Abstract
Double fertilization of flowering plants depends on the targeted transportation of sperm to the embryo sac by the pollen tube. Currently, little is known about the underlying molecular mechanisms that regulate pollen germination and pollen tube growth in maize (Zea mays). Here, a maize pollen-predominant gene Zm908, with several putative short open reading frames (sORFs), was isolated and characterized. The longest ORF of Zm908 encodes a small protein of 97 amino acids. This was designated as Zm908p11 and is distributed throughout the maize pollen tube. Western blot detected the small peptide in mature pollen. Quantitative reverse transcription–PCR and northern blot analysis revealed that Zm908p11 was expressed predominantly in mature pollen grains. Ectopic overexpression of full-length Zm908 and Zm908p11 in tobacco resulted in defective pollen, while transgenic tobacco plants with a site-specific mutation or a frameshift mutation of Zm908p11 showed normal pollen development. Overexpression of Zm908p11 in maize decreased pollen germination efficiency. Maize pollen cDNA library screening and protein–protein interaction assays demonstrated that Zm908p11 interacts with maize profilin 1 (ZmPRO1). A microarray analysis identified 273 up-regulated and 203 down-regulated genes in the overexpressing transgenic Zm908p11 pollen. Taken together, these results indicate that Zm908 functions as Zm908p11, and binds to profilins as a novel ligand, with a required role during pollen tube growth in maize. Accordingly, a model is proposed for the role of Zm908p11 during pollen tube growth in maize.
Key words: pollen tube, pollen-predominant, profilin, short open reading frame, Zea mays, Zm908p11.
Introduction
In flowering plants, male gametophyte (pollen) development is a complicated biological process. It takes place in the anther locules and comprises two essential phases: microsporogenesis and microgametogenesis (Borg et al., 2009). The mature pollen grain of maize (Zea mays) consists of three cells: two small sperm cells and a larger vegetative cell. After the pollen grain arrives at the compatible stigmatic hairs of maize silk, it hydrates. The pollen tube then starts to elongate in the style, which connects the stigma to the embryo sac. Ultimately, the sperm cells are delivered into the embryo sac of an ovule to achieve double fertilization. One sperm cell fuses with the egg cell, giving rise to the zygote, while the other one fuses with the central cell and develops into the primary endosperm (Mascarenhas, 1990; Taylor and Hepler, 1997).
Processes that control pollen germination and tube growth involve multiple signal transduction molecules, including Ca2+ (Holdaway-Clarke and Hepler, 2003), calmodulin (CaM; Moutinho et al., 1998), phosphoinositides (Moutinho et al., 2001), and GTPase (Cheung et al., 2002). These modulate vesicle targeting and the physical state of the actin cytoskeleton required for tube elongation (Miller et al., 1996). The actin cytoskeleton is a highly organized dynamic structure that plays prominent roles in pollen germination and tube growth. Specific roles include organelle positioning (Kadota et al., 2009), cytoplasmic streaming (Cárdenas et al., 2005), vesicle trafficking (Kim et al., 2005), and polar growth (Vidali et al., 2001). Various actin-binding proteins (ABPs) are involved in the regulation of actin dynamics, and these have the ability to bind to actin monomer and/or actin filaments (Higaki et al., 2007). ABPs regulate actin dynamics by maintaining the steady-state equilibrium between actin monomers and actin filaments (Staiger et al., 2010). In flowering plants, a subset of ABPs has been characterized from pollen (Lopez et al., 1996; Staiger et al., 1997), and includes actin-depolymerizing factors (ADFs) and profilins; these have similar or opposite effects on actin dynamics. ADFs bind to actin monomers and filaments, and preferentially depolymerize actin filaments by a complex mechanism. Increased levels of ADF in pollen tubes inhibit pollen tube growth and disrupt the actin cytoskeleton (Chen et al., 2002). Profilins are actin monomer-binding proteins that are able to regulate actin nucleation, causing either actin polymerization or depolymerization (Perelroizen et al., 1996; Kang et al., 1999). Profilins, whose activity is dependent on the Ca2+ concentration, are distributed throughout the pollen tube (Kovar et al., 2000). Five profilins have been identified in the maize PRO family (ZmPRO1–ZmPRO5). Of these, the ZmPRO1, 2, and 3 genes are highly expressed in pollen (Staiger et al., 1993; Gibbon et al., 1998; Kovar et al., 2000). Additionally, profilin can bind to polyphosphoinositides, poly-l-proline (PLP), proline-rich proteins, and several multiprotein complexes to regulate actin nucleation (Fedorov et al., 1994; Reinhard et al., 1995; Mahoney et al., 1999). Formins are a class of profilin-interactng proteins, which are a primary actin filament nucleation factors, and comprise two formin-homology domains, FH1 and FH2. The FH1 domain contains a proline-rich stretch which interacts with profilin and profilin–actin (Kovar et al., 2006). The FH2 domain is necessary for actin filament nucleation (Zigmond, 2004).
In recent years, it has been reported that a novel class of small peptide genes are involved in plant morphogenesis processes, including organ growth and development. They contain one or more short open reading frames (sORFs) of <100 amino acid residues that are able to translate small peptides (Kastenmayer et al., 2006). An increasing number of sORFs have been demonstrated to function as significant regulators in the control of cell proliferation, cell differentiation, and signal transduction in plants. For example, in the Arabidopsis mutant, inflorescence deficient in abscission (ida), the floral organs delay abscission after the shedding of mature seeds. This is because IDA is a ligand for the leucine-rich repeat receptor kinase HAESA, which influences floral organ abscission. IDA encodes a 77 amino acid protein with an N-terminal hydrophobic region, and is localized to the plasma membrane (Butenko et al., 2003). In Arabidopsis, CLAVATA3 (CLV3) encodes a 96 amino acid peptide with an 18 amino acid secretory signal peptide at the N-terminus. It has been reported that the clv3 floral meristem is larger than that of the wild type, and forms a large mass of additional carpels, thus confirming that CLV3 is a specific regulator in the coordination of cell differentiation and proliferation at the floral meristem throughout flower development (Clark et al., 1995; Fletcher et al., 1999). In addition to these, phytosulphokine-α (PSK-α) is a class of sulphated peptides. Its precursor peptide contains 80 amino acid residues and has a hydrophobic region at its N-terminus (Yang et al., 1999). In pollen, PSK-α regulates cell surface receptors of pollen grains to influence density-dependent pollen germination and tube growth (Chen et al., 2000).
By differential screening of the maize mature pollen cDNA library, two pollen-predominant genes with putative sORFs in maize were obtained, namely Zm401 (AY911609) (Li et al., 2001; Dai et al., 2004) and Zm908. Alhough full-length Zm401 is 1149bp, the longest ORF is only 270bp, and encodes the 89 amino acid peptide (~10kDa) Zm401p10. Zm401 plays a crucial role in the formation of Zm401p10 (Wang et al., 2009). In situ hybridization demonstrated that Zm401 is expressed specifically in tapetal cells and microspores (Ma et al., 2008). Overexpression of Zm401p10 causes retardation of tapetum degeneration and reduction of pollen viability (Wang et al., 2009).
Here, another gene, Zm908, is studied. Functional analyses demonstrated that Zm908 encodes a small functional peptide, Zm908p11 (with an estimated mol. wt of ~11kDa). This peptide is localized throughout the pollen tube. Maize with overexpressed Zm908p11 exhibited reduced pollen germination efficiency. This suggests that Zm908p11 functions in maize pollen germination. Protein–protein interaction assays revealed that Zm908p11 interacts with ZmPRO1. Moreover, transcript profiling data showed that overexpression of Zm908p11 affects the expression of many genes involved in pollen tube growth. A possible mechanism for the involvement of Zm908p11 in maize pollen germination and tube growth is discussed.
Materials and methods
Plant materials and growth conditions
Tobacco (Nicotiana tabacum cv Samsun NN and Nicotiana benthamiana) seeds were surface sterilized, and then sown on Murashige and Skoog (MS) solid medium (Murashige and Skoog, 1962) containing 3% sucrose and 0.8% agar. The plates were incubated at 28 °C under a 16h light/8h dark photoperiod for seed germination. The seedlings were then transferred to a bottle with the same medium and cultured under the same conditions as those for seed germination.
Maize inbred lines Z31 and Q31 were grown in a test field at China Agricultural University (Beijing). Immature embryos (~10 d after pollination) of the hybrid (Z31×Q31) were dissected and cultured on N6 solid medium containing 2% sucrose and 2.0mg l–1 2,4-D (pH 5.8) (Chu et al., 1975) for callus induction in darkness at 28 °C, and subcultured every 14 d until transformation.
Constructs for tobacco transformation
Details of the construction are given in the Supplementary Materials and methods available at JXB online.
35S-Zm908s and 35S-Zm908a
The sense and antisense strands of the full-length Zm908 were inserted into pBI121 between the Cauliflower mosaic virus (CaMV) 35S promoter and the Nos terminator to generate 35S-Zm908s (Supplementary Fig. S1A at JXB online) and 35S-Zm908a (Supplementary Fig. S1B).
35S-Zm908p11, 35S-Zm908p11ss, and 35S-Zm908p11fs
The longest ORF, Zm908p11 (+697 to +990), a site-specific mutation (the ATG start codon was replaced by AAG), and a frameshift mutation (the ATG start codon was replaced by ATGA) of Zm908p11 were inserted into pBI121 between the CaMV35S promoter and the Nos terminator to generate 35S-Zm908p11s (Supplementary Fig. S1C at JXB online), 35S-Zm908p11ss (Supplementary Fig. S1D), and 35S-Zm908p11fs (Supplementary Fig. S1E).
Lat52-Zm908s and Lat52-Zm908p11
The CaMV35S promoters in 35S-Zm908s and 35S-Zm908p11 were replaced by the Lat52 promoter to produce Lat52-Zm908s (Supplementary Fig. S1F at JXB online) and Lat52-Zm908p11 (Supplementary Fig. S1G).
Constructs for maize transformation
Zm908p11 controlled by a 282bp promoter fragment of Zm908 (Zm908p) and the Nos terminator was fused with an hpt gene (hygromycin B phosphotransferase) controlled by the CaMV35S promoter and the Nos terminator to produce the overexpression construct Zm908p-Zm908p11OE (Supplementary Fig. S1H at JXB online). The antisense strand of the 3’-untranslated region (UTR) of Zm908 (Zm908p11a), a part of the β-glucuronidase (GUS) gene (GUS ’), and the sense strand of the 3’-UTR of Zm908 (Zm908p11s) were fused to generate the stem–loop fragment. The stem–loop fragment controlled by Zm908p and the Nos terminator was fused with an hpt gene controlled by the CaMV35S promoter and the Nos terminator to produce Zm908p-Zm908p11Ri (Supplementary Fig. S1I). The detailed construction is shown in the Supplementary Materials and methods.
Plant transformation
Tobacco transformation
The 35S-Zm908s, 35S-Zm908a, 35S-Zm908p11, 35S-Zm908p11ss, 35S-Zm908p11fs, Lat52-Zm908s, and Lat52-Zm908p11 constructs were transformed into tobacco (N. tabacum cv Samsun NN) using the leaf disc method (Horsch et al., 1985). The regenerated plants were transplanted in the greenhouse at a temperature of 25 °C day/20 °C night with summer daylight and ~50% relative humidity.
Maize transformation
The Zm908p-Zm908p11OE and Zm908p-Zm908p11Ri constructs were transformed into calli induced from the immature embryos of the hybrid Z31×Q31 by particle bombardment. Resistant calli were screened by 20mg ml–1 hygromycin on NB medium, as described by Wang et al. (2006).
Quantitative RT–PCR
Maize total RNA was extracted from root, stem, leaf, seed, different anther stages, and mature pollen using TRIzol® Reagent (Invitrogen, USA) following the manufacturer’s instructions. A 2 μg aliquot of total RNA was reverse transcribed by M-MLV reverse transcriptase (Promega, USA) with oligo(dT)20 primer. Quantitative reverse transcription–PCR (RT–PCR) was conducted using TaKaRa SYBR® Premix Ex Taq™ (TaKaRa, Japan) on a Bio-Rad Real-Time System CFX96TM C1000 thermal cycler (Bio-Rad, USA). The PCR conditions were 95 °C for 10 s, followed by 40 cycles of 94 °C for 5 s, 60 °C for 20 s, and 72 °C for 20 s. Independent biological triplicates and technical triplicates were used for each experiment. Data analysis was performed using BIO-RAD CFX Manager software (Bio-Rad, USA). The maize Tubulin gene was used as an internal control. The primer pair for Zm908 was 9rtf and 9rtr, and for Tubulin it was trtf and trtr (Supplementary Table S1 at JXB online). The relative gene expression level was calculated using the 2–ΔΔCt analysis method.
Northern blot hybridization
Total RNAs (30 μg) extracted from root, stem, leaf, uninucleate and binucleate anthers, and mature pollen using TRIzol® Reagent (Invitrogen, USA) were separated on a 1.2% agarose–formaldehyde gel, and transferred onto nylon membranes (Boehringer Mannheim, Germany). The Zm908p11 fragment was labelled with [α-32P]dCTP as a probe using the Prime-a-Gene® Labeling System (Promega, USA). The membranes were pre-hybridized for 6h at 65 °C, and hybridization was performed for 20h at 65 °C with labelled probes. After hybridization, membranes were washed in 2× SSC/0.5% SDS for 30min and in 0.5× SSC/0.5% SDS for 30min at 65 °C. Finally, the hybridization signals were recorded on an X-omat BT Film (Kodak, Japan).
In vitro pollen germination assays
Pollen grains were harvested from freshly opened anthers, added to 20 μl of liquid pollen germination medium, then placed onto solid pollen germination medium [10mM CaCl2, 0.05mM KH2PO4, 0.01% (w/v) H3BO3, 0.1% (w/v) yeast extract, 10% (w/v) sucrose, and 6% (w/v) PEG-4000 solidified with 1% (w/v) agar for maize pollen; 1mM CaCl2, 1mM Ca(NO3)2·4H2O, 1mM MgSO4·7H2O, 0.01% (w/v) H3BO3, and 18% (w/v) sucrose solidified with 0.5% (w/v) agar for tobacco pollen]. The plates were incubated for 3h at 28 °C and 100% humidity in the dark. All the calculations were performed with technical triplicates and biological triplicates. At least 400 grains were used per assay. Statistical analysis was performed using Microsoft Excel.
Antiserum production, protein extraction, and western blot analysis
The antibody was produced by Beijing Protein Innovation Company, Ltd (Beijing, China; http://www.proteomics.org.cn/). A peptide corresponding to the amino acid sequence (GKWVRRGRLNPAPADC) of Zm908p11 cDNA was synthesized, and 200 μg of the synthesized peptide was used to immunize rabbits subcutaneously. Booster shots were given at 12 d intervals with the same amount of antigen three times.
Maize total protein from root, stem, leaf, seed, and mature pollen was extracted with an extraction buffer consisting of 2.5% (w/v) sucrose, 0.1% (v/v) β-mercaptoethanol, and 5mM potassium phosphate buffer, pH 6.0. A 20 μg aliquot of protein was separated by 19% Tricine-SDS–PAGE (Schägger, 2006), and blotted onto a polyvinylidene fluoride (PVDF) membrane (Millipore, USA). The western blot analysis was performed according to the method reported by Gallagher et al. (1993). The Zm908p11 antiserum was affinity purified and used as the primary antibody at a 1:1000 dilution. Alkaline phosphatase-conjugated goat anti-rabbit IgG (Promega, USA) was used as the secondary antibody (1: 5000). The signal was detected using the NBT/BCIP reaction kit (Promega, USA).
Scanning electron microscopy
Mature maize pollen grains from fresh dehiscent anthers were collected and mounted on stubs using double-sided carbon tape, and coated with palladium–gold by an ion coater (Eiko IB-3, Japan). The specimens were examined on a scanning electron microscope (Hitachi S-3400N, Japan) at an accelerating voltage of 15kV.
Alexander’s staining assay
Alexander’s staining assay was performed as previously described (Alexander, 1969). Mature pollen grains from fresh dehiscent anthers were collected and incubated with Alexander’s solution for 10–15min, and then observed by light microscopy (Leica, Germany).
Subcellular localization of Zm908p11 protein
For subcellular localization, Zm908p11-GFP (green fluorescent protein) was transiently transformed into tobacco pollen. For construction of Zm908p11-GFP, the Zm908p11 fragment was amplified by PCR using primer pair 9Gf and 9Gr (Supplementary Table S1 at JXB online), and fused to the C-terminus of GFP in pLat52-GFP with EcoRI and KpnI sites. Fresh pollen grains were harvested from tobacco anthers and transferred into liquid pollen germination medium. Transformation was conducted by particle bombardment. Following bombardment, the pollen grains were incubated to germinate for 3h, at 28 °C and 100% humidity in the dark. The fluorescence of GFP was monitored using a Zeiss LSM510 (Zeiss, Germany).
Yeast two-hybrid assay
To determine proteins that interact with Zm908p11, the MATCHMAKER GAL4 Two-Hybrid System3 (Clontech, USA) was used. Zm908p11 was amplified from pMD18-Zm908s with the primer pair y2h9Nf and y2h9Nr (Supplementary Table S1 at JXB online), and cloned in-frame into the BamHI and SalI sites of the yeast vector pGBKT7 to obtain BD-Zm908p11 which was used as the bait. For screening of maize pollen protein that interacted with Zm908p11, the maize mature pollen cDNA library was ligated into pGADT7 as the prey. The constructs were co-transformed into yeast strain AH109, and screened on SD/–Ade/–His/–Leu/–Trp medium according to protocol. This was followed by X-α-galactosidase staining to test the interaction. For analysis of the interaction between Zm908p11 and ZmPRO1, the ORF of ZmPRO1 was amplified from maize pollen cDNA with primer pair y2hPf and y2hPr (Supplementary Table S1), and cloned into the EcoRI and XhoI sites of pGADT7 to generate AD-ZmPRO1.
LCI assay
The luciferase complementation imaging (LCI) assay was performed as previously described (Chen et al., 2008). The cDNA of Zm908p11 was amplified by PCR using primers L9f and NL9r, and L9f and CL9r, and ZmPRO1 was amplified using LPf and NLPr, and LPf and CLPr (Supplementary Table S1 at JXB online). They were then cloned into the KpnI and SalI sites of pCAMBIA-NLuc and pCAMBIA-CLuc vectors, respectively. These constructs were transformed into Agrobacterium strain GV3101. Bacterial suspensions were infiltrated into fully expanded leaves of the 7-week-old N. benthamiana plants using a needleless syringe. Plants were grown in darkness for 12h, and then with 16h light/8h dark for 60h at 22 °C. Finally, the leaves were daubed with firefly luciferase and observed with an AndoriXon camera iKon-M (Andor, USA).
Microarray analysis
Total RNA was extracted from mature pollen grains, collected from fresh dehiscent anthers of Zm908p-Zm908p11OE and wild-type plants, using TRIzol® Reagent (Invitrogen, USA). RNA quantity and quality were measured with a Nanodrop ND-2000 spectrophotometer (Thermo, USA). Microarray analyses were performed using Affymetrix microarray technology by CapitalBio Corporation (http://www.capitalbio.com) following the Affymetrix GeneChip® expression analysis technical manual (Affymetrix, USA). Data analysis was carried out according to the Affymetrix statistical algorithms description document (Affymetrix, USA). P-values between Zm908p-Zm908p11OE and wild-type plants were calculated by Wilcoxon’s rank test (Wilcoxon, 1945). The gene expression analysis and gene ontology were performed using AgriGo (http://bioinfo.cau.edu.cn/agriGO/). Some of the differentially expressed genes were confirmed by quantitative RT–PCR using the primer pairs listed in Supplementary Table S3 at JXB online.
Results
Cloning and bioinformatic analysis of Zm908
Previously, a pollen-predominant cDNA fragment (682bp) was obtained from the maize mature pollen cDNA library by differential screening using cDNA probes synthesized from the mRNA of mature maize pollen and leaf tissue (Li et al., 2001). Using this cDNA fragment, two genomic clones were isolated by plaque hybridization experiments. One of these two clones, Zm401, has been previously described (Dai et al., 2004). The other clone, which contained a 14kb DNA fragment insertion, was digested with SalI and cloned into a pGEM-3Zf (+) vector. The ~4.5kb EcoRI/XbaI fragment was further subcloned into pGEM-3Zf (+). This clone was named Zm908 following sequencing. The use of RT–PCR and 5’ rapid amplification of cDNA ends (5’-RACE) enabled a 1758bp full-length cDNA of Zm908 to be cloned from mature pollen RNA.
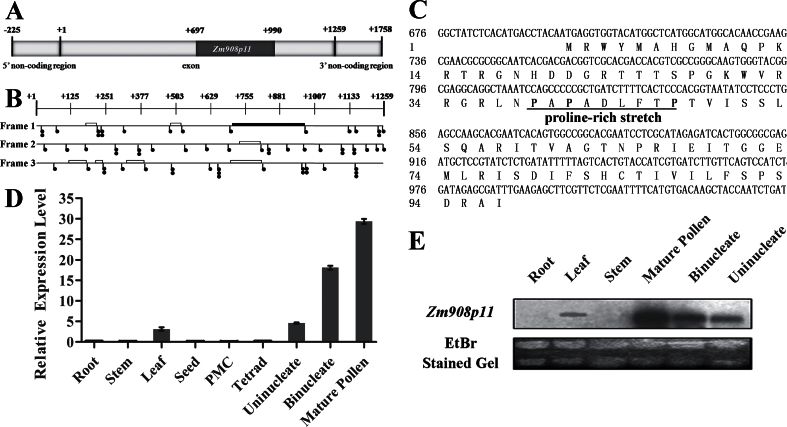
Comparison of the Zm908 cDNA with its genomic sequence revealed that the Zm908 genomic clone comprises a 5’-non-coding region (225bp), one exon (1259bp), and a 3’-non-coding region (274bp); it does not have any introns (Fig. 1A). This gene is located on chromosome 2 in the locus AC206253 (http://www.maizegdb.org). ORF prediction analysis of the Zm908 cDNA sequence using biological software identified many stop codons in all three possible reading frames, and no long ORFs were identified (Fig. 1B). The longest deduced ORF is 294bp in length (+697 to +990), and encodes a putative 97 amino acid peptide that is provisionally designated as Zm908p11 (Fig. 1C). The deduced Zm908p11 peptide has a molecular mass of 11kDa, with an isoelectric point (pI) of 11.6. It contains a proline-rich stretch and has no predicted signal peptide at the N-terminus (http://www.cbs.dtu.dk/services/SignalP/). A BLASTn search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) of Zm908 revealed that it was only homologous to Poaceae; no homologous genes in dicots or in other monocots were identified. The phylogenetic analysis of Zm908 using MEGA4 software is shown in Supplementary Fig. S2 at JXB online. Zm908 shared 65.8% identity with Zm401 (AY911609), 66.4% with Setaria italica Si401 (DQ981487), 66.7% with Oryza sativa Os401 (EU426833), and 66.7% with Triticum aestivum Ta401 (EU426832) cDNA, suggesting that Zm908 is a Poaceae-specific gene.
Fig. 1.
Gene structure and expression patterns of Zm908. (A) Gene structure of Zm908. The longest ORF Zm908p11 (+697 to +990) is shown by a black solid box. (B) Schematic representation of the Zm908 cDNA sequence. Each of the three possible reading frames of Zm908 is depicted separately. Stop codons (TAG, TAA, or TGA) are indicated by filled circles. Deduced ORFs are depicted as open boxes, and Zm908p11 is shown by a filled box. (C) Sequence of the 97 amino acid Zm908p11 peptide; the proline-rich stretch is underlined. (D) Expression pattern of Zm908 by quantitative RT–PCR analysis. The y-axis shows the relative expression level, and the x-axis shows different tissues. (E) Expression pattern of Zm908 by northern blot analysis. rRNAs were used as an internal control.
Zm908 transcript is predominantly expressed in mature maize pollen
The expression pattern of the Zm908 gene was initially investigated by quantitative RT–PCR. The expression of Zm908 was first detected at the uninucleate stages of anthers, and increased to a peak level in mature pollen. Additionally, a weak signal for Zm908 was detected in leaves (Fig. 1D).
To confirm the pollen-predominant expression pattern of Zm908, northern blot analysis was performed using an [α-32P]dCTP-labelled specific sequence of cDNA as a probe. As shown in Fig. 1E, the northern blot result was consistent with that of quantitative RT–PCR. The Zm908 transcript was detected from the uninucleate stage, and reached a maximum in mature pollen. No signal was detected in other tissues, with the exception of a weak band in the leaf. This indicated that Zm908 may function in the late stage of pollen development and/or pollen germination and tube growth.
Zm908 plays an important role as Zm908p11
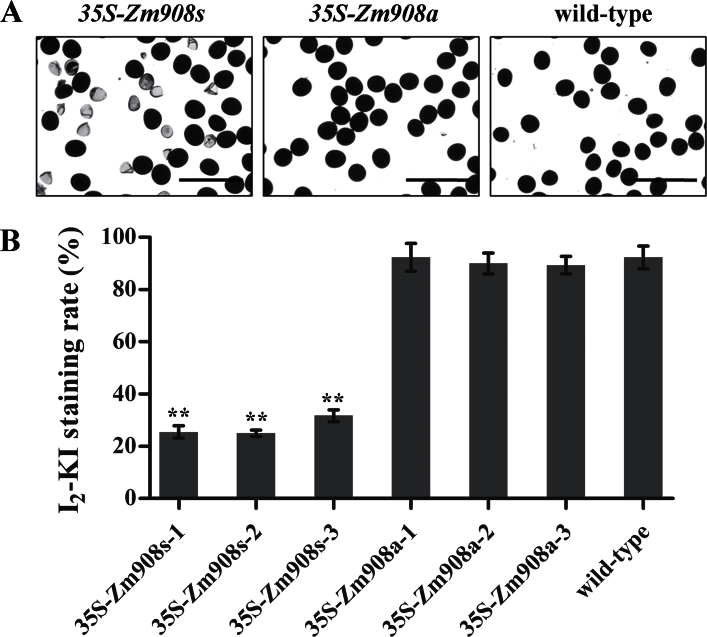
To investigate the function of Zm908 during pollen development, the full-length Zm908 cDNA was ectopically expressed in tobacco under the control of the CaMV35S promoter, and the Nos terminator (Supplementary Fig. S1A at JXB online). As a parallel reference, antisense Zm908 cDNA using the same regulatory elements (Supplementary Fig. S1B) was also transformed into tobacco. Three 35S-Zm908s lines and three 35S-Zm908a lines were selected for further investigation. Pollen grains from wild-type and transgenic lines were stained with I2–KI solution. The results were shown in Fig. 2. The majority of pollen grains from the 35S-Zm908s anthers were malformed and the pollen I2–KI staining rate was dramatically reduced. In contrast, the pollen grains from the 35S-Zm908a anthers were round and as fully stained as those of the wild type.
Fig. 2.
Transgenic pollen grain phenotype and pollen I2–KI staining rate analysis. Mature pollen grains were collected from wild-type and transgenic tobacco plants. (A) Tobacco pollen grains were stained with I2–KI solution. Normal pollen grains are deeply stained with a round shape, whereas malformed pollen grains are not stainable. Bars=200 μm. (B) The mature pollen I2–KI staining rate (%) in transgenic tobacco. 35S-Zm908s-1/2/3 and 35S-Zm908a-1/2/3 are transgenic tobacco lines. All the calculations were performed with technical triplicates and biological triplicates, and at least 400 grains were used per assay. Error bars represent the mean ±SD; **P < 0.01 (Student’s t-test).
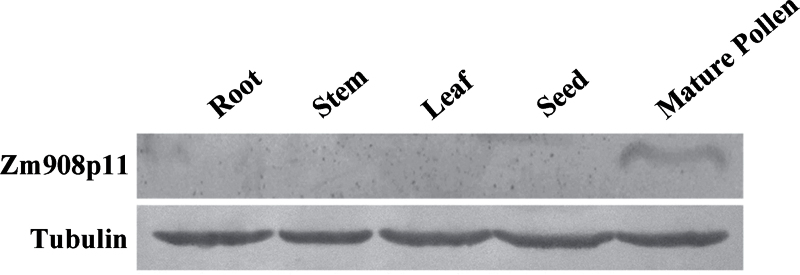
Considering that the Zm908 sequence has no long ORF, the longest ORF is 294bp and encodes a putative 97 amino acid peptide Zm908p11. Does the Zm908 function as Zm908p11? To answer this question, western blot analysis was performed using Zm908p11 antiserum to test for the existence of the Zm908p11 peptide in wild-type maize. Excitingly, a band at the expected size of 11kDa was detected in mature maize pollen, but not in other tissues (Fig. 3). This indicated that the small peptide Zm908p11 is present in mature pollen.
Fig. 3.
Western blot analysis of Zm908p11 peptide in maize. Immunoblot of 20 μg of total protein extracted from root, stem, leaf, seed, and mature pollen loaded as indicated.
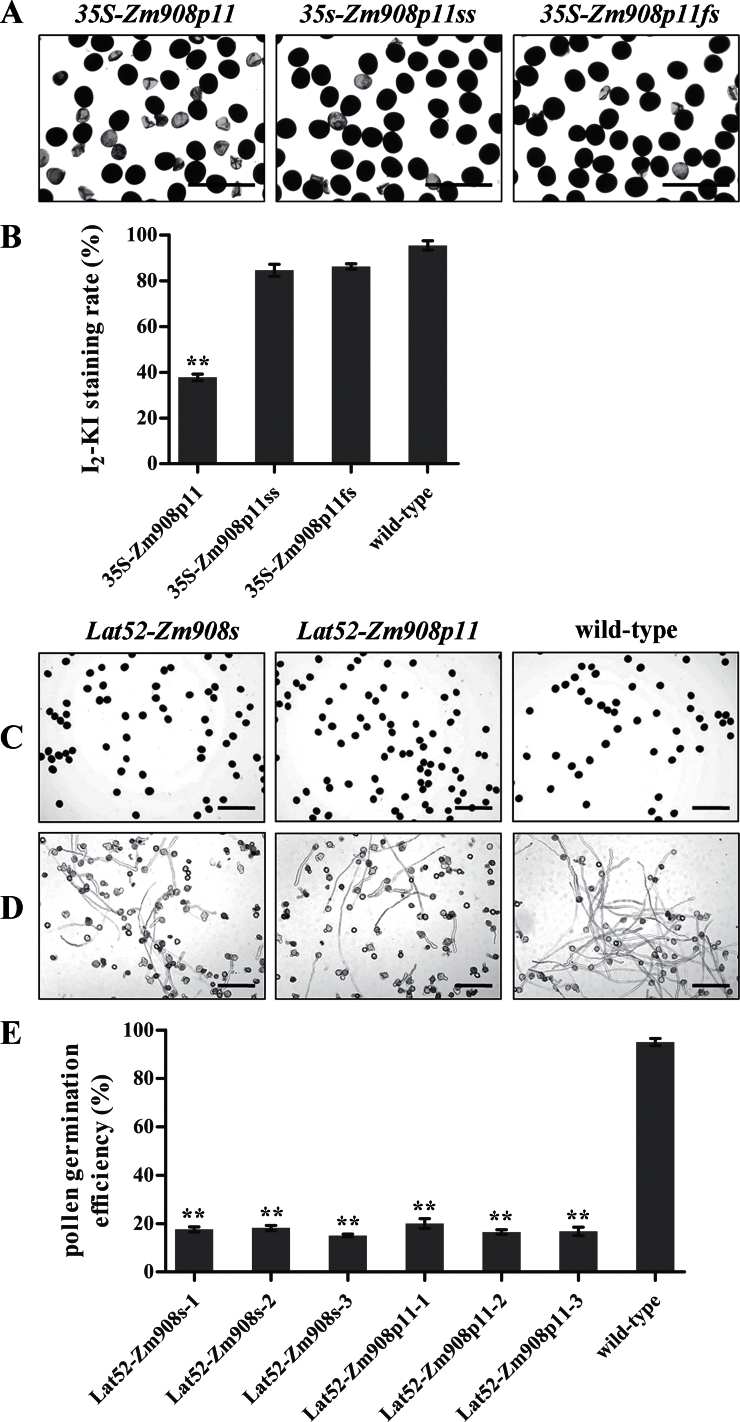
To confirm whether the transgenic phenotypes observed in 35S-Zm908s anthers were caused by Zm908p11, the construct 35S-Zm908p11 (Supplementary Fig. S1C at JXB online) was transformed into tobacco to produce ectopic overexpression of Zm908p11 lines. The transgenic lines did not show any noticeable differences in vegetative and floral development. However, I2–KI pollen grain staining revealed that only 38.2% of 35S-Zm908p11 pollen grains were normal in comparison with full-length Zm908 transgenic tobacco (Fig. 4A, B).
Fig. 4.
Transgenic pollen grain phenotype and analysis. Mature pollen grains were collected from wild-type and transgenic tobacco plants. (A) and (C) Tobacco pollen grains were stained with I2–KI solution. Normal pollen grains are deeply stained with a round shape, whereas malformed pollen grains are not stainable. (A) Bars=200 μm; (C) bars=250 μm. (B) The mature pollen I2–KI staining rate (%) in transgenic tobacco. (D) In vitro pollen tube germination assay. Pollen grains were incubated in vitro for 3h at 28 °C and 100% humidity in the dark. Bars=250 μm. (E) In vitro pollen germination efficiency analysis in transgenic tobacco. Lat52-Zm908s-1/2/3 and Lat52-Zm908p11-1/2/3 are transgenic tobacco lines. All the calculations were performed with technical triplicates and biological triplicates, and at least 400 grains were used per assay. Error bars represent the mean ±SD; **P < 0.01 (Student’s t-test).
To elucidate further the requirement for an intact ORF of Zm908p11, a site-specific mutation (Zm908p11ss; Supplementary Fig. S1D at JXB online) and a frameshift mutation (Zm908p11fs; Supplementary Fig. S1E) were generated and ectopically expressed in tobacco. I2–KI staining showed that both 35S-Zm908p11ss and 35S-Zm908p11fs transgenic pollen grains were round and dark-stained like those of the wild type (Fig. 4A, B). This indicated that both the site-specific and the frameshift mutation, which abolished production of Zm908p11 peptide, failed to show any of the phenotypic changes found in 35S-Zm908p11 transgenic lines. These results demonstrated that Zm908 functions as Zm908p11.
To check whether the transgenic phenotypes that were found were a consequence of constitutive expression of Zm908p11 controlled by the CaMV35S promoter, two constructs Lat52-Zm908s (Supplementary Fig. S1F at JXB online) and Lat52-Zm908p11 (Supplementary Fig. S1G), controlled by the pollen-specific promoter Lat52, were introduced into tobacco. Unlike the transgenic lines with constitutive expression of Zm908 and Zm908p11, both Lat52-Zm908s and Lat52-Zm908p11 pollen showed normal I2–KI staining (Fig. 4C), and decreased germination efficiency (15.6–18.3% for Lat52-Zm908 and 16.5–20.1% for Lat52-Zm908p11) compared with wild-type pollen (93.6–98.1%, Fig. 4D, E) in an in vitro germination test. These results implied that Zm908p11 is functional in maize pollen germination.
Zm908p11 is localized in the cytoplasm of pollen tubes
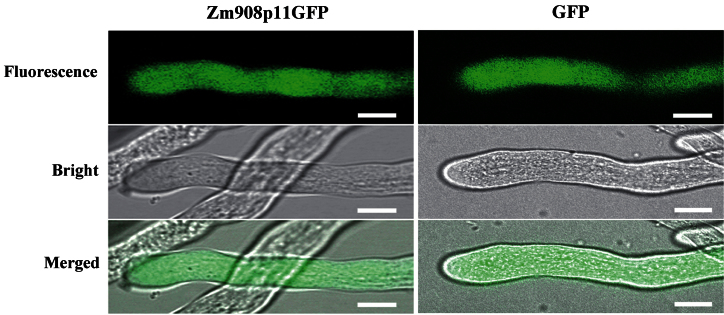
To investigate whether Zm908p11 peptide is localized in the pollen tube, Lat52-Zm908p11GFP was constructed and introduced into fresh tobacco pollen grains by particle bombardment transformation. Lat52-GFP was used as a control. The green fluorescence signal of Zm908p11–GFP was observed throughout the pollen tube cytoplasm at the same levels as free GFP (Fig. 5); this indicated that Zm908p11 peptide was distributed in the pollen tube.
Fig. 5.
Subcellular localization of Zm908p11 peptide in tobacco pollen tube in vitro. Bars=10 μm.
Overexpression of Zm908p11 decreases pollen germination efficiency in maize
To analyse the function of Zm908p11 in maize, 282bp of the Zm908 promoter region (Zm908p) was obtained from the Zm908 genomic clone. The construct of Zm908p fused with GUS was transformed into tobacco. Five independently transgenic lines were selected to characterize promoter activity. The results showed that GUS expression was mainly detected in the mature pollen grains of the transgenic anthers (Supplementary Fig. S3 at JXB online). In contrast, the anthers from untransformed control plants did not show any detectable GUS activity (data not shown).
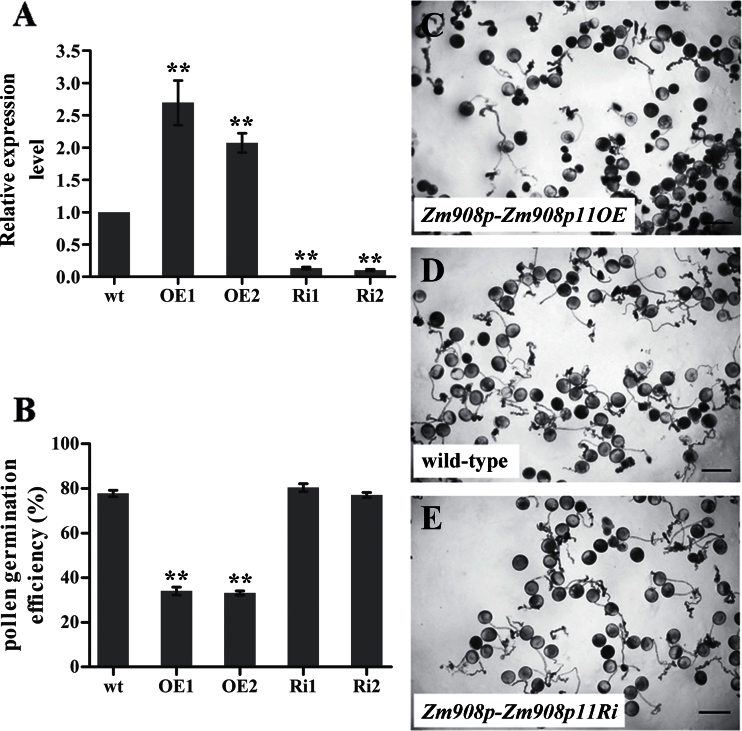
Using Zm908p, the plant expression constructs Zm908p-Zm908p11OE (Supplementary Fig. S1H at JXB online) and Zm908p-Zm908p11Ri (Supplementary Fig. S1I) were designed and transformed into maize calli by particle bombardment to generate Zm908p11-overexpressing and RNAi (RNA interference) transgenic plants, respectively. Quantitative RT–PCR was conducted to analyse the relative expression level of Zm908p11 in pollen grains (Fig. 6A). Two overexpressing lines and two RNAi lines were selected for further investigation. The vegetative and reproductive development of both transgenic lines was normal. Additionally, the pollen grains were observed using scanning electron microscopy (SEM), and Alexander, I2–KI, and 4’,6-diamiaino-2-phenylindole (DAPI) staining. The results are shown in Supplementary Fig. S4 at JXB online. No differences were observed between the mature pollen grains from transgenic lines and those of the wild type, indicating that Zm908p11 may not function in pollen grain development.
Fig. 6.
Phenotypic analysis of pollen from Zm908-Zm908p11OE, Zm908p-Zm908p11Ri, and wild-type maize plants. (A) Relative expression level of Zm908p11 in transgenic and wild-type maize pollen. (B) In vitro pollen germination efficiency of transgenic and wild-type plants. (C–E) In vitro germination of pollen grains from transgenic and wild-type plants. (C) Pollen grains from Zm908p-Zm908p11OE plants. (D) Pollen grains from wild-type plants. (E) Pollen grains from Zm908p-Zm908p11Ri plants. Pollen grains were cultured in vitro for 3h at 28 °C and 100% humidity in the dark. Bars=100 μm. Error bars represent the mean ±SD; **P < 0.01 (Student’s t-test).
In vitro pollen germination assays were further carried out. Only 33.1–37.7% of pollen grains from Zm908p11-overexpressing anthers yielded normal pollen tubes under these conditions (Fig. 6B, C), whereas 78.3% of pollen grains from the wild type (Fig. 6B, D) and 77.2–81.2% from Zm908p11 RNAi anthers (Fig. 6B, E) could produce normal pollen tubes. These findings indicated that Zm908p11 may function in maize pollen germination and tube growth.
Zm908p11 interacts with ZmPRO1
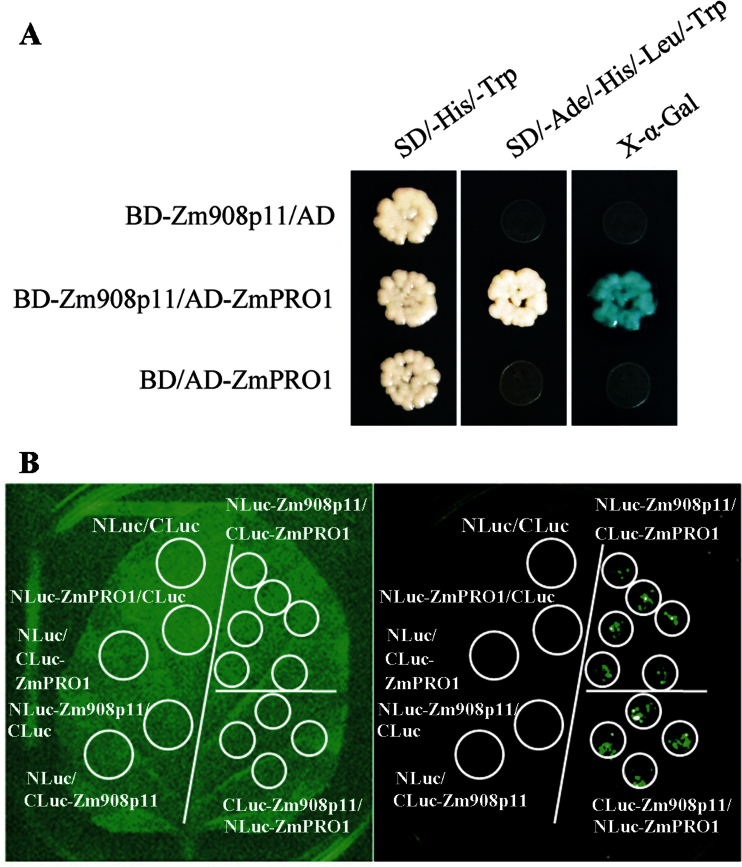
Protein–protein interaction assays were performed to investigate the function of Zm908p11 further. Zm908p11 was designed as a bait to screen a prey cDNA library prepared from mature maize pollen using yeast two-hybrid assay. A maize profilin 1 (ZmPRO1, P35081.1) was identified. A yeast transformant carrying a pair of constructs (BD-Zm908p11/AD-ZmPRO1) could grow on the SD/–Trp/–Leu/–His/–Ade selection medium, indicating direct interaction of Zm908p11 and ZmPRO1. The X-α-galactosidase assay was employed to verify the results (Fig. 7A).
Fig. 7.
Interaction of Zm908p11 with ZmPRO1. (A) Yeast two-hybrid assay. The yeast strains AH109 harbouring the construct pairs BD-Zm908p11/AD, BD-Zm908p11/AD-ZmPRO1, and BD/AD-ZmPRO1 grow on SD/–His/–Trp and SD/–Ade/–His/–Leu/–Trp agar medium, respectively, and X-α-galactosidase assays were performed to verify protein–protein interactions. (B) LCI assay. The 7-week-old tobacco leaf was transformed by infiltration with the bacterial suspensions containing construct pairs using a needleless syringe. The left panel shows the bright field image and the right panel shows the fluorescence field image of the treated leaf.
In addition to the yeast two-hybrid assay, the interaction between Zm908p11 and ZmPRO1 has also been verified by a firefly LCI assay (Chen et al., 2008). To conduct this assay, the following pairs of constructs, NLuc-Zm908p11/CLuc-ZmPRO1, CLuc-Zm908p11/NLuc-ZmPRO1, NLuc/CLuc, NLuc-ZmPRO1/CLuc, NLuc/CLuc-ZmPRO1, NLuc-Zm908p11/CLuc, and NLuc/CLuc-Zm908p11, were designed. Each pair of constructs was transformed into Agrobacterium strain GV3101, followed by infiltration of tobacco (N. benthamiana) leaves. The pairs NLuc-Zm908p11/CLuc-ZmPRO1 and CLuc-Zm908p11/NLuc-ZmPRO1 generated fluorescence signals in the transformed tobacco leaf (Fig. 7B). In contrast, no fluorescent signals were detected using the negative control sets.
Transcript profiling analysis in Zm908p11-overexpressing transgenic pollen
Affymetrix microarray analysis was employed to explore the gene expression of Zm908p11 transgenic maize. Total RNA extracted from mature pollen grains collected from fresh dehiscent anthers of Zm908p-Zm908p11OE and wild-type plants was used. Compared with wild-type pollen, 476 genes showed differential expression in Zm908p11 transgenic pollen. Among these, 273 genes were up-regulated and 203 down-regulated (fold change ≥2). Gene ontology (GO) analysis revealed that these differentially expressed genes were involved in cellular and metabolic processes, developmental processes, and localization (Supplementary Table S2 at JXB online). Among the up-regulated genes, 23 genes (4.8%) belonged to the categories of developmental processes and cellular component biogenesis, whereas 14.8% of down-regulated genes belonged to localization and establishment of localization processes.
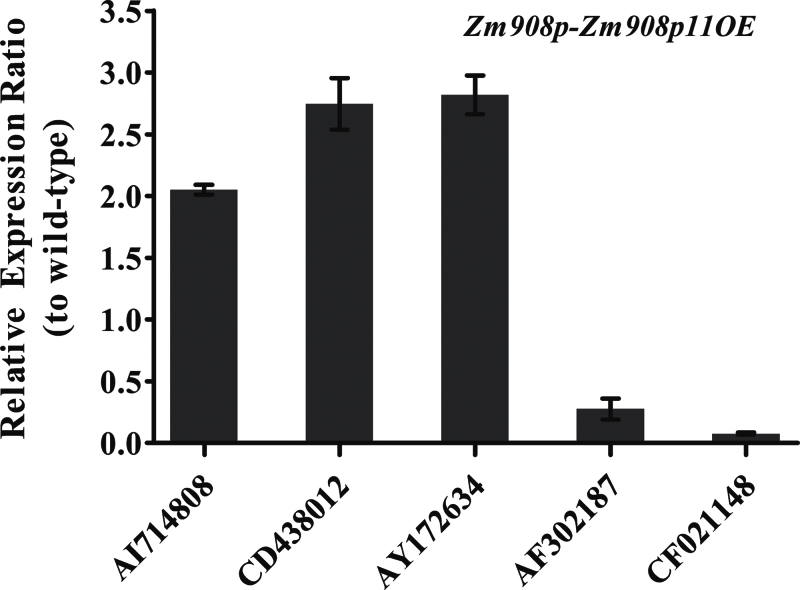
To validate the microarray data, three up-regulated genes (AI714808, protein phosphatase methylesterase 1, +2.076; CD438012, heat shock protein18c, +2.285; and AY172634, isoamylase-type starch debranching enzyme ISO3, +2.001) and two down-regulated genes (AF302187, sucrose export defective1, –2.583; and CF021148, CaM, –4.78), which are involved in pollen germination and tube growth, were selected for quantitative RT–PCR analysis. The quantitative RT–PCR results confirmed the microarray data (Fig. 8).
Fig. 8.
Quantitative RT–PCR of five genes identified from microarray analysis. AI714808, protein phosphatase methylesterase 1; CD438012, heat shock protein18c; AY172634, isoamylase-type starch debranching enzyme ISO3; AF302187, sucrose export defective1; CF021148, CaM.
Discussion
Zm908 functions as Zm908p11
Transcripts encoding sORFs are under-represented in transcriptome profiling. In maize, >8% of full-length cDNAs characterized encode ORFs of <100 amino acids and >4% encode ORFs of <40 amino acids (Soderlund et al., 2009). Kastenmayer et al. (2006) provided the first evidence that small peptides encoded by sORF genes could be translated and are functional in Saccharomyces cerevisiae. These findings spur on the further investigation of small peptide genes. In this study, the focus is on a new maize small peptide, Zm908p11, encoded by an sORF gene involved in pollen germination and tube growth. Furthermore the molecular mechanisms for its function are proposed.
Previously two sORF genes, Zm401 and Zm908, were obtained. Wang et al. (2009) demonstrated that Zm401 actually functions as a small peptide, Zm401p10, rather than as a non-coding RNA as previously believed (Dai et al., 2004, 2007). The Zm401p10 transcript was first detected in microspores and tapetal cells at the tetrad stage of anthers, and continued to accumulate thereafter, reaching its maximum level in mature pollen. The expression of Zm908p11 was initially weak at post-meiotic uninucleate stages, gradually increased thereafter, and reached its maximum level in mature pollen (Fig. 1D, E). Therefore, the expression patterns of these two genes are very similar in that there is low expression in early stages, building to a maximum in pollen. Overexpression of Zm401p10 in transgenic maize under the control of the native Zm401 promoter delayed tapetal layer degradation, and most microspores were sterile. In contrast, pollen maturation appeared to be normal in maize plants that overexpressed Zm908p11, but the germination efficiency of pollen grains was significantly reduced (Fig. 6B).
The function of Zm908 as an sORF product of Zm908p11 is based on the following evidence. First, overexpression of the full-length Zm908 gene, controlled by CaMV35S or Lat52 promoters, in tobacco caused abnormality in pollen development or tube growth. The same phenotypes were observed when the same methods were used to overexpress Zm908p11 in tobacco (Figs 2, 4). Secondly, a protein with the expected size of Zm908p11 was detected in mature pollen by western blot analysis (Fig. 3). Finally, the pollen development of transgenic tobacco carrying constructs containing a site-specific or frameshift mutation of Zm908p11 was the same as seen in the wild type (Fig. 4A, B). These results indicated that Zm908 functions as Zm908p11.
Transgenic tobacco with Zm908 or Zm908p11 controlled by the promoters CaMV35S and Lat52, respectively, revealed different phenotypes. Pollen development was defective under the control of the CaMV35S promoter, whereas pollen germination efficiency was decreased and pollen development was normal under the control of the Lat52 promoter. The distinct phenotypes may be due to the specificity of these two promoters. The genes Zm908 and Zm908p11 controlled by the CaMV35S promoter were constitutively expressed in all tissues of transgenic tobacco, whereas genes controlled by Lat52 were exclusively expressed in pollen.
Zm908p11 interacts with profilin and may be a novel ligand involved in the regulation network of pollen tube growth
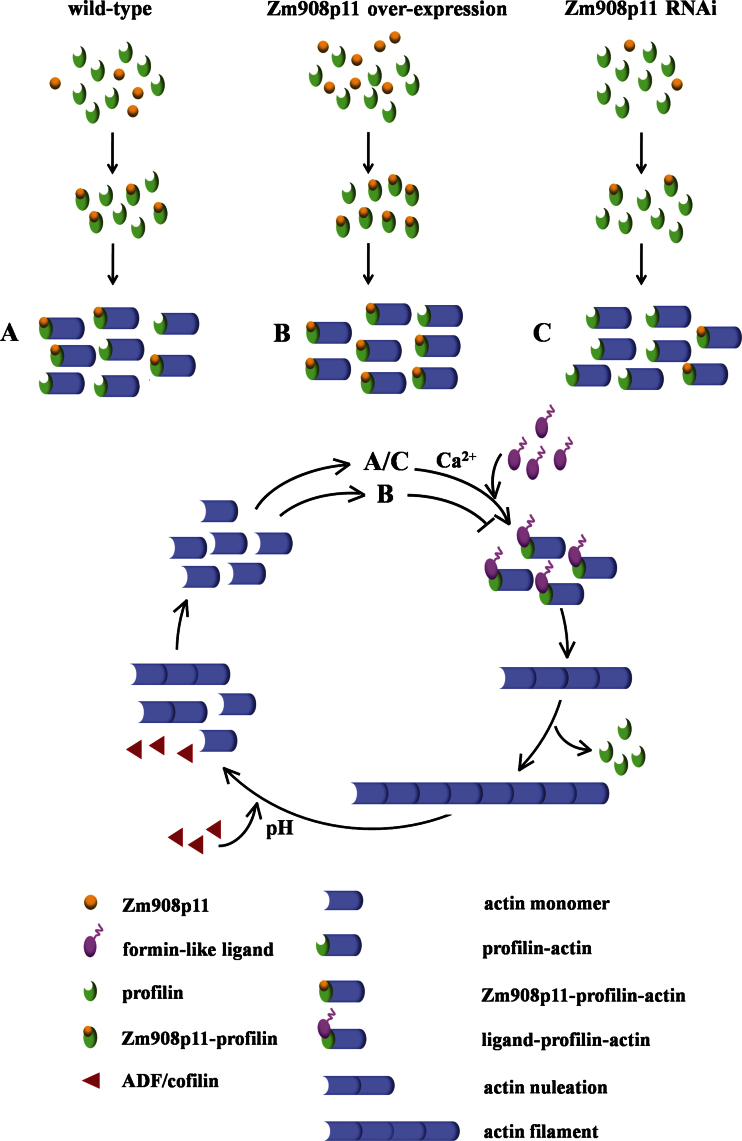
Actin dynamics are essential for pollen tube growth, and are maintained by the interaction of actin with a multitude of ABPs. Profilins can bind to actin monomers, and also bind to ligands containing a proline-rich stretch, such as formin (Mahoney et al., 1999). Profilin–actin complexes are able to prevent new actin filament nucleation, which is the rate-limiting step for actin polymerization (Pollard and Cooper, 1984). While barbed ends of actin filaments are present, formins promote profilin–actin nucleation due to binding to profilin, and then new monomers continuously add to the elongating filament (Romero et al., 2004).
In the present study, a proline-rich stretch was identified in Zm908p11 (Fig. 1C). The detected interaction between Zm908p11 and profilin may be a result of this structural feature. According to these results and a model for regulation of actin turnover reviewed by Staiger et al. (2010), the following scenario for Zm908p11 in the regulation of pollen tube growth is proposed (Fig. 9). In wild-type pollen grains, it is suggested that a normal level of Zm908p11–profilin–actin is in dynamic equilibrium with profilin–actin. Zm908p11–profilin–actin is more stable and prevents actin nucleation. Once the pollen tube grows, actin nucleation occurs following the release of profilin from profilin–actin, which is triggered by the interaction between formin-like ligand and profilin, and then actin monomer adds to the barbed end of the actin filament to elongate, while Zm908p11–profilin–actin does not participate in nucleation and elongation (Fig. 9, process A).
Fig. 9.
A simple mechanism for Zm908p11 in regulating actin dynamics. In wild-type pollen grains, Zm908p11–profilin–actin is in dynamic equilibrium with profilin–actin. Actin nucleation occurs following the release of profilin from profilin–actin, which is triggered by the interaction between formin-like ligand and profilin, and then actin monomer adds to the barbed end of the actin filament to elongate, while Zm908p11–profilin–actin does not participate in nucleation and elongation (process A). In Zm908p11-overexpressing maize pollen, the increase in Zm908p11–profilin–actin may dramatically suppress the nucleation of actin filaments, or the remaining level of profilin–actin is not sufficient to maintain the elongation of actin filaments (process B). In contrast, in Zm908p11 RNAi maize pollen, the decreased level of Zm908p11 leads to an increase in profilin–actin. Profilin–actin assembles on the barbed end of the actin filament in the presence of formin-like ligand. Thus, the actin filament nucleated and elongated, as in the wild type (process C).
In Zm908p11-overexpressing maize, the increased level of Zm908p11 disturbs the dynamic equilibrium between profilin–actin and Zm908p11–profilin–actin. The increase in Zm908p11–profilin–actin may dramatically suppress the nucleation of actin filaments, or the remaining level of profilin–actin may not be sufficient to maintain the elongation of actin filaments. Therefore, the deficiency of pollen germination and pollen tube growth was observed in overexpressing maize (Fig. 9, process B). In contrast, in Zm908p11 RNAi maize, the decreased level of Zm908p11 leads to an increase of profilin–actin. Profilin–actin assembles on the barbed end of the actin filament in the presence of formin-like ligand. Thus, the actin filament was nucleated and elongated, as in the wild type, resulting in the normal growth of pollen tubes (Fig. 9, process C).
It is possible that Zm908 may also act as a signalling factor to regulate gene expression in plants. Therefore, microarray technology was used to analyse the gene expression patterns of Zm908p11-overexpressing maize. This identified 203 genes as being down-regulated, including several genes that are involved in pollen tube growth. An example of a significantly down-regulated gene is CaM (CF021148, fold change: –4.78); this shares 97–99% identity with Arabidopsis CaM genes. CaM is a small conserved Ca2+-binding protein and has no enzymatic activity. After binding to Ca2+, the complex can modulate many downstream cellular target proteins, and this leads to a series of different physiological responses (Snedden and Fromm, 1998, 2001). In the Arabidopsis cam2-2 mutant, pollen germination efficiency was reduced nearly 30% compared with the wild type in vitro (Landoni et al., 2010).
In conclusion, a novel maize small peptide Zm908p11 was identified which plays a required role during pollen tube growth. Zm908p11 may be a ligand of profilins to prevent profilin–actin nucleation. Additionally, it may participate in a molecular network in association with pollen tube development.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Diagram of the constructs used in the present study.
Figure S2. Phylogenetic analysis of Zm908.
Figure S3. GUS staining of pollen grains and anther from Zm908p-GUS transgenic tobacco.
Figure S4. Phenotypic analysis of pollen grains from Zm908-Zm908p11OE, Zm908p-Zm908p11Ri, and wild-type maize.
Table S1. List of primers used for gene cloning and quantitative RT–PCR assays.
Table S2. List of genes up-regulated and down-regulated which were changed ≥2-fold in the microarray analysis.
Table S3. List of primers used for microarray analysis.
Acknowledgements
We are grateful to Dr Zhen Su (China Agricultural University) for assisting with the Affymetrix GeneChip analysis; Dr Ying Fu (China Agricultural University) for help with protein localization in the pollen tube; Dr Dapeng Zhang (Tsinghua University) for help with the LCI assay; and Haihong Liu and Junzhen Jia at the ‘985’ Technology Platform (China Agricultural University) for their assistance in scanning electron microscopy. We also thank Dr Virginia Walbot (Stanford University, USA) and Dr Roger B. Deal (Emory University, USA) for their kind reviews. This work was supported by research grants from the Natural Science Foundation of China (grant no. 30971555) and the National Basic Research Program of China (2012CB215301).
References
- Alexander MP. 1969. Differential staining of aborted and nonaborted pollen. Stain Technology 44, 117–122. [DOI] [PubMed] [Google Scholar]
- Borg M, Brownfield L, Twell D. 2009. Male gametophyte development: a molecular perspective. Journal of Experimental Botany 60, 1465–1478. [DOI] [PubMed] [Google Scholar]
- Butenko MA, Patterson SE, Grini PE, Stenvik GE, Amundsen SS, Mandal A, Aalen RB. 2003. INFLORESCENCE DEFICIENT IN ABSCISSION controls floral organ abscission in Arabidopsis and identifies a novel family of putative ligands in plants. The Plant Cell 15, 2296–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas L, Lovy-Wheeler A, Wilsen KL, Hepler PK. 2005. Actin polymerization promotes the reversal of streaming in the apex of pollen tubes. Cell Motility and the Cytoskeleton 61, 112–127. [DOI] [PubMed] [Google Scholar]
- Chen CY, Wong EI, Vidali L, Estavillo A, Hepler PK, Wu H-m, Cheung AY. 2002. The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes. The Plant Cell 14, 2175–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zou Y, Shang Y, Lin H, Wang Y, Cai R, Tang X, Zhou JM. 2008. Firefly luciferase complementation imaging assay for protein–protein interactions in plants. Plant Physiology 146, 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Matsubayashi Y, Sakagami Y. 2000. Peptide growth factor phytosulfokine-α contributes to the pollen population effect. Planta 211, 752–755. [DOI] [PubMed] [Google Scholar]
- Cheung AY, Chen CY-h, Glaven RH, de Graaf BHJ, Vidali L, Hepler PK, Wu H-m. 2002. Rab2 GTPase regulates vesicle trafficking between the endoplasmic reticulum and Golgi bodies and is important to pollen tube growth. The Plant Cell 14, 945–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CC, Wang CC, Sun CS, Hsn C, Yin KC, Chu CY, Bi FY. 1975. Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Scientia Sinica 18, 659–668. [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. 1995. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1 . Development 121, 2057–2067. [Google Scholar]
- Dai XY, Yu JJ, Ao GM, Zhao Q. 2007. Overexpression of Zm401, an mRNA-like RNA, has distinct effects on pollen development in maize. Plant Growth Regulation 52, 229–239. [Google Scholar]
- Dai XY, Yu JJ, Zhao Q, Zhu DY, Ao GM. 2004. Non-coding RNA for ZM401, a pollen-specific gene of Zea mays . Acta Botanica Sinica 46, 497–504. [Google Scholar]
- Fedorov AA, Magnus KA, Graupe MH, Lattman EE, Pollard TD, Almo SC. 1994. X-ray structures of isoforms of the actin binding protein profilin that differ in their affinity for phosphatidylinositol phosphates. Proceedings of the National Academy of Sciences, USA 91, 8636–8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM. 1999. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283, 1911–1914. [DOI] [PubMed] [Google Scholar]
- Gallagher S, Winston SE, Fuller SA, Hurrell JGR. 1993. Immunoblotting and immunodetection. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Sedtman JG, Smith JA, Struhl K, eds. Current protocols in molecular biology , Vol. 2 New York: John Wiley & Sons, 10.8.1–10.8.16. [Google Scholar]
- Gibbon BC, Zonia LE, Kovar DR, Hussey PJ, Staiger CJ. 1998. Pollen profilin function depends on interaction with proline-rich motifs. The Plant Cell 10, 981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki T, Sano T, Hasezawa S. 2007. Actin microfilament dynamics and actin side-binding proteins in plants. Current Opinion in Plant Biology 10, 549–556. [DOI] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Hepler PK. 2003. Control of pollen tube growth: role of ion gradients and fluxes. New Phytologist 159, 539–563. [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT. 1985. A simple and general method for transferring genes into plants. Science 227, 1229–1231. [DOI] [PubMed] [Google Scholar]
- Kadota A, Yamada N, Suetsugu N, Hirose M, Saito C, Shoda K, Ichikawa S, Kagawa T, Nakano A, Wada M. 2009. Short actin-based mechanism for light-directed chloroplast movement in Arabidopsis. Proceedings of the National Academy of Sciences, USA 106, 13106–13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang F, Purich DL, Southwick FS. 1999. Profilin promotes barbed-end actin filament assembly without lowering the critical concentration. Journal of Biological Chemistry 274, 36963–36972. [DOI] [PubMed] [Google Scholar]
- Kastenmayer JP, Ni L, Chu A, et al. 2006. Functional genomics of genes with small open reading frames (sORFs) in S. cerevisiae . Genome Research 16, 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Park M, Kim SJ, Hwang I. 2005. Actin filaments play a critical role in vacuolar trafficking at the Golgi complex in plant cells. The Plant Cell 17, 888–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR, Drobak BK, Staiger CJ. 2000. Maize profilin isoforms are functionally distinct. The Plant Cell 12, 583–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR, Harris ES, Mahaffy R, Higgs HN, Pollard TD. 2006. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell 124, 423–435. [DOI] [PubMed] [Google Scholar]
- Landoni M, Francesco AD, Galbiati M, Tonelli C. 2010. A loss-of-function mutation in Calmodulin2 gene affects pollen germination in Arabidopsis thaliana . Plant Molecular Biology 74, 235–247. [DOI] [PubMed] [Google Scholar]
- Li C, Liu J, Yu JJ, Zhao Q, Ao GM. 2001. Cloning and expression analysis of pollen-specific cDNA ZM401 from Zea mays. Journal of Agricultural Biotechnology 9, 374–377. [Google Scholar]
- Lopez I, Anthony RG, Maciver SK, Jiang C-J, Khan S, Weeds AG, Hussey PJ. 1996. Pollen specific expression of maize genes encoding actin depolymerizing factor-like proteins. Proceedings of the National Academy of Sciences, USA 93, 7415–7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JX, Yan BX, Qu YY, Qin FF, Yang YT, Hao XJ, Yu JJ, Zhao Q, Zhu DY, Ao GM. 2008. Zm401, a short-open reading-frame mRNA or noncoding RNA, is essential for tapetum and microspore development and can regulate the floret formation in maize. Journal of Cellular Biochemistry 105, 136–146. [DOI] [PubMed] [Google Scholar]
- Mahoney NM, Rozwarski DA, Fedorov E, Fedorov AA, Almo SC. 1999. Profilin binds proline-rich ligands in two distinct amide backbone orientations. Nature Structural Biology 6, 666–671. [DOI] [PubMed] [Google Scholar]
- Mascarenhas JP. 1990. Gene activity during pollen development. Annual Review of Plant Physiology and Plant Molecular Biology 41, 317–338. [Google Scholar]
- Miller DD, Lancelle SA, Hepler PK. 1996. Actin filaments do not form a dense meshwork in Lilium longiflorum pollen tube tips. Protoplasma 195, 123–132. [Google Scholar]
- Moutinho A, Hussey PJ, Trewavas AJ, Malhó R. 2001. cAMP acts as a second messenger in pollen tube growth and reorientation. Proceedings of the National Academy of Sciences, USA 98, 10481–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutinho A, Love J, Trewavas AJ, Malhó R. 1998. Distribution of calmodulin protein and mRNA in growing pollen tubes. Sexual Plant Reproduction 11, 131–139. [Google Scholar]
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15, 473–497. [Google Scholar]
- Perelroizen I, Didry D, Christensen H, Chua NH, Carlier MF. 1996. Role of nucleotide exchange and hydrolysis in the function of profilin in actin assembly. Journal of Biological Chemistry 271, 12302–12309. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Cooper JA. 1984. Quantitative analysis of the effect of Acanthamoeba profilin on actin filament nucleation and elongation. Biochemistry 23, 6631–6641. [DOI] [PubMed] [Google Scholar]
- Reinhard M, Giehl K, Abel K, Haffner C, Jarchau T, Hoppe V, Jockusch BM, Walter U. 1995. The proline-rich focal adhesion and microfilament protein VASP is a ligand for profilins. EMBO Journal 14, 1583–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero S, Clainche CL, Didry D, Egile C, Pantaloni D, Carlier M-F. 2004. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell 119, 419–429. [DOI] [PubMed] [Google Scholar]
- Schägger H. 2006. Tricine–SDS–PAGE. Nature Protocols 1, 16–22. [DOI] [PubMed] [Google Scholar]
- Snedden WA, Fromm H. 1998. Calmodulin, calmodulin-related proteins and plant responses to the environment. Trends in Plant Science 3, 299–304. [Google Scholar]
- Snedden WA, Fromm H. 2001. Calmodulin as a versatile calcium signal transducer in plants. New Phytologist 151, 35–66. [DOI] [PubMed] [Google Scholar]
- Soderlund C, Descour A, Kudrna D, et al. 2009. Sequencing, mapping, and analysis of 27,455 maize full-length cDNAs. PLoS Genetics 5, e1000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger CJ, Gibbon BC, Kovar DR, Zonia LE. 1997. Profilin and actin depolymerizing factor: modulators of actin organization in plants. Trends in Plant Science 2, 275–281. [Google Scholar]
- Staiger CJ, Goodbody KC, Hussey PJ, Valenta R, Drøbak BK, Lloyd CW. 1993. The profilin multigene family of maize: differential expression of three isoforms. The Plant Journal 4, 631–641. [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Poulter NS, Henty JL, Franklin-Tong VE, Blanchoin L. 2010. Regulation of actin dynamics by actin-binding proteins in pollen. Journal of Experimental Botany 61, 1969–1986. [DOI] [PubMed] [Google Scholar]
- Taylor LP, Hepler PK. 1997. Pollen germination and tube growth. Annual Review of Plant Physiology and Plant Molecular Biology 48, 461–491. [DOI] [PubMed] [Google Scholar]
- Vidali L, McKenna ST, Hepler PK. 2001. Actin polymerization is essential for pollen tube growth. Molecular Biology of the Cell 12, 2534–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DX, Li CX, Zhao Q, Zhao LN, Wang MZ, Zhu DY, Ao GM, Yu JJ. 2009. Zm401p10, encoded by an anther-specific gene with short open reading frames, is essential for tapetum degeneration and anther development in maize. Functional Plant Biology 36, 73–85. [DOI] [PubMed] [Google Scholar]
- Wang DX, Zhao Q, Zhu DY, Ao GM, Yu JJ. 2006. Particle-bombardment-mediated co-transformation of maize with a lysine rich protein gene (sb401) from potato. Euphytica 150, 75–85. [Google Scholar]
- Wilcoxon F. 1945. Individual comparisons by ranking methods. Biometrics Bulletin 1, 80–83. [Google Scholar]
- Yang H, Matsubayashi Y, Nakamura K, Sakagami Y. 1999. Oryza sativa PSK gene encodes a precursor of phytosulfokine-α, a sulfated peptide growth factor found in plants. Proceedings of the National Academy of Sciences, USA 96, 13560–13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond SH. 2004. Formin-induced nucleation of actin filaments. Current Opinion in Cell Biology 16, 99–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.