycopersicon esculentum . Biologia Plantarum 52, 792–795. [Google Scholar]
- Horváth E, Szalai G, Janda T. 2007. Induction of abiotic stress tolerance by salicylic acid signaling. Journal of Plant Growth Regulation 26, 290–300. [Google Scholar]
- Hosy E, Vavasseur A, Mouline K, et al. 2003. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proceedings of the National Academy of Sciences, USA 100, 5549–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashikina N, Becker D, Ache P, Meyerhoff O, Felle HH, Hedrich R. 2001. K+ channel profile and electrical properties of Arabidopsis root hairs. FEBS Letters 508, 463–469. [DOI] [PubMed] [Google Scholar]
- Jayakannan M, Babourina O, Rengel Z. 2011. Improved measurements of Na+ fluxes in plants using calixarene-based microelectrodes. Journal of Plant Physiology 168, 1045–1051. [DOI] [PubMed] [Google Scholar]
- Kerkeb L, Donaire JP, Rodríguez-Rosales MP. 2001. Plasma membrane H+-ATPase activity is involved in adaptation of tomato calli to NaCl. Physiologia Plantarum 111, 483–490. [DOI] [PubMed] [Google Scholar]
- Kováčik J, Klejdus B, Hedbavny J, Bačkor M. 2009. Salicylic acid alleviates NaCl-induced changes in the metabolism of Matricaria chamomilla plants. Ecotoxicology 18, 544–554. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu H, Pan Q, Yang H, Zhan J, Huang W. 2009. The plasma membrane H+-ATPase is related to the development of salicylic acid-induced thermotolerance in pea leaves. Planta 229, 1087–1098. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang J, Liu H, Huang W. 2008. Salicylic acid or heat acclimation pre-treatment enhances the plasma membrane-associated ATPase activities in young grape plants under heat shock. Scientia Horticulturae 119, 21–27. [Google Scholar]
- Ma L, Zhang H, Sun L, Jiao Y, Zhang G, Miao C, Hao F. 2012. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. Journal of Experimental Botany 63, 305–317. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Amtmann A. 1999. K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Annals of Botany 84, 123–133. [Google Scholar]
- Marschner H. 1995. Mineral nutrition of higher plants. London, UK.: Academic Press. [Google Scholar]
- Miller G, Shulaev V, Mittler R. 2008. Reactive oxygen signaling and abiotic stress. Physiologia Plantarum 133, 481–489. [DOI] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. 2009. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell and Environment 33, 453–467. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. 2004. Reactive oxygen gene network of plants. Trends in Plant Science 9, 490–498. [DOI] [PubMed] [Google Scholar]
- Newman IA. 2001. Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant, Cell and Environment 24, 1–14. [DOI] [PubMed] [Google Scholar]
- Niu X, Narasimhan ML, Salzman RA, Bressan RA, Hasegawa PM. 1993. NaCl regulation of plasma membrane H+-ATPase gene expression in a glycophyte and a halophyte. Plant Physiology 103, 713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman C, Howell KA, Millar AH, Whelan JM, Day DA. 2004. Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant Physiology 134, 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfi C, Pottosin I, Cuin T, Mancuso S, Shabala S. 2010. Specificity of polyamine effects on NaCl-induced ion flux kinetics and salt stress amelioration in plants. Plant and Cell Physiology 51, 422–434. [DOI] [PubMed] [Google Scholar]
- Sahu BB, Shaw BP. 2009. Salt-inducible isoform of plasma membrane H+-ATPase gene in rice remains constitutively expressed in natural halophyte, Suaeda maritima . Journal of Plant Physiology 166, 1077–1089. [DOI] [PubMed] [Google Scholar]
- Shabala L, Cuin TA, Newman IA, Shabala S. 2005. Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta 222, 1041–1050. [DOI] [PubMed] [Google Scholar]
- Shabala S, Cuin TA. 2008. Potassium transport and plant salt tolerance. Physiologia Plantarum 133, 651–669. [DOI] [PubMed] [Google Scholar]
- Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Miller AJ, Davies JM, Newman IA. 2006. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiology 141, 1653–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Hariadi Y. 2005. Effects of magnesium availability on the activity of plasma membrane ion transporters and light-induced responses from broad bean leaf mesophyll. Planta 221, 56–65. [DOI] [PubMed] [Google Scholar]
- Shabala S, Shabala L, Van Volkenburgh E. 2003. Effect of calcium on root development and root ion fluxes in salinised barley seedlings. Functional Plant Biology 30, 507–514. [DOI] [PubMed] [Google Scholar]
- Shabala SN, Newman IA, Morris J. 1997. Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiology 113, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HZ, Ishitani M, Kim CS, Zhu JK. 2000. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proceedings of the National Academy of Sciences, USA 97, 6896–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog DA, West DM, Holler FJ, Crouch SR. 2000. Analytical chemistry: an introduction. Philadelphia, PA, USA: Saunders College Publishing; (pp 594–631). [Google Scholar]
- Smethurst CF, Rix K, Garnett T, Auricht G, Bayart A, Lane P, Wilson SJ, Shabala S. 2008. Multiple traits associated with salt tolerance in lucerne: revealing the underlying cellular mechanisms. Functional Plant Biology 35, 640–650. [DOI] [PubMed] [Google Scholar]
- Tuteja N, Sopory SK. 2008. Chemical signaling under abiotic stress environment in plants. Plant Signaling and Behavior 3, 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y-J, Xu S, Han B, Wu M-Z, Yuan X-X, Han Y, Gu Q, Xu D-K, Yang Q, Shen W-B. 2011. Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role of RbohD-derived reactive oxygen species synthesis. The Plant Journal 66, 280–292. [DOI] [PubMed] [Google Scholar]