Abstract
Dehydration is a major factor resulting in huge loss from cut flowers during transportation. In the present study, dehydration inhibited petal cell expansion and resulted in irregular flowers in cut roses, mimicking ethylene-treated flowers. Among the five floral organs, dehydration substantially elevated ethylene production in the sepals, whilst rehydration caused rapid and elevated ethylene levels in the gynoecia and sepals. Among the five ethylene biosynthetic enzyme genes (RhACS1–5), expression of RhACS1 and RhACS2 was induced by dehydration and rehydration in the two floral organs. Silencing both RhACS1 and RhACS2 significantly suppressed dehydration- and rehydration-induced ethylene in the sepals and gynoecia. This weakened the inhibitory effect of dehydration on petal cell expansion. β-glucuronidase activity driven by both the RhACS1 and RhACS2 promoters was dramatically induced in the sepals, pistil, and stamens, but not in the petals of transgenic Arabidopsis. This further supports the organ-specific induction of these two genes. Among the five rose ethylene receptor genes (RhETR1–5), expression of RhETR3 was predominantly induced by dehydration and rehydration in the petals. RhETR3 silencing clearly aggravated the inhibitory effect of dehydration on petal cell expansion. However, no significant difference in the effect between RhETR3-silenced flowers and RhETR-genes-silenced flowers was observed. Furthermore, RhETR-genes silencing extensively altered the expression of 21 cell expansion-related downstream genes in response to ethylene. These results suggest that induction of ethylene biosynthesis by dehydration proceeds in an organ-specific manner, indicating that ethylene can function as a mediator in dehydration-caused inhibition of cell expansion in rose petals.
Key words: cut roses, dehydration, ethylene biosynthesis, ethylene perception, petal expansion, rehydration.
Introduction
Dehydration typically causes numerous morphological and developmental changes in plants, such as a reduced life cycle, inhibition of shoot and leaf growth, promotion of root growth, and early flowering (Xiong and Zhu, 2002). In the past two decades, the dehydration signalling pathway has been thoroughly studied (Seki et al., 2007). Although abscisic acid is generally regarded as the major hormonal signalling molecule for plants in response to dehydration (Seki et al., 2007), previous reports have demonstrated that ethylene can also play an important role in this biological process (Wilkinson and Davies, 2010).
The role of ethylene appears to be highly species and/or organ specific. Dehydration cannot induce ethylene production in an organ attached to a plant in many plant species, including wheat, beans, cotton, and miniature rose (Morgan et al., 1990; Narayana et al., 1991). However, dehydration can result in ethylene production in detached plant organs, such as cotton bolls and petioles (McMichael et al., 1972; Guinn, 1976), wheat leaves (McKeon et al., 1982), carnation and valencia orange flowers (Ben-Yehoshua and Aloni, 1974; Yakimova and Woltering, 1997), and avocado and persimmon fruits (Adato and Gazit, 1974; Nakano et al., 2003). In addition, ethylene has also been reported to function in rehydration, which usually occurs after dehydration during post-harvest handling. In Cleopatra mandarin seedlings, a rapid and substantial increase in ethylene production is observed in the leaves of water-stressed plants after rehydration, resulting in leaf abscission (Tudela and Primo-Millo, 1992). In wheat ears, rehydration of plants at full turgor after desiccation cause a high level of ethylene production (Beltrano et al., 1997).
As elevated ethylene production is reported in both dehydration and rehydration, it is of interest to study how the regulation of ethylene biosynthesis is involved in a plant’s response to dehydration and rehydration. Over the past three decades, the ethylene biosynthesis mechanism has been well documented in higher plants. The rate-limiting step of ethylene biosynthesis is conversion of AdoMet to 1-aminocyclopropane-1-carboxylic acid (ACC) by ACC synthase (ACS). ACC is then oxidized to ethylene by ACC oxidase (Yang and Hoffman, 1984). Ethylene is perceived by a family of endoplasmic reticulum-localized receptors (ETR1, ERS1, ETR2, EIN4, and ERS2) that are similar to the bacterial two-component system and function as negative regulators of the ethylene response (Chen et al., 2005).
ACS plays a key role in the rate-limiting step in ethylene biosynthesis, even though ACC oxidase can control ethylene production in some cases (Shi et al., 2006). ACS is encoded by a divergent multigene family and is regulated at both the transcriptional and post-transcriptional levels by various internal and external factors (Argueso et al., 2007). Expression of ACS genes exhibits spatial- and temporal-specific patterns in post-pollinated orchid flowers: PhalACS2 and PhalACS3 correlate with higher ACS activity in the stigma and ovary. A sequential increase in ACS activity in the labellum is attributed to the increased expression of ethylene-inducible PhalACS1 (Bui and O’Neill, 1998). In carnations, DCACS2 and DCACS3 are preferentially expressed in the styles, whereas DCACS1 mRNA is most abundant in the petals (Jones and Woodson, 1999). In detached persimmon fruits, water-loss-induced expression of DkACS2, a wound-induced ACS gene, in the calyx, caused large amounts of ethylene production. This triggers expression of DKACS1 and DKACS2, which leads to ethylene production in other tissues, such as the pulp, peel, and core (Nakano et al., 2003).
The rose is thought of as one of the most beautiful flowers in the world, with many romantic and sentimental associations. Cut roses account for approximately 31 and 21% of all cut-flower trade business in European and Chinese markets, respectively (Heinrichs, 2008). As a fresh product, rose flowers are particularly susceptible to dehydration-induced stress, which usually results in damage, such as the failure of buds to expand, wilted flowers, and bent necks (Jin et al., 2006). In cut roses, after ethylene treatment, the rapid and substantial increase in ethylene production in the gynoecia is associated with a rapid and enhanced expression of RhACS2 and RhACS3 (Xue et al., 2008). Expression of two receptor genes, RhETR1 and RhETR3, is enhanced by ethylene (Ma et al., 2006), and RhETR3 has been proven to be expressed in an organ-specific manner (Xue et al., 2008).
Currently, there is no direct evidence showing that members of the ACS gene family correspond to dehydration- and rehydration-induced ethylene biosynthesis in detached plant organs. It is also unclear which receptor gene plays a crucial role in the perception of ethylene during dehydration and rehydration.
In the work presented here, the temporal and spatial expression of ACS genes in rose floral organs was measured during dehydration and rehydration. Furthermore, the role of members of the ACS gene family in rose petal expansion in response to dehydration and rehydration was investigated using a virus-induced gene silencing (VIGS) approach. The perception of ethylene and the effect of the key receptor genes on the expression of potential ethylene-downstream genes related to cell expansion in rose petals were also investigated. The results suggested that induction of ethylene biosynthesis during dehydration proceeds in a tissue-specific manner and allows ethylene to function as a mediator in inhibition of cell expansion of rose petals caused by dehydration.
Materials and methods
Plant materials
Cut rose (Rosa hybrida) cv. Samantha was produced in a local solar greenhouse using standard commercial practices in Beijing, PR China. The flowers were harvested at flower opening stage 2 (completely opened bud) (Ma et al., 2005) and placed immediately in water. The flowers were delivered to the laboratory within 1h of harvesting and the stems were re-cut to 30cm in length under water and placed in deionized water (DW) until further processing.
Dehydration treatment and phenotype observations
For the dehydration treatment, the flowers were placed horizontally on test beds and exposed to air for 6, 12, 18, and 24h. The environmental conditions were: 25 °C, 40–50% relative humidity, and continuous light (140 μmol m–2 s–1). After dehydration treatment, the bottom stems of the flowers were re-cut under water, removing approximately 1cm from the 30cm total length, and were then placed in DW for rehydration and further evaluation of their opening.
To investigate the role of ethylene in dehydration, the rose flowers were sealed in a 64 l chamber with 2 ppm of 1-methylcyclopropene (1-MCP) for 12h and then with 10 ppm of ethylene for 24h at 25 °C, 40–50% relative humidity. The flowers exposed to ordinary atmospheric air served as the control group. NaOH (1M) was used to prevent the accumulation of CO2.
For observations of the phenotype and cell counting, one petal was chosen randomly from the second layer of each flower on the third day after dehydration. Photos of petals were taken using a USB scanner (Microtek Scanmaker 8700), and petal areas were measured independently using Adobe Photoshop 7.0 software. Abaxial subepidermis (AbsE) cell photography and cell counting were performed as described by Ma et al. (2008). A tissue sample (0.5×0.4cm) was excised at 25% of the petal length from the petal tip. The tissue slices were fixed in formaldehyde and cleared using ethanol. The number of AbsE cells was counted using ImageJ software in a visual field of 1360×1024 μm2.
Ethylene production of whole flowers and different floral tissues, including sepals, petals, androecia, gynoecia, and the receptacle, was determined as described by Xue et al. (2008). Based on previous work, the floral organs can produce wound ethylene when the incubation time is longer than a particular time. To avoid contamination of wound-induced ethylene, the gas chromatography vials were capped and incubated at 25 °C for 1h for sepals, petals, androecia, and receptacle, and for 40min for gynoecia (Xue et al., 2008).
RNA extraction and semi-quantitative RT-PCR analysis
For RNA extraction, one flower was defined as an independent sample. All sepals and gynoecia from the flower were collected. Petals from the second and third whorl were taken from the flower. Total RNA from the sepals and petals was extracted using the hot borate method described by Wan and Wilkins (1994). Total RNA from the gynoecia was extracted using the hot phenol method (Ma et al., 2005).
For semi-quantitative RT-PCR analysis, cDNAs were synthesized from 1 μg of total RNA using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA). The rose ubiquitin1 gene (Ubi1, JK622648) was used as the internal control. The primer sequences are listed in Table S1 at JXB online. The specificity of each primer pair was checked by sequencing of the PCR products. PCRs were carried out with 31 cycles for RhACS1–5, 27 cycles for Ubi1, and 27 cycles for RhETR1–5. The linearity of response with these cycle numbers was tested using cDNA dilutions. To control for background DNA contamination, a reaction using each gene primers but no reverse transcriptase, was performed. The relative transcript levels were determined by the densitometry of the signals using AlphaImagerTM2200 software (Alpha Innotech, USA), and a statistical analysis was performed using Duncan’s multiple range tests (P <0.05).
Silencing of RhACS and RhETR genes in rose flowers by VIGS
A tobacco rattle virus (TRV)-based vector, including pTRV1 and pTRV2 VIGS vectors (Liu et al., 2002), used in this work were graciously provided by Dr Yule Liu (Tsinghua University, PR China). The gene silencing in rose flowers by VIGS was performed according to the procedures described by Ma et al. (2008) with some modifications. VIGS experiments were performed a minimum of five times.
For ACS gene silencing, a 360bp gene-specific fragment at the 3′ end of RhACS1 and a 325bp fragment at the 3′ end of RhACS2 were amplified using cDNA as template. For RhACS1/2 silencing, the fragments of RhACS1 and RhACS2 were fused by overlapping PCR. The resulting products were inserted into pGEM-T Easy vector and subjected to sequencing. The vector was digested to produce fragments of 360bp for RhACS1, 325bp for RhACS2, and 685bp for RhACS1/2, which were then inserted into TRV2 plasmids. For ethylene receptor gene silencing, a 410bp gene-specific fragment at the 3’ end of RhETR3 and a 852bp fragment possessing a conserved domain of the ethylene receptor gene was used to construct the pTRV2-RhETR3 and pTRV2-RhETRs vectors, respectively, as described above (Table S2 at JXB online).
The constructs were transformed into Agrobacterium GV3101 by electroporation. Agrobacterium containing pTRV1, pTRV2, pTRV2-RhACS1, pTRV2-RhACS2, pTRV2-RhACS1/2, pTRV2-RhETR3, or pTRV2-RhETRs were grown at 28 °C in Luria–Bertani medium supplemented with 10mM MES, 20mM acetosyringone, and 50mg l–1 of kanamycin for approximately 24h. Agrobacterium cells were harvested and suspended in the infiltration buffer (10mM MgCl2, 200mM acetosyringone, and 10mM MES, pH 5.6) to a final optical denisty at 600nm of 1.5. A mixture of Agrobacterium cultures containing pTRV1 and pTRV2 or its derivatives (pTRV2-RhACS1, -RhACS2, -RhACS1/2, -RhETR3, or -RhETRs) at a ratio of 1:1 (v/v) were placed at room temperature for 4h before vacuum infiltration.
For vacuum infiltration, flower stems were placed upside down in an 81.64 l container, with the whole flower immersed into the bacterial suspension solution. They were then infiltrated by vacuum at 30 mmHg for 2min and allowed to slowly recover. The rose flowers were washed with DW and kept in DW for 3 d at 8 °C before the dehydration treatment.
A preliminary experiment observing the phenotype showed that it was difficult to see the difference in petal areas between TRV control and VIGS-silenced petals from the outer layers, because the petals of the outer layers partially extended during virus infection for 3 d at 8 °C before dehydration treatment. Therefore, petals were chosen randomly from the fourth layer of each flower on the second day after the treatment for measurements. Petal areas and cell numbers were determined according to the methods described above.
Results
Induction of ethylene production by dehydration in rose floral parts
After 24h of dehydration, flowers lost 22.8% of their initial weight and the flower water potential decreased to −3.2MPa from −0.5MPa (Supplementary Fig. S1 at JXB online). The flowers were capable of recovery with full opening once they had been rehydrated in water. However, after 36h dehydration, flower opening was severely impeded resulting in buds that would not open in over 50% of flowers during rehydration (data not shown). Therefore, in the following experiments, the dehydration treatment was only performed for a measurement of 24h.
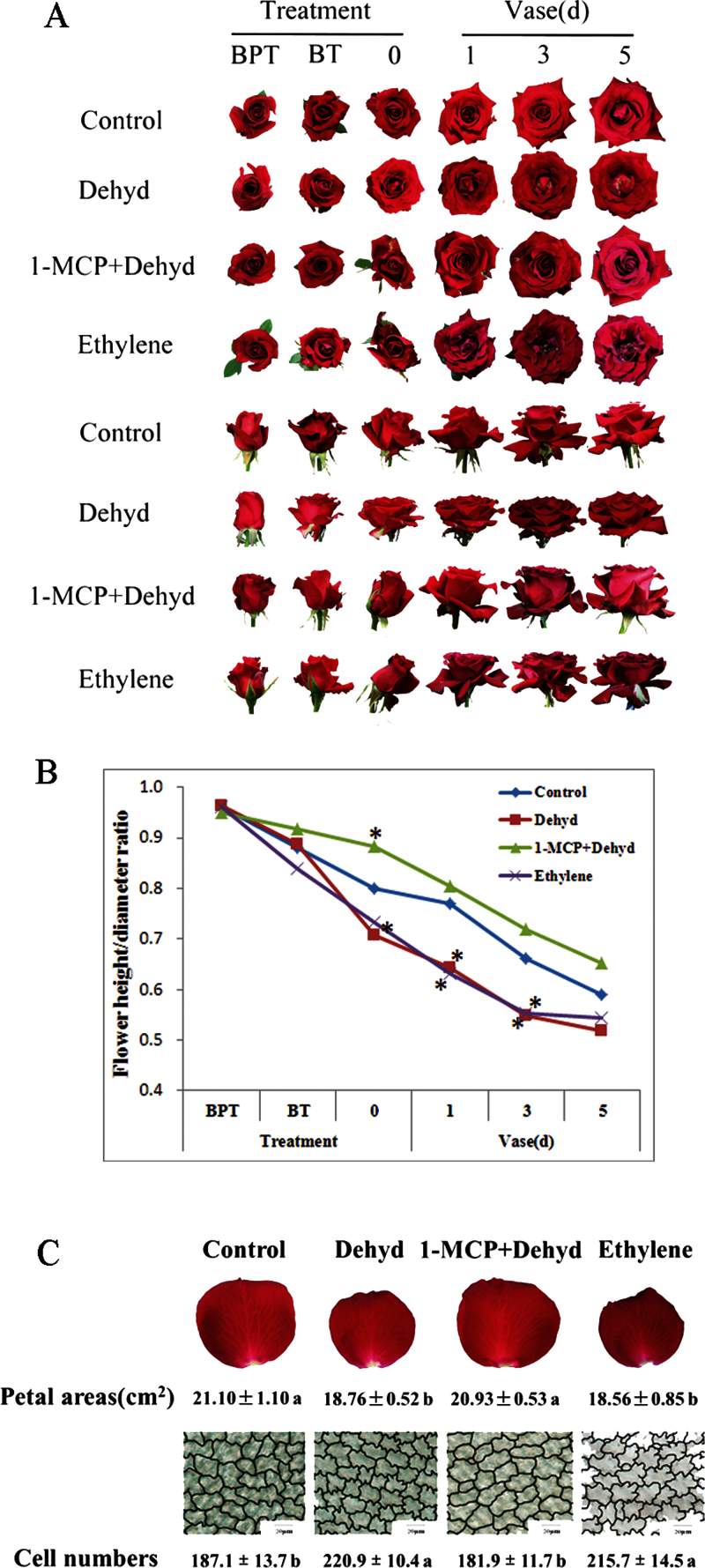
Compared with the control, flowers subjected to dehydration developed irregular shapes including uneven unfurling of the outer layer petals and curly edges (Fig. 1A). Dehydration also resulted in vertically compressed flowers with a decreased flower height-to-diameter ratio (Fig. 1B). This phenomenon was similar to those that were treated with ethylene. Pre-treatment with 1-MCP, an ethylene action inhibitor, clearly weakened the dehydration-induced negative effects on flower opening (Fig. 1A, B). Dehydration significantly decreased the petal area of the second layer in comparison with control flowers, similar to the observations on ethylene-treated flowers. As expected, 1-MCP pre-treatment effectively prevented petals from all negative influence caused by dehydration (Fig. 1C).
Fig. 1.
Effect of dehydration on flower opening and petal expansion in cut roses. (A) Flower opening processes in a vase after dehydration. Flowers at stage 2 were used for all treatments. Control, flowers placed in DW and under air throughout; Dehyd, flowers placed in DW were under air for 12h and then subjected to dehydration for 24h; 1-MCP+Dehyd, flowers placed in DW were pre-treated with 2 ppm 1-MCP for 12h and then subjected to dehydration for 24h; Ethylene, flowers placed in DW were pre-treated with 10 ppm ethylene for 12h and then moved to air. BPT, before pre-treatment; BT, before treatment; 0, 1, 3, 5, d in a vase after treatment. Each treatment comprised a minimum of 30 replicates, and representative results are shown. (B) Ratio of flower height-to-diameter. Asterisks indicate significant differences between control and dehydration-, 1-MCP+dehydration-, or ethylene-treated flowers according to Duncan’s multiple range test (n=30, P <0.05). (C) Petal expansion after dehydration. Upper panels: petal size (n=30). Lower panels: outline of petal AbsE cells. Bars, 200 μm. The traces were drawn using Photoshop 7.0 software. Cell numbers were counted using ImageJ software in a visual field of 1360×1024 μm2 (n=15). Different letters indicate significant differences among the treatments according to Duncan’s multiple range test (P <0.05).
The cell size of the petals was also measured to determine whether or not dehydration decreased petal area by inhibiting cell expansion. As shown in Fig. 1C, dehydration significantly increased cell density by 18.1% compared with the control. This indicated that dehydration caused a decrease in the cell size of the petals. Moreover, dehydration also resulted in a severely interlocking AbsE cell shape that mimicked the ethylene-treated petal cells. As expected, 1-MCP pre-treatment weakened the influence of dehydration on AbsE cell size and shape, indicating that ethylene may mediate the influence of dehydration on petal expansion of rose flowers.
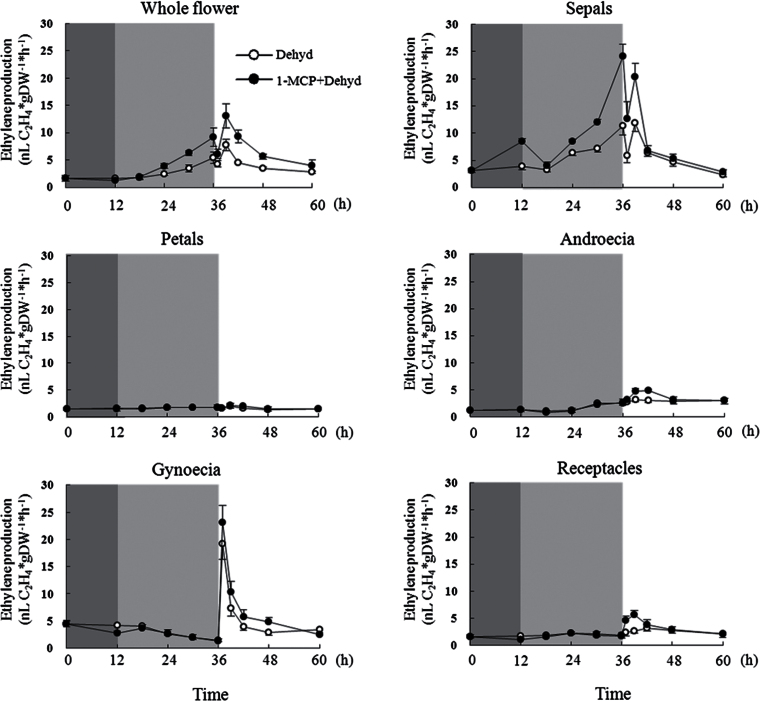
Ethylene production was determined in different floral parts during dehydration and rehydration. For the whole flower, ethylene production levels were low in untreated controls in a vase (Supplementary Fig. S2 at JXB online). It substantially increased from 18 to 24h in the control during dehydration. Surprisingly, 1-MCP pre-treatment promoted ethylene production during dehydration (Figs 2 and S2). Among the five floral organs, sepal-produced ethylene exhibited a clear increase from 12 to 24h, accounting for 44.3 and 65.4% of ethylene production in whole flowers at 18 and 24h (Table S4 at JXB online). Additionally, 1-MCP pre-treatment showed a significantly elevated effect on ethylene production in sepals among the five floral organs (Fig. 2).
Fig. 2.
Ethylene production in whole flowers and five floral organs of cut roses treated by dehydration. Dark grey shading indicates 1-MCP pre-treatment duration; light grey shading indicates the duration of dehydration, and a white background indicates the rehydration duration. Results are shown as means ±standard error (SE) (n=30).
During rehydration for the whole flower, ethylene slightly and transiently decreased at 1h and then sharply increased at 3h, followed by a gradual decline until 24h. Additionally, ethylene production of flowers was further promoted by 1-MCP pre-treatment during rehydration (Figs 2 and S2). In the gynoecia, ethylene production increased dramatically and attained a peak value, accounting for 20-fold that before rehydration, at 1h. In sepals, rehydration-induced ethylene production decreased at 1h and then recovered to a level close to that before rehydration. After recovery, it then decreased gradually. Ethylene production in the androecia and the receptacle maintained a constant level after slightly increasing at 3h. During the entire rehydration period, ethylene production in petals remained at a low level. 1-MCP pre-treatment further elevated ethylene production in all five tissues at an earlier time during rehydration.
Dehydration-induced expression of RhACS genes in the sepals and gynoecia
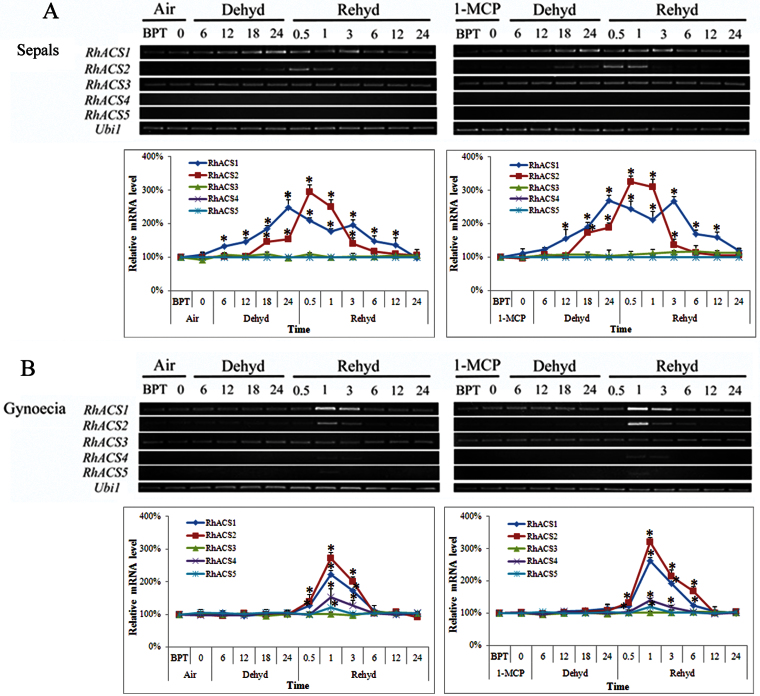
As ACS is the rate-limiting enzyme in ethylene biosynthesis, the mRNA levels of five known ACS genes in rose were determined during dehydration and rehydration. As dehydration-induced ethylene production is organ specific, more focus was given to the sepals and gynoecia, which are more responsible for ethylene production, during gene expression. RhACS1 and RhACS2 displayed substantially increasing levels of expression throughout the dehydration period (Fig. 3). RhACS3 mRNA levels maintained a constant level, whereas the mRNA levels of the RhACS4 and RhACS5 genes were hardly detectable. During dehydration, in sepals, expression of RhACS1 was slightly enhanced at 12h and then increased linearly. Expression of RhACS2 was increased at 18h. In the sepals, there was a significant difference in gene expression at late times of dehydration for RhACS2 but not for RhACS1 between the air control and 1-MCP pre-treatment.
Fig. 3.
Expression of RhACS1–5 in sepals (A) and gynoecia (B) of cut roses. Air, flowers placed in DW under air for 12h; 1-MCP, flowers placed in DW and pre-treated with 2 ppm 1-MCP for 12h. BPT, before pre-treatment. The number of hours of dehydration or rehydration treatment is indicated. One flower was defined as an independent biological sample. Each time point in the figure is the mean±SE of five biological replicates. The relative transcript levels were determined by densitometry of the signals using AlphaImagerTM2200. Asterisks indicate significant differences between BPT and each time point for the same gene according to Duncan’s multiple range tests (P <0.05). (This figure is available in colour at JXB online.)
In the gynoecia, the RhACS1 mRNA levels strongly increased at 1h during rehydration and remained at a high level until 3h, before gradually decreasing. Meanwhile, RhACS2 expression increased slightly at 0.5h and attained a peak value at 1h, before dramatically decreasing during rehydration. In the sepals, expression of RhACS1 exhibited a fluctuating pattern, and the expression decreased within 1h, began to increase at 3h, and then decreased gradually during rehydration. Expression of RhACS2 clearly increased at 0.5h and then declined (Fig. 3).
Effect of RhACS1/2 silencing on petal expansion of rose flowers during dehydration
In order to understand the contribution of RhACS1 and RhACS2 in the dehydration-caused ethylene production, RhACS1, RhACS2 or RhACS1/2 were silenced in rose flowers using a VIGS approach (Liu et al., 2002; Ma et al., 2008) (Supplementary Fig. S3 at JXB online).
As described above, increased levels of ethylene were observed in the sepals from 12 to 24h in the dehydration treatment. In the gynoecia and sepals, elevated ethylene levels occurred within 6h of rehydration. Therefore, ethylene production was determined at 18h of dehydration and 3h of rehydration in the sepals and gynoecia of gene-silenced flowers.
At 18h of dehydration, when compared with the TRV control, ethylene production was significantly reduced in RhACS1-, RhACS2-, or RhACS1/2-silenced sepals (Fig. 4A). RhACS1/2-silenced sepals had a greater reduction (79%) in ethylene production when compared with RhACS1- or RhACS2-silenced petals. At 3h of rehydration, in contrast to TRV, silencing of RhACS1, RhACS2, or RhACS1/2 significantly reduced ethylene production in the sepals and gynoecia (Fig. 4B).
Fig. 4.
Ethylene production of RhACS1-, RhACS2-, and RhACS1/2-silenced flowers during dehydration and rehydration. Rose flowers were infiltrated with Agrobacterium containing TRV alone (TRV: pTRV1+pTRV2) or TRV carrying a fragment of RhACS1, RhACS2, or RhACS1/2 (TRV-RhACS1: pTRV1+pTRV2-RhACS1; TRV-RhACS2: pTRV1+pTRV2-RhACS2; TRV-RhACS1/2: pTRV1+pTRV2-RhACS1/2). (A) Ethylene production in sepals. Upper panel, RT-PCR analysis of RhACS1 and RhACS2 in RhACS1-, RhACS2-, and RhACS1/2-silenced flowers. Lower panel, ethylene production at 18h dehydration and 3h rehydration. Different letters indicate significant differences between different treatments according to Duncan’s multiple range test (P <0.05). (B) Ethylene production in gynoecia at 3h rehydration. (C) Phenotype of RhACS1-, RhACS2-, and RhACS1/2-silenced flower petals after dehydration. Upper panel, petal size. Lower panel, outline of AbsE cells of petals. Cell numbers were counted using ImageJ software in a visual field of 1360×1024 μm2.
Flower opening was then observed in the RhACS1/2-silenced flowers. Consistent with ethylene production, the ratio of flower height to diameter was increased significantly in RhACS1-, RhACS2-, and RhACS1/2-silenced flowers after 24h of dehydration (Supplementary Fig. S4 at JXB online). This was in stark contrast to the TRV control. Again using TRV as a comparison, petal areas in the fourth layer of flowers significantly increased in RhACS1-, RhACS2-, and RhACS1/2-silenced flowers (Fig. 4C). Furthermore, the cell density of AbsE cells decreased significantly in RhACS1-, RhACS2-, and RhACS1/2-silenced petals (Fig. 4C).
Induction of the RhACS1/2 promoter activities in Arabidopsis floral parts by dehydration
To test whether or not dehydration directly influenced the expression of RhACS1 and RhACS2, the 2080bp 5’-upstream sequence of RhACS1 and 1584bp 5’-upstream sequence of RhACS2 were isolated from rose flowers. A number of cis-elements, including abscisic acid, dehydration-responsive, and tissue-specific elements, were identified in the two promoters (Supplementary Fig. S5 at JXB online) using the PLACE program (Higo et al., 1999). The RhACS1 promoter contained eight putative MYC (CANNTG) and two MYB (WAACCA) motifs, and the RhACS2 promoter possessed six putative MYC motifs and one MYB motif. Putative GATA boxes and OSE were also found in both promoters, which are considered to be involved in tissue-specific expression (Aird et al., 1994; Vieweg et al., 2004). A promoter assay using β-glucuronidase as reporter showed that the expression of RhACS1 and RhACS2 genes could be induced by dehydration, and the two genes showed an organ-specific induced expression (Figs S6 and S7 at JXB online).
Perception of dehydration-induced ethylene by rose petals
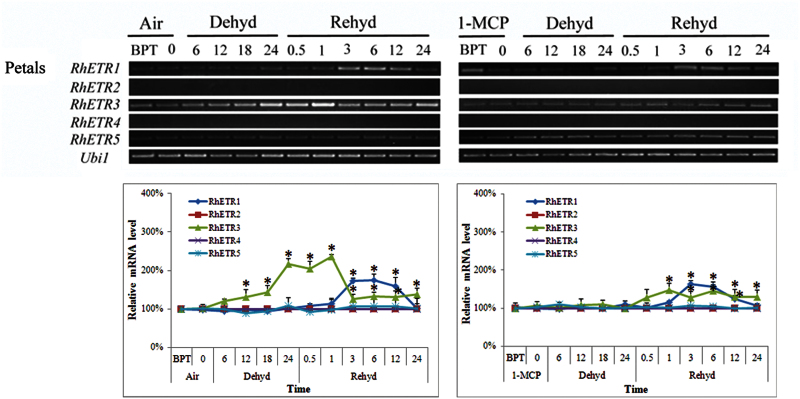
As ethylene may mediate the effect of dehydration on petal expansion, tests were performed to determine whether petals perceived the signal of ethylene induced by dehydration in the sepals and gynoecia. mRNA levels of RhETR2 and RhETR4 were undetectable, whilst RhETR5 expression was constant. Similarly, RhETR1 expression was not induced by dehydration. In contrast, RhETR3 expression was induced gradually in petals during the 24h dehydration treatment. During rehydration, RhETR3 expression maintained high levels until 1h of rehydration and then visibly decreased at 3h, and maintained an almost constant level within 24h of determination. 1-MCP pre-treatment strongly inhibited the expression of RhETR3 during dehydration and rehydration (Fig. 5).
Fig. 5.
Expression of RhETR1–5 genes in petals of cut roses. BPT, before pre-treatment. The number of hours of dehydration or rehydration treatment is indicated. One flower was defined as an independent biological sample. Each time point in the figure represents five biological replicates, and each band in the figure is a representative result. The relative transcript levels were determined by the methods used in Fig. 3. (This figure is available in colour at JXB online.)
In addressing the role of RhETR3 in petal expansion during dehydration, the effect of RhETR3 or RhETR-genes silencing on petal expansion was explored in rose flowers treated by dehydration using a VIGS approach (Liu et al., 2002; Ma et al., 2008) (Supplementary Fig. S8 at JXB online). Following infiltration treatment for the whole flower, RhETR3- and RhETR-genes-silenced flowers were successfully obtained, although the degree of silencing varied among the three RhETR members (Fig. 6A).
Fig. 6.
Silencing of RhETR3 or the RhETR genes in rose flowers by VIGS. Rose flowers were infiltrated with Agrobacterium containing TRV alone (TRV: pTRV1 + pTRV2) or TRV carrying a fragment comprising RhETR3 or the RhETR genes (TRV-RhETR3: pTRV1+pTRV2-RhETR3; TRV-RhETRs: pTRV1+pTRV2-RhETRs). (A) RT-PCR analysis of RhETR1, RhETR3, and RhETR5 genes in RhETR3- or RhETR-genes-silenced flower petals. (B) The phenotype of RhETR3- or RhETR-genes-silenced flower petals after dehydration. Upper panel, petal size. Lower panel, outline of AbsE cells of petals. Cell numbers were counted using ImageJ software in a visual field of 1360×1024 μm2. Different letters indicate significant differences between different treatments according to Duncan’s multiple range tests (P <0.05).
On the second day after dehydration treatment, dehydration-caused flower-opening phenotypes, such as irregular shape, were further aggravated in RhETR3-silenced flowers compared with TRV (Supplementary Fig. S9 at JXB online). Dehydration-caused inhibition of petal expansion and cell expansion was also significantly aggravated in RhETR3-silenced rose flowers (Fig. 6B).
In addition, the changes in petal cell expansion contrasted between RhETR3- and RhETRs-silenced flowers. The decrease in petal area in RhETR3-silenced petals (11.3%) was equivalent to 85.0% of that in RhETRs-silenced petals (13.3%). The increased in cell density accounted for 73.7% of that in RhETRs-silenced petals (Fig. 6B). These findings illustrated that RhETR3 plays an important role in perception of ethylene production induced by dehydration.
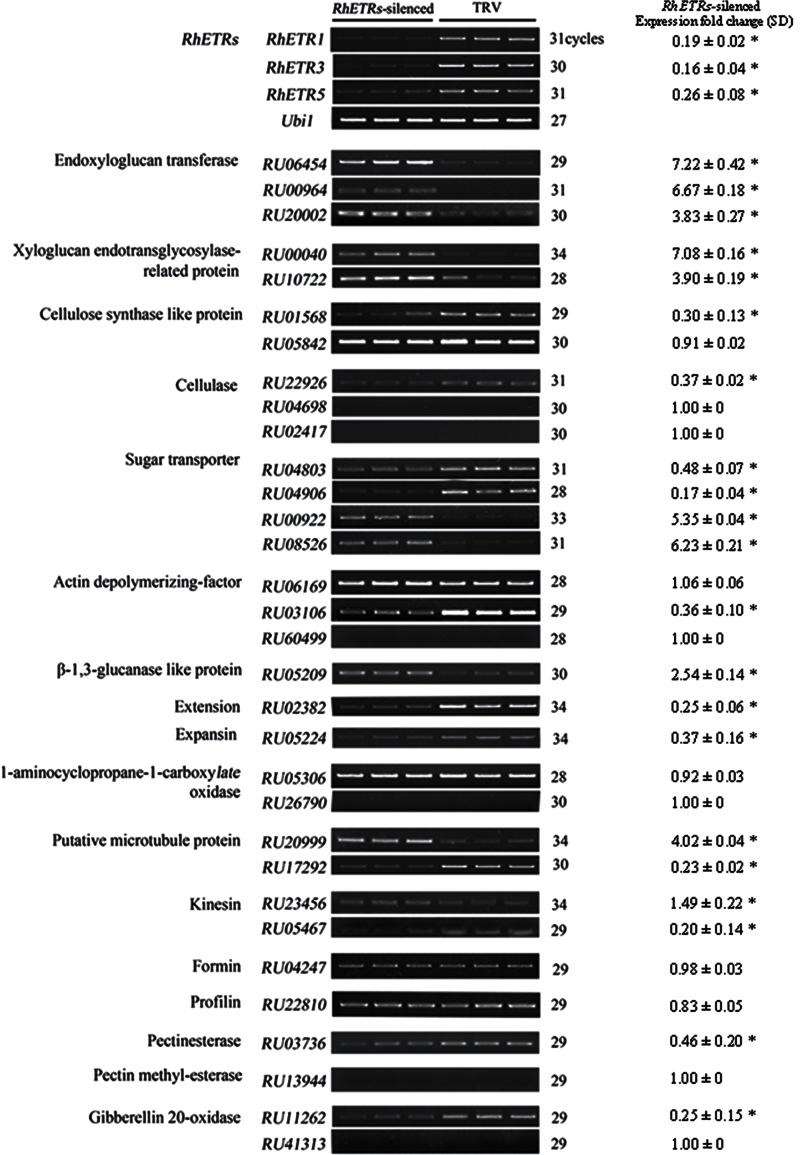
The expression of relevant ethylene-downstream genes was also determined in RhETRs-silenced flowers on the third day after 24h of dehydration. The results showed that, among the 32 genes, RhETRs silencing substantially upregulated ten genes, including endoxyloglucan transferase, xyloglucan endotransglycosylase-related protein, β-1,3-glucanase-like protein, two of the sugar transporters and one of the putative microtubule and kinesin proteins. This was in contrast to the TRV controls. Moreover, RhETRs silencing also substantially downregulated 11 genes including extensin and expansin, cellulose synthase-like protein, cellulase, sugar transporter, actin depolymerizing-factor, putative microtubule proteins, kinesin, and gibberellin 20-oxidase (Fig. 7).
Fig. 7.
Semi-quantitative RT-PCR analysis of 32 ethylene-signalling downstream genes in RhETR-genes-silenced rose petals. Ubi1 was used as the internal control. TRV-RhETRs, petals infiltrated by TRV-RhETRs with RhETR1, -3, and -5 silenced; TRV, petals infiltrated by empty vector. At least three biological replicates, were tested for each gene and representative results are shown. The relative transcript levels were determined by densitometry of the signals using AlphaImagerTM2200, and fold change in expression of each gene was calculated by using the relative transcript levels in RhETR-genes-silenced compare to TRV-silenced petals. SD, standard deviation. Asterisks indicates significant differences between the TRV- and RhETR-genes-silenced petals for the same gene according to Duncan’s multiple range tests (P <0.05).
These results indicated that the inhibitory effect of dehydration-caused ethylene on petal expansion may occur partially through regulating the expression of relevant downstream genes related to cell expansion.
Discussion
Spatial-specific induction of ethylene biosynthesis by dehydration in rose flowers
Decades ago, several reports indicated that detached vegetative organs usually produce an ethylene burst in response to dehydration. Detached wheat and orange leaves produce large amounts of ethylene under dehydration (Ben-Yehoshua and Aloni, 1974; McKeon et al., 1982). In cotton, bracts as green organs, lose much more water and produce greater amounts of ethylene than the rest of the boll during dehydration (Guinn, 1976). For detached reproductive organs, ethylene has been reported to be induced rapidly and dramatically in the calyx in detached persimmon fruit under dehydration (Nakano et al., 2003). Here, we found that ethylene was primarily produced in the sepals among the five floral organs of detached rose flowers during dehydration treatment. Interestingly, the response of the calyx in persimmon suggests that different plants respond similarly to dehydration stress.
Surprisingly, we found that ethylene production appeared to be highly induced in the gynoecia during the initial period of rehydration, and in the sepals (Fig. 2); it is suggested here that gynoecia are more sensitive than the other floral organs in the production of ethylene as a response to rehydration.
In most reported plants, tissue-specific ethylene induction is attributed to the temporal and spatial regulation of ACS genes (Lin et al., 2009). In post-pollinated orchid flowers, elevated expression of PhalACS2 and PhalACS3 in stigma and ovary subsequently resulted in increased expression of PhalACS1 in the labellum (Bui and O’Neill, 1998). In carnation, ethylene treatment affects the induction of ethylene biosynthetic genes with different kinetics in all flower organs. Expression of DCACS2 and DCACS3 in styles causes enhanced ethylene production, which is able to induce DCACS1 expression in petals (Jones and Woodson, 1999). In cut roses, after ethylene treatment, the rapid and substantial increase in ethylene production in the gynoecia is attributed to rapid and enhanced expression of RhACS2 and RhACS3 (Xue et al., 2008).
Here, we found that expression of RhACS1, a wounding-inducible gene, and RhACS2, a senescence-inducible gene (Ma et al., 2005), exhibited spatial and temporal specificity during dehydration and rehydration in detached rose flowers (Fig. 3) in a pattern consistent with the ethylene production in the two organs (Fig. 2). Gene silencing by VIGS further confirmed the role of the RhACS1 and RhACS2 genes in producing ethylene. This ethylene resulted in sequential phenotypic changes in response to dehydration and rehydration (Fig. 4).
Similarly, in detached persimmon fruit, water loss also induced expression of a wounding-induced ACS gene, DkACS2, in the calyx and caused large amounts of ethylene production.
Together, these findings indicate that, in detached rose flowers during dehydration and rehydration, the sepals and gynoecia are the main organs of ethylene induction, and RhACS1 and RhACS2 primarily contribute to ethylene induction at the transcriptional level.
Regulation of ETR genes in response to dehydration and rehydration
It has been well documented that the ethylene receptors act as negative regulators of ethylene responses. This means that reducing the levels of receptor increases signal output, and vice versa (Hua and Meyerowitz, 1998). Ethylene receptor genes exhibit higher expression levels in reproductive tissues, such as the androecia and gynoecia of Arabidopsis (Sakai et al., 1998), anthers of rice (Yau et al., 2004), pollen and embryo of tobacco (Zhang et al., 2001), flowers and fruits of tomato (Tieman and Klee, 1999), and gynoecia of rose flowers (Xue et al., 2008). Here, among the five rose ETR genes, RhETR1 and RhETR3 were upregulated during dehydration and rehydration in petals (Fig. 5).
As the ethylene receptors are negative regulators of ethylene signalling, silencing of the ethylene receptor might increase ethylene sensitivity in plants. A double mutant of subfamily I receptor, etr1ers1, exhibited hypersensitivity to ethylene, whereas single- and double-gene knockouts of the other subfamily did not clearly display different phenotypes. This observation indicates that the subfamily I receptors are more important than the subfamily II receptors in determining competency in response to ethylene in Arabidopsis (Wang et al., 2003). In tomato, however, silencing of the LeETR4 or LeETR6 gene, both subfamily II members, displays exaggerated ethylene response phenotypes, including epinastic growth, premature flower senescence, and early fruit ripening (Tieman et al., 2000). In the present study using the VIGS approach, it was confirmed that silencing of RhETR3, a subfamily II receptor, significantly aggravated dehydration-caused inhibition of petal expansion in rose flowers (Fig. 6), indicating that RhETR is a negative regulator for ethylene signalling in rose flowers. Therefore, ethylene perception and signalling might vary in different plant species.
A dehydration-caused abnormal phenotype, such as inhibition of petal expansion, might result from inappropriate cell expansion. Generally, cell expansion is thought to depend on degradation and resynthesis of cell-wall substrates, changes in cell turgor, and remodelling of the cytoskeleton. In the present work, it was found that, in RhETR-genes-silenced petals, there were 21 substantially altered genes among 32 rose genes that were related to cell expansion.
Of the genes related to the cell wall, RU00040 and RU10722 encode xyloglucan endotransglycosylase-related protein, which is a key enzyme in catalysing the biosynthesis of major structural polysaccharides for primary cell walls (Hayashi and Kaida, 2011). RU06454, RU00964, and RU20002 encode endoxyloglucan transferase, which can catalyse the cleavage and molecular grafting of xyloglucan polymers. A gene encoding endoxyloglucan transferase has been suggested as one of the most likely agents responsible for cell-wall loosening (Nishitani, 1997).
Of the genes related to cell turgor, RU04803, RU04906, RU00922, and RU08526 encode sugar-transport proteins, which play a crucial role in long-distance and cell-to-cell distribution of sugars. These are key signalling molecules that can potentially regulate cell growth throughout the plant (Williams et al., 2000). In diffusely growing cells, microtubules provide tracks for the movements of cellulose synthases and hence provide directional deposition of cellulose, the major factor controlling cell expansion. The binding of microtubules and microfilaments in plant cells appears to regulate cytoskeleton bundling (Petrásek and Schwarzerová, 2009).
Taken together, these results indicated that RhETR-genes silencing leads to substantial expression changes in genes associated with the cell wall, cytoskeleton, and cell turgor, which regulate cell expansion.
Conclusions
Based on the present findings, a working model of the role of ethylene under dehydration in rose petal expansion is proposed. As shown in Supplementary Fig. S10 at JXB online, for ethylene biosynthesis, dehydration- and rehydration-caused ethylene primarily contributes to RhACS1 and RhACS2 gene expression in the sepals and gynoecia, and the perception of ethylene is chiefly attributable to RhETR3 in petals. The perception of ethylene by RhETR3 further influences the expression of genes related to cell expansion and finally influences petal cell expansion.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Changes in fresh weight loss rate during dehydration.
Supplementary Fig. S2. Ethylene production in whole flowers.
Supplementary Fig. S3. Alignment of the cDNA sequence of RhACS1–5 genes and the specific fragments used in VIGS.
Supplementary Fig. S4. Phenotype of RhACS1-, RhACS2-, and RhACS1/2-silenced flowers (upper) and ratio of height to diameter (lower).
Supplementary Fig. S5. The promoters of RhACS1 and RhACS2.
Supplementary Fig. S6. Induction of RhACS1 promoter activity by dehydration in flowers of transgenic Arabidopsis.
Supplementary Fig. S7. Induction of RhACS2 promoter activity by dehydration in flowers of transgenic Arabidopsis.
Supplementary Fig. S8. Alignment of the cDNA sequence with RhETR1–5 genes and the specific fragments used in VIGS.
Supplementary Fig. S9. The phenotype of RhETR3- and RhETR-genes-silenced flowers (upper) and the ratio of height to flower diameter (lower).
Supplementary Fig. S10. Proposed model of dehydration-affected flower opening mediated by ethylene in rose flowers.
Supplementary Table S1. Primer sequences of the various genes in RT-PCR analysis.
Supplementary Table S2. Primer sequences of the various genes for construction of VIGS vectors.
Supplementary Table S3. Primer sequences of the various genes in RT-PCR analysis for gene silencing.
Supplementary Table S4. Proportion of each floral tissue contributing to increased ethylene production.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (grant nos 31130048 and 30671480) and the ‘948’ project (grant no. 2011-G17) from the Ministry of Agriculture. We thank Mr Gabriel M. Garcia (University of New Mexico, USA) for careful proofreading of our manuscript.
Glossary
Abbreviations:
- 1-MCP
1-methylcyclopropene
- AbsE
abaxial subepidermis
- ACC
1-aminocyclopropane-1-carboxylic acid
- ACS
ACC synthase
- DW
deionized water
- SE
standard error
- TRV
tobacco rattle virus
- VIGS
virus-induced gene silencing.
References
- Adato I, Gazit S. 1974. Water-deficit, ethylene production, and ripening in avocado fruits. Plant Physiology 53, 45–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aird WC, Parvin JD, Sharp PA, Rosenberg RD. 1994. The interaction of GATA-binding proteins and basal transcription factors with GATA box containing core promoters: a model of tissue specific gene expression. Journal of Biological Chemistry 269, 883–889. [PubMed] [Google Scholar]
- Argueso CT, Hansen M, Kieber JJ. 2007. Regulation of ethylene biosynthesis. Journal of Plant Growth Regulation 26, 92–105. [Google Scholar]
- Beltrano J, Montaldi E, Bartoli C, Carbone A. 1997. Emission of water-stress ethylene in wheat (Triticum aestivum L.) ears: effects of rewatering. Plant Growth Regulation 21, 121–126. [Google Scholar]
- Ben-Yehoshua S, Aloni B. 1974. Effect of water stress on ethylene production by detached leaves of Valencia orange (Citrus sinensis Osbeck). Plant Physiology 53, 863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui AQ, O’Neill SD. 1998. Three 1-aminocyclopropane-1-carboxylate synthase genes regulated by primary and secondary pollination signals in orchid flowers. Plant Physiology 116, 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Etheridge N, Schaller GE. 2005. Ethylene signal transduction. Annals of Botany 95, 901–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinn G. 1976. Water deficit and ethylene evolution by young cotton bolls. Plant Physiology 57, 403–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Kaida R. 2011. Function of xyloglucan in plant cell. Molecular Plant 4, 17–24. [DOI] [PubMed] [Google Scholar]
- Heinrichs F. 2008. International statistics flowers and plants. AIPH/Union Fleurs 56, 16–90. [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Iorenaga T. 1999. Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Research 27, 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. 1998. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana . Cell 94, 261–271. [DOI] [PubMed] [Google Scholar]
- Jin JS, Shan NW, Ma N, Bai JH, Gao JP. 2006. Regulation of ascorbate peroxidase at the transcript level is involved in tolerance to postharvest water deficit stress in the cut rose (Rosa hybrida L.) cv. Samantha . Postharvest Biology and Technology 40, 236–243. [Google Scholar]
- Jones ML, Woodson WR. 1999. Differential expression of three members of the 1-aminocyclopropane-1-carboxylate synthase gene family in carnation. Plant Physiology 119, 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ZF, Zhong SL, Grierson D. 2009. Recent advances in ethylene research. Journal of Experimental Botany 60, 3311–3336. [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP. 2002. Virus-induced gene silencing in tomato. The Plant Journal 31, 777–786. [DOI] [PubMed] [Google Scholar]
- Ma N, Cai L, Lu WJ, Tan H, Gao JP. 2005. Exogenous ethylene influences flower opening of cut roses (Rosa hybrida) by regulating the genes encoding ethylene biosynthesis enzymes. Science in China Series: Life Science 48, 434–444. [DOI] [PubMed] [Google Scholar]
- Ma N, Tan H, Liu XH, Xue JQ, Li YH, Gao JP. 2006. Transcriptional regulation of ethylene receptor and CTR genes involved in ethylene-induced flower opening in cut rose (Rosa hybrida) cv. Samantha . Journal of Experimental Botany 57, 2763–2773. [DOI] [PubMed] [Google Scholar]
- Ma N, Xue JQ, Li YH, Liu XJ, Dai FW, Jia WS, Luo YB, Gao JP. 2008. Rh-PIP2;1, a rose aquaporin gene, is involved in ethylene-regulated petal expansion. Plant Physiology 148, 894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon TA, Hoffman NE, Yang SF. 1982. Effect of plant hormone pretreatments on ethylene production and synthesis of 1-aminocyclopropane-l-carboxylic acid in water stressed wheat leaves. Planta 155, 437–443. [DOI] [PubMed] [Google Scholar]
- McMichael BL, Jordan WR, Powell RD. 1972. An effect of water stress on ethylene production by intact cotton petioles. Plant Physiology 49, 658–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PW, He CJ, De, Greef JA, De, Proft MP. 1990. Does water deficit stress promote ethylene synthesis by intact plants? Plant Physiology 94, 1616–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano R, Ogura E, Kubo Y, Inaba A. 2003. Ethylene biosynthesis in detached young persimmon fruit is initiated in calyx and modulated by water loss from the fruit. Plant Physiology 131, 276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana I, Lalonde S, Saini HS. 1991. Water stress-induced ethylene production in wheat: a fact or artifact? Plant Physiology 96, 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani K. 1997. The role of endoxyloglucan transferase in the organization of plant cell walls. International Review of Cytology 173, 157–206. [DOI] [PubMed] [Google Scholar]
- Petrásek J, Schwarzerová K. 2009. Actin and microtubule cytoskeleton interactions. Current Opinion in Plant Biology 12, 728–734. [DOI] [PubMed] [Google Scholar]
- Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM. 1998. ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis . Proceedings of the National Academy of Sciences, USA 95, 5812–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Umezawa T, Urano K, Shinozaki K. 2007. Regulatory metabolic networks in drought stress responses. Current Opinion in Plant Biology 10, 296–302. [DOI] [PubMed] [Google Scholar]
- Shi YH, Zhu SW, Mao XZ, Feng JX, Qin YM, Zhang L, Cheng J, Wei LP, Wang ZY, Zhu YX. 2006. Transcriptome profiling, molecular biological and physiological studies reveal a major role for ethylene in cotton fiber cell elongation. The Plant Cell 18, 651–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman D, Taylor M, Ciardi J, Klee HJ. 2000. The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proceedings of the National Academy of Sciences, USA 97, 5663–5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman DM, Klee HJ. 1999. Differential expression of two novel members of the tomato ethylene receptor family. Plant Physiology 120, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudela D, Primo-Millo E. 1992. 1-Aminocyclopropane-1-carboxylic acid transported from roots to shoots promotes leaf abscission in Cleopatra Mandarin (Citrus reshni Hort. ex Tan.) seedlings rehydrated after water stress. Plant Physiology 100, 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieweg MF, Fruhling M, Quandt HJ, Heim U, Baumlein H, Puhler A, Kuster H, Andreas MP. 2004. The promoter of the Vicia faba L. leghemoglobin gene VfLb29 is specifically activated in the infected cells of root nodules and in the arbuscule-containing cells of mycorrhizal roots from different legume and nonlegume plants. Molecular Plant–Microbe Interactions 17, 62–69. [DOI] [PubMed] [Google Scholar]
- Wan CY, Wilkins TA. 1994. A modified hot borate method significantly enhances the yield of high quality RNA from cotton (Gossypium hisrstum L.). Analytical Biochemistry 223, 7–12. [DOI] [PubMed] [Google Scholar]
- Wang WY, Hall AE, O’Malley R, Bleecker AB. 2003. Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission. Proceedings of the National Academy of Sciences, USA 100, 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ. 2010. Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant, Cell & Environment 33, 510–525. [DOI] [PubMed] [Google Scholar]
- Williams LE, Lemoine R, Sauer N. 2000. Sugar transporters in higher plants: a diversity of roles and complex regulation. Trends in Plant Science 5, 283–290. [DOI] [PubMed] [Google Scholar]
- Xiong L, Zhu JK. 2002. Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell & Environment 25, 131–139. [DOI] [PubMed] [Google Scholar]
- Xue JQ, Li YH, Tan H, Yang F, Ma N, Gao JP. 2008. Expression of ethylene biosynthetic and receptor genes in rose floral tissues during ethylene-enhanced flower opening. Journal of Experimental Botany 59, 2161–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakimova E, Woltering E. 1997. Stress-induced ethylene production in flower parts of cut carnation flowers cv. Light pink tasman. Bulgarian Journal of Plant Physiology 23, 43–56. [Google Scholar]
- Yang SF, Hoffman NE. 1984. Ethylene biosynthesis and its regulation in higher plants. Annual Review of Plant Physiology 35, 155–189. [Google Scholar]
- Yau CP, Wang L, Yu M, Zee SY, Yip WK. 2004. Differential expression of three genes encoding an ethylene receptor in rice during development, and in response to indole-3-acetic acid and silver ions. Journal of Experimental Botany 55, 547–556. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Xie C, Shen YG, Chen SY. 2001. A two-component gene (NTHK1) encoding a putative ethylene receptor homolog is both developmentally- and stress-regulated in tobacco. Theoretical and Applied Genetics 102, 815–824. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.