Abstract
Isohydric plants tend to maintain a water potential homeostasis primarily by controlling water loss via stomatal conductance. However, there is accumulating evidence that plants can also modulate water uptake in a dynamic manner. The dynamics of water uptake are influenced by aquaporin-mediated changes in root hydraulics. Most studies in this area have been conducted on herbaceous plants, and less is known about responses of woody plants. Here a study was conducted to determine how roots of hybrid poplar plants (Populus trichocarpa×deltoides) respond to a step change in transpirational demand. The main objective was to measure the expression of selected aquaporin genes and to assess how transcriptional responses correspond to changes in root water flow (Q R) and other parameters of water relations. A subset of plants was grown in shade and was subsequently exposed to a 5-fold increase in light level. Another group of plants was grown at ~95% relative humidity (RH) and was then subjected to lower RH while the light level remained unchanged. Both plant groups experienced a transient drop in stem water potentials. At 28h after the increase in transpirational demand, water potentials recovered. This recovery was associated with changes in the expression of PIP1 and PIP2 subfamily genes and an increase in Q R. Stomata of plants growing at high RH were larger and showed incomplete closure after application of abscisic acid. Since stomatal conductance remained high and unchanged in these plants, it is suggested that the recovery in water potential in these plants was largely driven by the increase in Q R.
Key words: Aquaporins, poplar, root hydraulics, stomatal control, transpirational demand, water uptake.
Introduction
Plants face ever-changing environmental conditions. Throughout their lifetime, trees may not only experience gradual changes in soil moisture, temperature, and other variables, but also have to respond to sudden changes in light and transpirational demand. Dynamic physiological adjustments are required to respond to sudden environmental changes, for example the opening of a gap in the canopy.
In isohydric plants, active stomatal control of water loss maintains leaf water potential relatively constant during periods of water stress (Jones and Tardieu, 1998). By dynamically controlling stomatal conductance, plants can effectively regulate long-distance water flow and water potential over the short term (Jones and Sutherland, 1991; Sperry and Pockman, 1993; Hacke and Sauter, 1995). However, plants can also modulate water uptake in a dynamic fashion. Water taken up by roots flows through living cells, and root water flow (Q R) is influenced by the modulation of aquaporin abundance and regulation of aquaporin activity (Henzler et al., 1999; Kamaluddin and Zwiazek, 2004; Aroca et al., 2012).
Aquaporins are water channel proteins and are present in a wide range of animal, microbial, and plant membranes (Henzler et al., 1999; Baiges et al., 2002). Fifty-six full-length aquaporin sequences have been identified in the Populus trichocarpa genome (Gupta and Sankararamakrishnan, 2009; Almeida-Rodriguez et al., 2010; Lopez et al., 2012). The plasma membrane intrinsic protein subfamily (PIPs), with their phylogenetic subgroups PIP1 and PIP2, is composed of 15 members in poplar (Supplementary Fig. S1 available at JXB online). Both PIP1-type (Siefritz et al., 2002; Postaire et al., 2010) and PIP2-type aquaporins (Vandeleur et al., 2009) show significant water transport activity in planta. Moreover, PIP1 and PIP2 aquaporins may interact to increase water permeability (Zelazny et al., 2007; Secchi and Zwieniecki, 2010). PIPs are generally localized in organs and tissues characterized by high fluxes of water, including root tissues (Javot and Maurel, 2002; Gomes et al., 2009; Secchi et al., 2009). Thus, plants have the ability to adjust their water uptake capacity to changing environmental conditions by regulating aquaporins in the plasma membrane of root cells. How dynamic above-ground changes are perceived by roots and how root aquaporins are subsequently regulated is not well understood.
In rice, root-specific aquaporins, such as OsPIP2;3, OsPIP2;4, and OsPIP2;5, were strongly induced by transpirational demand (Sakurai-Ishikawa et al., 2011); these aquaporins could play important roles in the adjustment of radial water transport in rice roots. That transpirational demand can strongly affect water uptake has also been shown in poplar (Almeida-Rodriguez et al., 2011) and other woody plants (McElrone et al., 2007). Almeida-Rodriguez et al. (2011) identified gene candidates in poplar that could play similar roles to those of the rice genes mentioned above. However, in their study, plant responses were measured 40–46h after plants were exposed to higher light levels, providing little temporal resolution of molecular and physiological changes that occurred prior to this time.
The first objective of the present study was to measure absolute transcript abundance of key PIP1 and PIP2 genes 4h and 28h after hybrid poplar plants were exposed to an increase in transpirational demand, and to assess how transcriptional responses correspond to changes in Q R and other parameters of water relations. The second objective was to determine whether changes in aquaporin expression and Q R would require an increase in light level per se, or whether such changes could also be triggered by lowering relative humidity (RH) at a constant light level.
To test this, plants were grown under contrasting irradiance and RH conditions, and were subsequently exposed to a sudden increase in transpirational demand with or without changing the light level. It was hypothesized that a step change in environmental conditions would lead to a transient perturbation of the water potential homeostasis, but that transcript accumulation of key PIP genes and associated dynamic changes in Q R would correspond to at least a partial recovery of water potentials.
Materials and methods
Plant material and growing conditions
Saplings of hybrid poplar (Populus trichocarpa×deltoides, clone H11-11) were produced in 2 litre pots from rooted cuttings and maintained in a growth chamber under the following growing conditions: 18/6h day/night cycle; 24/18 °C day/night temperature; ~75% RH. Plants were watered daily and fertilized on a weekly basis with a 2g l–1 solution of 15:30:15 N:P:K. Plants were grown in turface calcined clay in order to facilitated the separation of roots from soil particles (Almeida-Rodriguez et al., 2011).
After a 2 month period of sapling establishment, plants were randomly assigned to one of three groups and were kept under specific growing conditions for 6 weeks. A control group (subsequently referred to as ‘light control’) was kept at an irradiance level of 350 μmol m–2 s–1 (measured at plant level) under the same growing conditions as outlined above. A second group of plants (subsequently referred to as ‘shaded plants’) was placed in shading structures, which resulted in 80% reduction in irradiance from 350 μmol m–2 s–1 to 70 μmol m–2 s–1 at plant level. A third group of plants (subsequently referred to as ‘high humidity plants’) was placed in a humidified box. The humidified box allowed the RH to be increased to 95% while the light level, temperature, and day/night cycles remained the same as in the control conditions.
Experimental treatments
Experiments were designed to examine changes in hydraulic parameters and aquaporin gene expression in response to an increase in light (shaded plants) and a decrease in relative air humidity (high humidity plants), respectively. A subset of plants was removed from the shade and high humidity boxes at 07:00h. This was always done at the same time to minimize any effect of time of day on the physiological and molecular measurements. Measurements (or tissue sampling in the case of gene expression and immunolocalization assays) were carried out 4h (same day) and 28h (next day) after shaded and high humidity plants had been removed from their respective environment. All measurements were conducted between 10:30h and 11:30h. Control plants were also measured at this time.
Plant morphology
Morphological measurements included plant height above pots, root dry weight, and total leaf area. Root dry weight was measured after washing and drying entire root systems at 70 °C for 48h. Leaf areas were determined with a LI-3100C leaf area meter (Li-Cor Inc., Lincoln, NE, USA). The root dry weight to leaf area ratio is considered as a measure of biomass partitioning (Blake and Filho, 1988; Barigah et al., 2006).
Stomatal parameters
The youngest fully expanded leaf of five plants per treatment was used for measurements of stomatal length, density, and pore aperture. Images were recorded in eight randomly selected fields of view of each leaf. Fields of view were located near the point of maximum leaf width on the abaxial (lower) leaf surface. Images were recorded with a digital camera (DFC420C, Leica, Wetzlar, Germany) attached to a light microscope (DM3000, Leica) at ×400 magnification. Analysis was performed with Fiji software (Schindelin et al., 2012). To test if there was an effect of growing conditions on stomatal responses to abscisic acid (ABA), ABA was applied to detached leaves as described by Nejad and van Meeteren (2007) and Arend et al. (2009). Leaf samples were pre-incubated for 2h under light (~100 μmol m–2 s–1 photosyntheic photon flux density) in a stomata-opening medium (10mM MES-KOH, pH 6.15, 50mM KCl) to achieve stomatal opening. Stomatal closure was induced by supplementing the solution with 100 μM ABA (Sigma-Aldrich, St Louis, MO, USA) for 1h.
Water potential, stomatal conductance, and transpiration
The water potential of leaves (ΨL) and stems (ΨS) was measured using a Scholander-type pressure chamber (Model 1000; PMS Instruments, Albany, OR, USA). One leaf per plant was measured, from five plants per group. Stem water potential was measured after leaves had been sealed in aluminium foil and plastic bags the night before harvesting to promote equilibration of water potentials. Stomatal conductance and transpiration were measured with a steady-state porometer (LI-1600, Li-Cor Inc.) on five plants per group. High humidity plants were removed from the humidity box (and kept inside the growth chamber) immediately prior to measurements. Stomatal conductance and transpiration could not be measured in the humidity box because the high RH was outside the recommended operating range of the LI-1600. To minimize potential artefacts which might be caused by water desorption from the leaf surface immediately following a transition from high to low RH, leaf surfaces were wiped with Kimwipes (laboratory tissues) prior to measurements.
Root water flow
The Q R of five plants per group was measured according to the hydrostatic pressure method (Kamaluddin and Zwiazek, 2004). Entire root systems were immersed in a beaker filled with measuring solution (20mM KCl, 1mM CaCl2) and placed in a pressure chamber. A constant pressure of 0.3MPa was applied. This pressure allowed stable flow rates to be recorded within ~15min. The protruding stem was fitted to a graduated pipette and the volume of exudate was measured. Since root function was related to above-ground changes, Q R was normalized by the total leaf area of each plant. Normalizing by leaf area provides a measure of the ‘sufficiency’ of the roots to supply water to leaves (Lo Gullo et al., 1998; Tyree et al., 1998).
Gene transcript measurements by quantitative real-time PCR
For molecular analysis, representative root samples were collected, immediately frozen in liquid nitrogen, and stored at –80°C until analysed. Total RNA was extracted from root tissue of 3–4 plants per treatment using the RNeasy Plant Extraction Mini Kit (Qiagen, Valencia, CA, USA) with hexadecyltrimethylammonium bromide extraction buffer. RNA quality was assessed on an agarose gel and quantified with a spectrophotometer (Nanodrop ND-1000; Thermo Scientific; Wilmington, DE, USA). A 1 μg aliquot of total RNA was treated with DNase I (Invitrogen, Carlsbad, CA, USA) and used as template for first-strand cDNA synthesis with SuperScript II (Invitrogen) following the manufacturer’s instructions. cDNA quality was checked by PCR with intron-spanning actin (POPTR_0001s45780) primers (TCCCTCAGCACTTTCCAACAG/ACAAGCCATATTACTCGGCCTCAC).
Candidate genes were selected according to their expression patterns in previous experiments (Secchi et al., 2009; Wilkins et al., 2009; Almeida-Rodriguez et al., 2011) and due to their close similarity to rice genes induced by transpirational demand (Sakurai-Ishikawa et al., 2011) (Supplementary Table S1, Supplementary Fig. S1 at JXB online). Specific primers (Supplementary Table S2) were designed according to Rutledge and Stewart (2010) using the QuantPrime online tool (Arvidsson et al., 2008). PCR efficiency (E) was determined from a five-point cDNA serial dilution, according to: E=10[–1/slope]. All selected primer pairs showed correlation coefficients of R 2 > 0.98 and primer efficiency values ranging between 1.95 and 2.01.
Real-time quantitative PCR (qPCR) was performed on a 7900 HT Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) using cDNA equivalent to 2.5ng of RNA following instructions provided by Rutledge and Stewart (2008) and using lambda genomic DNA as a quantitative standard. Each reaction was carried out in triplicate using master mix containing 0.2mM dNTPs, 0.3U of Platinum Taq polymerase, and 0.25× SYBR Green. The PCR conditions were as follows: 15min activation at 95 °C, 40 cycles of 95 °C for 10 s, 65 °C for 2min, and a dissociation stage including two cycles of 95 °C for 15 s, 60 °C for 1min. Each run was completed with a melting curve analysis to confirm the specificity of amplification and absence of primer dimers. Data analysis was performed according to the sigmoidal method with LRE (linear regression of efficiency) analyzer software (Rutledge, 2011) to assess the absolute quantity of transcripts expressed as number of molecules per ng of total RNA.
Immunolocalization
Root segments were fixed in formaldehyde–acetic acid medium (FAA; 10% formaldehyde, 5% acetic acid, 50% ethanol) under vacuum for 1h and stored in FAA for 16h at 4 °C. Next, samples were embedded, sectioned, dewaxed, and rehydrated as described before (Almeida-Rodriguez et al., 2011). Before the first immunoreaction, sections were incubated for 45min with blocking solution [BS; 1.5% glycine, 5% (w/v) bovine serum albumin, 0.1% Tween-20 in phosphate-buffered saline (PBS)] following the protocol of Gong et al. (2006). Primary antibody directed against the first 42 N-terminal amino acids of AtPIP1;3 (Kammerloher et al., 1994; Henzler et al., 1999) was applied overnight at 4 °C. Slides were washed as described previously (Gong et al., 2006). DyLight 549-conjugated rabbit anti-chicken secondary antibody was pre-absorbed with plant tissue extract (1:500 in BS) before it was applied for 2h at 37 °C. Slides were rinsed several times and were coverslipped with Permount. Controls with no primary and/or secondary antibody were also prepared. Images were taken with a Leica DMRXA fluorescence microscope (filter cube N2.1, excitation range 515–560nm, suppression filter LP 590nm) equipped with a Nikon DXM1200 camera (Melville, NY, USA) at a standardized exposure time.
Statistical analysis
Differences due to the effect of treatments and growing conditions were analysed using a one-way analysis of variance (ANOVA) followed by a Tukey’s test. Data are presented as means ±SE. Differences were considered significantly different at P ≤ 0.05. All statistical analyses were carried out using SigmaPlot 12.3 (Systat, Point Richmond, CA, USA).
Results
Morphology and stomatal characteristics
Morphological traits of the different plant groups are shown in Table 1. Shaded plants had 54% lower root dry mass (DW R) and 50% lower leaf area (A L) than control plants. As a result of this proportional decrease, the A L:DW R ratio did not differ between shaded and control plants. Plants growing at high humidity had the lowest A L:DW R ratio of any plant group.
Table 1.
Morphological traits of hybrid poplar saplings grown under control (‘Light control’), shade, and high humidity (‘High RH’) conditions.
| Experimental treatment | Height (m) | DW R (g) | A L (m2) | A L:DW R (m2 g–1) |
|---|---|---|---|---|
| Light control | 0.98 (0.03) a | 1.14 (0.12) a | 0.32 (0.04) a | 0.28 (0.01) a |
| Shade | 0.73 (0.03) b | 0.61 (0.06) b | 0.16 (0.02) b | 0.27 (0.02) a |
| High RH | 1.21 (0.05) c | 1.61 (0.19) a | 0.27 (0.04) a,b | 0.16 (0.01) b |
The standard error of the mean is given in parentheses, n=5. Different letters indicate significant differences between treatments (P ≤ 0.05). Variables shown are plant height above pots, total root dry weight (DW R), total leaf area (A L), and leaf area to root dry weight ratio (A L:DW R).
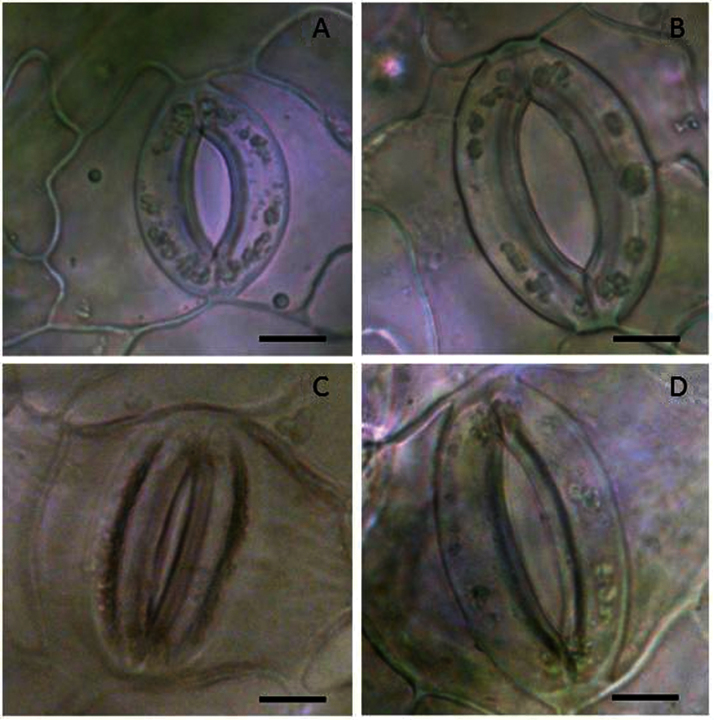
Stomatal characteristics did not differ between shaded and control plants (Table 2), although stomatal density of shaded plants tended to be more heterogeneous than in controls. High humidity plants had larger stomata and pore apertures as well as higher stomatal densities than other plant groups. Moreover, after application of 100 μM ABA to leaves, the pore apertures of high humidity plants remained larger than those of other plant groups; that is, stomata of high humidity plants exhibited incomplete closure (Fig. 1, Table 2).
Table 2.
Stomatal characteristics of hybrid poplar saplings grown under control (‘Light control’), shade, and high humidity (‘High RH’) conditions All parameters were measured on abaxial leaf surfaces.
| Experimental treatment | Stomatal length (μm) | Stomatal density (no. per mm2) | Pore aperture (μm) before/after application of ABA |
|---|---|---|---|
| Light control | 35.18 (0.58) a | 132.6 (4.5) a | 6.83 (0.36) a/4.20 (0.36) a |
| Shade | 32.84 (0.53) a | 118.1 (8.7) a | 6.81 (0.30) a/4.18 (0.20) a |
| High RH | 39.36 (0.77) b | 161.7 (6.1) b | 8.55 (0.10) b/7.07 (0.22) b |
The standard error of the mean is given in parentheses. Values are grand means of five plants. Different letters indicate significant differences between treatments (P ≤ 0.05).
In the case of pore apertures, two separate statistical analyses were conducted; one on apertures measured before application of ABA and one after ABA application. i.e. apertures were not compared before and after ABA application.
Fig. 1.
Light microscope images of stomata from poplar leaves growing in moderate (~75% RH) (A) and high (95% RH) relative humidity (B). The images were taken from the abaxial side of the leaves. Leaves that developed under high RH had larger stomatal length and aperture. While application of 100 μM ABA triggered stomatal closure in plants growing at moderate RH (C), the large stomata of high humidity grown plants failed to close fully (D). Bars = 10 μm. (This figure is available in colour at JXB online.)
Water potential and stomatal conductance
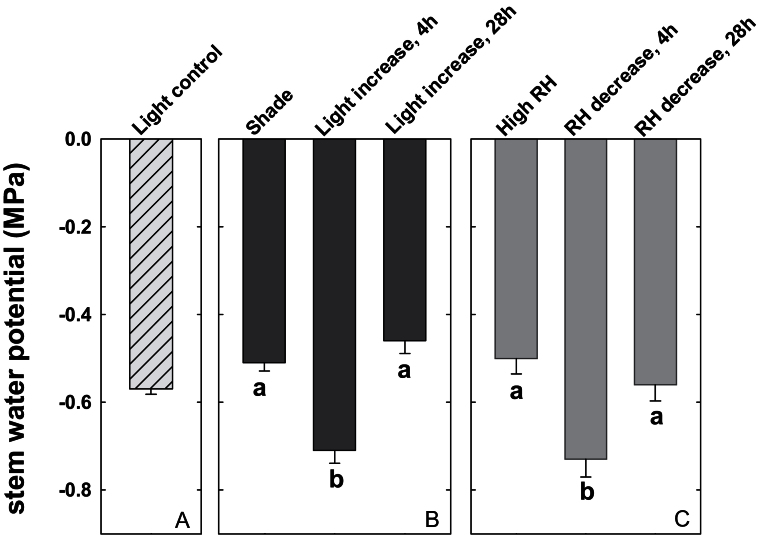
Control plants had a ΨS of –0.57±0.01MPa (Fig. 2A, ‘Light control’). At 4h after shaded plants were exposed to an increase in light level, their ΨS dropped from –0.51±0.02MPa to –0.71±0.03MPa (Fig. 2B). Leaf water potential showed a similar drop (data not shown). At 28h after the increase in light level, ΨS recovered to –0.46±0.03MPa. Plants experiencing a sudden drop in RH showed a very similar ΨS pattern (Fig. 2C).
Fig. 2.
Effect of a sudden change in transpirational demand on stem water potential. (A) Stem water potential of control plants grown under full light conditions in the growth chamber (‘Light control’). (B) Stem water potentials of shaded plants (‘Shade’), of plants removed from shade after 4h (‘Light increase, 4h’), and of plants removed from shade after 28h (‘Light increase, 28h’). (C) Stem water potentials of plants growing at high relative humidity (‘High RH’), of plants removed from high RH after 4h (‘RH decrease, 4h’), and of plants removed from high RH after 28h (‘RH decrease, 28h’). Data show the means ±SE; n=5 plants. Significant differences are indicated by unique letters (P ≤ 0.05).
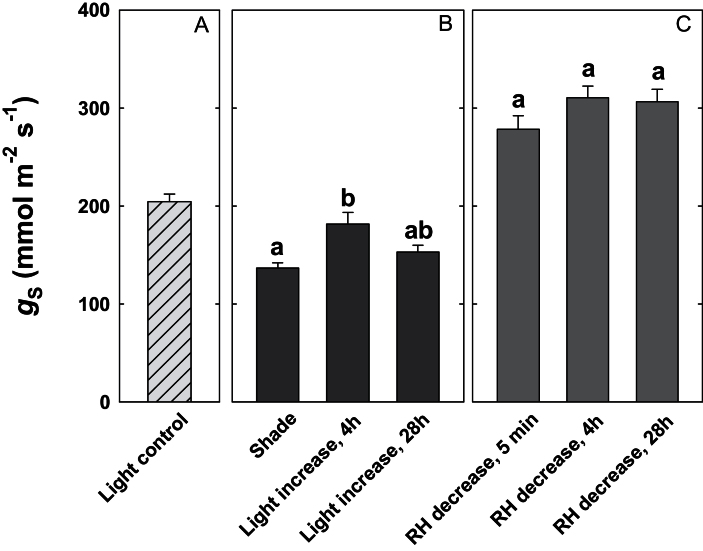
Shaded plants exhibited a temporary increase in stomatal conductance 4h after the increase in light level (Fig. 3). In contrast, plants that were exposed to decreasing RH maintained high stomatal conductances and transpiration rates throughout the experiment (Fig. 3, Supplementary Fig. S2 at JXB online).
Fig. 3.
Effect of a sudden change in transpirational demand on stomatal conductance. (A) Stomatal conductance of control plants (‘Light control’). (B) Stomatal conductance of shaded plants (‘Shade’), of plants removed from shade after 4h (‘Light increase, 4h’), and of plants removed from shade after 28h (‘Light increase, 28h’). (C) Stomatal conductance of plants growing at high relative humidity (RH) after a step change in RH. Stomatal conductance was measured 5min (‘RH decrease, 5 min’), 4h (‘RH decrease, 4h’), and 28h (‘RH decrease, 28h’) after the decrease in humidity. Data show the means ±SE; n=5 plants. Significant differences are indicated by unique letters (P ≤ 0.05).
Root water flow and aquaporin expression patterns in light-exposed plants
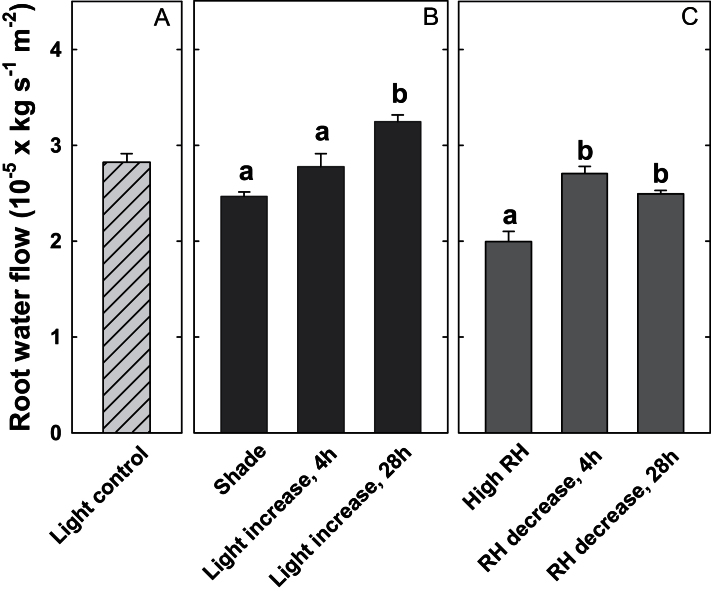
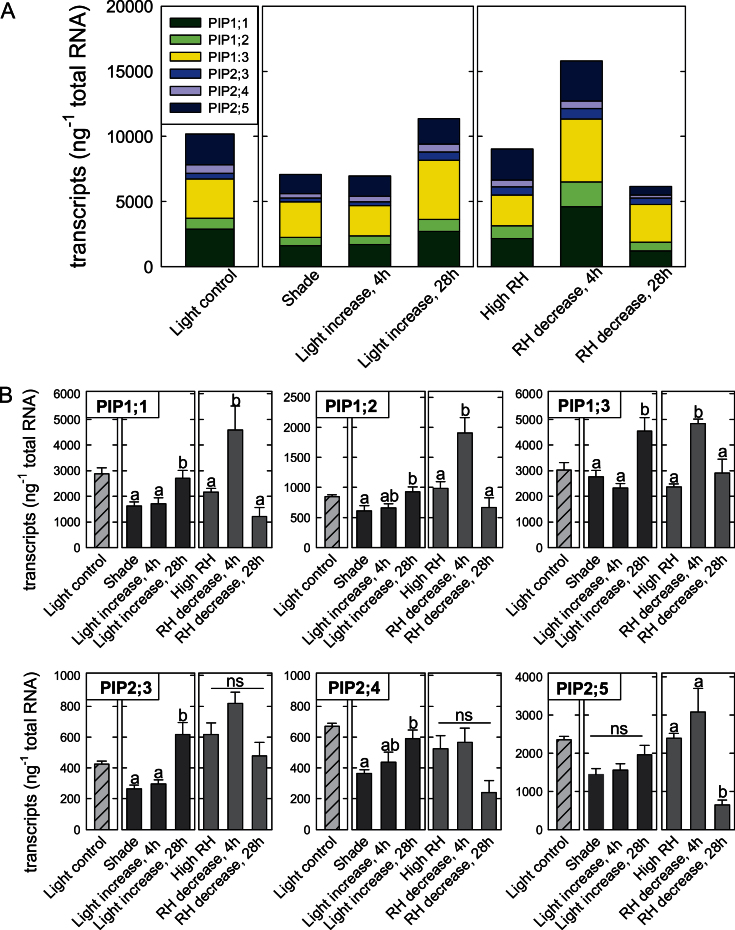
Q R increased in response to increased evaporative demand. In shaded plants, this increase was significant 28h after the increase in light level, but not after 4h (Fig. 4). The delayed increase in Q R corresponded to aquaporin expression patterns (Fig. 5). The total amount of PIP transcripts and the relative proportions of transcripts remained unchanged after 4h (Fig. 5A, compare ‘Shade’ and ‘Light increase, 4h’), but increased by 60% after 28h (Fig. 5A, ‘Light increase, 28h’). Of the aquaporin genes studied here, PIP1;3 ranked first in terms of its proportion to the total number of mRNA molecules (Fig. 5A, yellow portion of the bars). Moreover, this gene contributed substantially to the dynamic response shown in Fig. 5A. PIP2;5 was also highly expressed in roots (Fig. 5A, dark blue portion of the bars), but did not show significant changes in expression in response to an increase in light level.
Fig. 4.
Effect of a sudden change in transpirational demand on root water flow (scaled by leaf area). (A) Root water flow of control plants (‘Light control’). (B) Root water flow of shaded plants (‘Shade’), of plants removed from shade after 4h (‘Light increase, 4h’), and of plants removed from shade after 28h (‘Light increase, 28h’). (C) Water flow of plants growing at high relative humidity (‘High RH’), of plants removed from high RH after 4h (‘RH decrease, 4h’), and of plants removed from high RH after 28h (‘RH decrease, 28h’). Data show the means ±SE; n=5 plants. Significant differences are indicated by unique letters (P ≤ 0.05).
Fig. 5.
Effect of a sudden change in transpirational demand on aquaporin transcript amounts in poplar roots. (A) Cumulative aquaporin transcript amounts in roots. Individual genes are labelled with different colours. One subset of plants was grown at an adequate light level in the growth chamber (‘Light control’). Other subsets of plants were grown in shade (‘Shade’) or in a humidified box at ~95% relative humidity (‘High RH’). Shaded plants were exposed to a 5-fold increase in light level. Gene expression was measured 4h (‘Light increase, 4h’) and 28h (‘Light increase, 28h’) after the increase in light level. Plants growing at high humidity were removed from their humidified box and were exposed to an ~4-fold increase in vapour pressure deficit while light levels remained adequate. Gene expression was measured 4h and 28h after the decrease in relative humidity. (B) Transcript abundance of PtPIP1;1, PtPIP1;2, PtPIP1;3, PtPIP2;3, PtPIP2;4, and PtPIP2;5. Values are the means ±SE from three biological samples which were tested in triplicate. Significant differences are indicated by unique letters (P ≤ 0.05).
Figure 5B shows the expression patterns of individual genes. All of the three PIP1 genes exhibited a significant 52–66% increase in expression after 28h relative to plants that remained in shade; expression of PIP2;3 even increased >2-fold after 28h (Fig. 5B, black bars).
Root water flow and aquaporin expression patterns in plants experiencing a sudden drop in humidity
In plants that were removed from the high humidity environment, Q R increased by 35% after 4h and remained unchanged after 28h (Fig. 4C). The rapid increase in Q R corresponded to a 75% increase in the cumulative transcript copy numbers of all six PIP genes (Fig. 5A). This increase in transcripts after 4h was mainly due to a 2-fold increase in the transcript copy numbers of the three PIP1 genes (Fig. 5B, grey bars). No significant changes in the expression of PIP2 genes occurred after 4h.
After 28h, expression levels of PIP1 genes had returned to values found prior to the change in RH while Q R remained relatively high. While transcript copy numbers of PIP2;3 and PIP2;4 did not change significantly in response to the change in humidity, transcript numbers of PIP2;5 had decreased sharply after 28h (Fig. 5B).
Immunolabelling
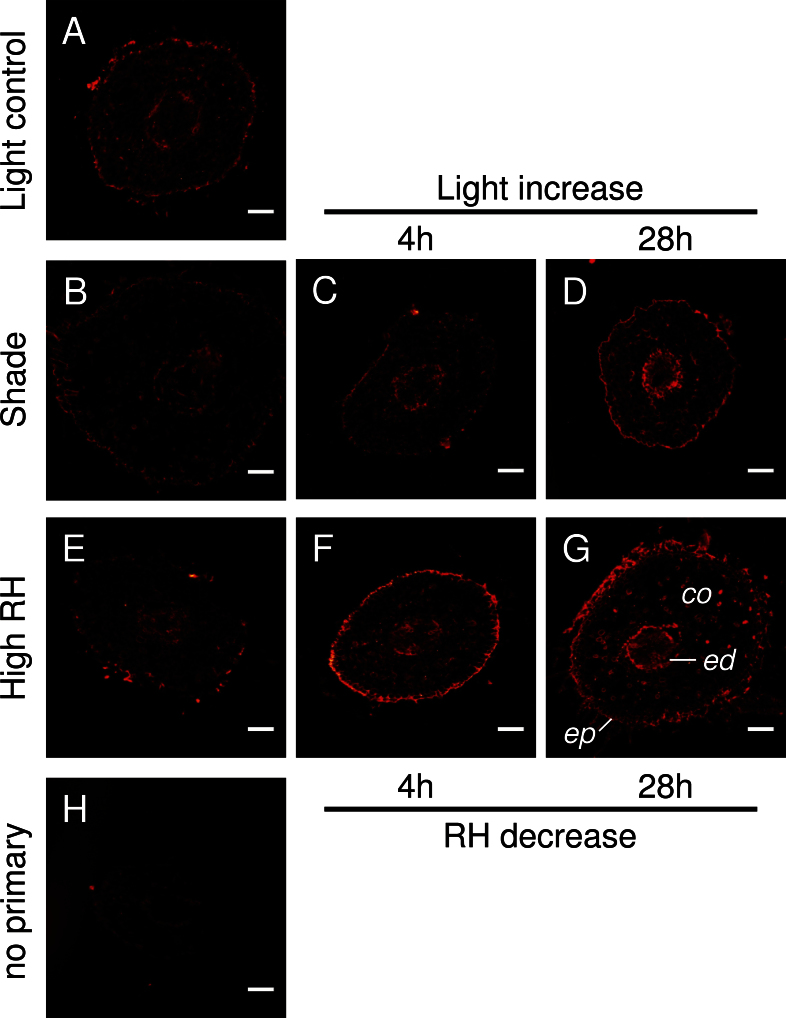
Immunofluorescence labelling was performed on cross-sections taken at 25–30mm from the root tip (Fig. 6). The intensity of the red colour is equivalent to the abundance of PIP1 protein. In roots of control plants, PIP1 was present in epidermis and cortex cells as well as in the endodermis and in vascular tissue (Fig. 6A). Weak labelling was observed in roots of shaded plants (Fig. 6B). In contrast, root sections taken after the increase in light level exhibited strong immunolabelling of the epidermis, endodermis, and of cells adjacent to the endodermis. Labelling was particularly abundant after 28h when a continuous fluorescence signal occurred in the epidermis (Fig. 6D). A similar trend was observed in plants that were exposed to decreasing humidity (Fig. 6E–G), although strong signals were already detected after 4h (Fig. 6F). Controls without primary antibody exhibited minimal fluorescence (Fig. 6H).
Fig. 6.
Immunolocalization of PIP1 protein in root cross-sections. Transverse sections were taken at 25–30mm from the root tip. PIP1 antibody is specific to all PIP1s. (A) Roots of control plants growing at full light in the growth chamber. (B–D) Roots of shaded plants before (B) and after a step change in light level (C, D). (E–G) Roots of plants growing at high relative humidity before (E) and after a step change in humidity (F, G). (H) Control with no primary antibody indicates minimal background autofluorescence. co, cortex; ed, endodermis; ep, epidermis. Bars=100 μm.
Discussion
Although much has been learned about the possible physiological roles of aquaporins in plants, many questions remain unanswered (Baiges et al., 2002; Aroca et al., 2012). The present study was conducted to gain a better understanding of how aquaporins in roots are regulated and how their function relates to whole-plant–water relations in woody plants (Hacke et al., 2012).
Aquaporin gene expression and root hydraulics are affected by changes in transpirational demand
The first objective of this present study was to measure absolute transcript abundance of key PIP1 and PIP2 genes 4h and 28h after hybrid poplar plants were exposed to an increase in transpirational demand, and to assess how transcriptional responses correspond to changes in Q R and other parameters of water relations. To minimize the effect of a circadian rhythm (Henzler et al., 1999; Clarkson et al., 2000; Lopez et al., 2003) on the data collected in the present study, all measurements were conducted between 10:30h and 11:30h.
Among the 11 PIP genes that were studied by Almeida-Rodriguez et al. (2011), the authors reported the differential expression of nine PIP genes in roots of poplars exposed to different light regimes. Based on this and on available literature data (Secchi et al., 2009; Supplementary Table S1 at JXB online), six PIP genes that were highly expressed in roots were chosen for gene expression analysis.
The three PIP1 genes exhibited remarkably similar expression patterns (Fig. 5B). Interestingly, these genes are orthologues of the rice OsPIP1 genes whose transcription in roots increased with transpirational demand (Sakurai-Ishikawa et al., 2011). Furthermore, the closely related PtPIP1;1 and PtPIP1;2 (95% amino acid identity; Supplementary Fig. S1 at JXB online) were found to be induced in response to xylem embolism (Secchi and Zwieniecki, 2010) and by osmotic stress (Bae et al., 2010). Expression changes of the studied PIP2 genes were smaller and more variable than those of the three PIP1 genes, a pattern which has also been described in drought-stressed stems of P. trichocarpa (Secchi and Zwieniecki, 2010).
In terms of transcript copy numbers, PIP1;1 and PIP1;3 ranked first among the PIP genes measured in this study (Fig. 5B). The transcripts of all three PIP1 genes represented nearly three-quarters of the total transcript amount while Q R increased. It is therefore suggested that these genes play crucial roles in modifying root water uptake in poplar in response to changes in transpirational demand. Aquaporin activity is regulated at both the transcriptional and the post-translational levels. While the present study focused on transcriptional regulation, it is noted that responses to a change in environmental conditions can also be realized by other mechanisms, including aquaporin gating, translocation of aquaporins into the membrane, and interactions of membrane proteins (e.g. Hedfalk et al., 2006; Zelazny et al., 2007; Maurel et al., 2008). Nonetheless, the fact that expression patterns, particularly those of PIP1 genes, closely corresponded to changes in Q R suggests that transcriptional control was an important mechanism involved in the regulation of root physiology.
Striking differences between trends in transcript abundance and Q R only occurred 28h after plants were transferred to lower humidity (compare Figs 4C and 5A ‘RH decrease, 28h’). At that time, transcript copy numbers of several genes reached low levels (Fig. 5B, grey ‘RH decrease, 28h’ bars) while Q R was still nearly as high as it was 4h after the change in humidity (Fig. 4C, grey bars). It is suggested that the peak in transcription seen 4h after the change in humidity resulted in an accumulation of water channel proteins, and that proteins were still present 24h later. This conclusion is supported by immunolabelling experiments, which revealed that PIP1 protein remained highly abundant in root cross-sections 28h after the transfer to lower humidity (Fig. 6).
Differences between plants grown in shade and in high humidity
The adaptive significance of aquaporin-mediated changes in whole-plant hydraulic conductance is that it would provide plants with a mechanism to maintain their water potential homeostasis despite changing environmental conditions through modifying water transport in roots and leaves. While the present study focused on roots, it is noted that whole-plant hydraulic conductance will probably also be affected by aquaporins in leaves (Heinen et al., 2009). A fine-tuned balance between water loss and water uptake is especially important in plants that are vulnerable to xylem cavitation and lack efficient mechanisms to repair xylem dysfunction. The poplar clone studied here (H11-11) is very vulnerable to cavitation. In a previous study (Plavcova and Hacke, 2012) on H11-11 plants growing under similar conditions, 50% loss of hydraulic conductivity occurred at –1.14MPa and –0.62MPa in basal and distal stem segments, respectively. This is close to or within the range of stem water potentials measured in the present study. It is therefore concluded that the recovery of stem water potentials 28h after the increase in transpirational demand was necessary to prevent excessive and irreversible levels of embolism.
Shaded plants would probably have benefited from a faster increase in Q R to take advantage of increased light levels (Almeida-Rodriguez et al., 2011). The relatively slow increase of Q R in shaded plants may be due to the stressful growing conditions that these plants experienced. Poplars are light-demanding plants, and shade-grown plants were probably energy starved. To the degree that new expression and activation of aquaporins are energy dependent, water uptake dynamics may have been constrained by limited resources in the roots of shaded plants.
Interestingly, changes in the transcript levels of PIP1 genes and in Q R occurred sooner in high humidity plants than in shaded plants. This may in part be due to the fact that stomatal conductance and transpiration in high humidity plants remained high throughout the experiment (Fig. 3; Supplementary Fig. S2 at JXB online). Stomata of these plants were larger and more frequent than in other plant groups, and were unable to close (Fig. 1; see also Arve et al., 2013). Hence, fast aquaporin-mediated responses of Q R to changes in the above-ground environment may have compensated for a lack of stomatal control.
An increase in light level is not required to trigger changes in gene expression and root hydraulics
The second objective of this study was to determine whether changes in gene expression and Q R would require an increase in light level per se, or whether such changes could also be triggered by lowering RH at a constant light level. Altering RH without changing irradiance had a profound effect on both PIP transcript levels and Q R (see above). It was therefore concluded that an increase in light level is not required to trigger changes in PIP expression and Q R in poplar. This conclusion agrees with recent work on rice (Sakurai-Ishikawa et al., 2011). Levin et al. (2009) found that some aquaporin genes were differentially expressed in Arabidopsis thaliana plants subjected to low RH. How exactly changes in the above-ground environment are transmitted to and sensed by roots remains unknown. The most parsimonious hypothesis is that root cells sense xylem pressure pulses (McElrone et al., 2007) or changes in water potential (Levin et al., 2009), and/or cell turgor (Hill et al., 2004), which all would correspond to changes in transpirational demand.
In conclusion, hybrid poplar plants were subjected to a sudden increase in transpirational demand, either by increasing the light level or by reducing the RH. Both treatments led to a transient perturbation of water potentials. At 28h after plants were removed from shade or from their high humidity environment, respectively, stem water potentials recovered to their original values (measured prior to treatments). The recovery of water potentials was associated with an increase in Q R and an increase in the transcript abundance of aquaporin genes in roots. In both experiments, transcript levels of three PIP1 genes closely matched trends in Q R. While stomata of plants grown in high humidity were unable to close properly, the Q R of these plants quickly responded to increased transpirational demand. In contrast, the Q R of shaded plants increased 28h after the increase in light, but not 4h after the removal from the shade environment. The fact that aquaporin gene expression and Q R responded to a drop in RH while light levels were unchanged indicates that an unknown signal was involved in this case of shoot–root communication. Future work will probably be directed at unravelling the nature of this signalling process and will study how the signal is perceived by root aquaporins.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Phylogenetic relationships of plasma membrane intrinsic proteins (PIPs) in Arabidopsis thaliana, Oryza sativa, and Populus trichocarpa.
Figure S2. Effect of step changes in light level and relative humidity on transpiration rate.
Table S1. Summary of previously reported PIP expression patterns in Populus trichocarpa.
Table S2. Primer sequences used in the qRT-PCR assays
Acknowledgements
UH acknowledges support from an NSERC Discovery grant and the Canada Research Chair program. We thank Anton Schäffner (Helmholtz Zentrum München, Germany) for the kind gift of the PIP1 antibody.
References
- Almeida-Rodriguez AM, Cooke JEK, Yeh F, Zwiazek JJ. 2010. Functional characterization of drought-responsive aquaporins in Populus balsamifera and Populus simonii×balsamifera clones with different drought resistance strategies. Physiologia Plantarum 140, 321–333. [DOI] [PubMed] [Google Scholar]
- Almeida-Rodriguez AM, Hacke UG, Laur J. 2011. Influence of evaporative demand on aquaporin expression and root hydraulics in hybrid poplar. Plant, Cell and Environment 34, 1318–1331. [DOI] [PubMed] [Google Scholar]
- Arend M, Schnitzler JP, Ehlting B, Hansch R, Lange T, Rennenberg H, Himmelbach A, Grill E, Fromm J. 2009. Expression of the Arabidopsis mutant abi1 gene alters abscisic acid sensitivity, stomatal development, and growth morphology in gray poplars. Plant Physiology 151, 2110–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca R, Porcel R, Ruiz-Lozano JM. 2012. Regulation of root water uptake under abiotic stress conditions. Journal of Experimental Botany 63, 43–57. [DOI] [PubMed] [Google Scholar]
- Arve LE, Terfa MT, GislerØD HR, Olsen JE, Torre S. 2013. High relative air humidity and continuous light reduce stomata functionality by affecting the ABA regulation in rose leaves. Plant, Cell and Environment 36, 382–392. [DOI] [PubMed] [Google Scholar]
- Arvidsson S, Kwasniewski M, Riano-Pachon DM, Mueller-Roeber B. 2008. QuantPrime—a flexible tool for reliable high-throughput primer design for quantitative PCR. BMC Bioinformatics 9, 465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae EK, Lee H, Lee JS, Noh EW. 2010. Isolation and characterization of osmotic stress-induced genes in poplar cells by suppression subtractive hybridization and cDNA microarray analysis. Plant Physiology and Biochemistry 48, 136–141. [DOI] [PubMed] [Google Scholar]
- Baiges I, Schäffner AR, Affenzeller MJ, Mas A. 2002. Plant aquaporins. Physiologia Plantarum 115, 175–182. [DOI] [PubMed] [Google Scholar]
- Barigah TS, Ibrahim T, Bogard A, Faivre-Vuillin B, Lagneau LA, Montpied P, Dreyer E. 2006. Irradiance-induced plasticity in the hydraulic properties of saplings of different temperate broad-leaved forest tree species. Tree Physiology 26, 1505–1516. [DOI] [PubMed] [Google Scholar]
- Blake TJ, Filho WS. 1988. Drought tolerance, growth partitioning and vigor in eucalypt seedlings and rooted cuttings. Tree Physiology 4, 325–335. [DOI] [PubMed] [Google Scholar]
- Clarkson DT, Carvajal M, Henzler T, Waterhouse RN, Smyth AJ, Cooke DT, Steudle E. 2000. Root hydraulic conductance: diurnal aquaporin expression and the effects of nutrient stress. Journal of Experimental Botany 51, 61–70. [PubMed] [Google Scholar]
- Gomes D, Agasse A, Thiébaud P, Delrot S, Gerós H, Chaumont F. 2009. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochimica et Biophysica Acta 1788, 1213–1228. [DOI] [PubMed] [Google Scholar]
- Gong HQ, Peng YB, Zou C, Wang DH, Xu ZH, Bai SN. 2006. A simple treatment to significantly increase signal specificity in immunohistochemistry. Plant Molecular Biology Reporter 24, 93–101. [Google Scholar]
- Gupta AB, Sankararamakrishnan R. 2009. Genome-wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa: characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant Biology 9, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke UG, Jacobsen AL, Pratt RB, Maurel C, Lachenbruch B, Zwiazek J. 2012. New research on plant–water relations examines the molecular, structural, and physiological mechanisms of plant responses to their environment. New Phytologist 196, 345–348. [DOI] [PubMed] [Google Scholar]
- Hacke U, Sauter JJ. 1995. Vulnerability of xylem to embolism in relation to leaf water potential and stomatal conductance in Fagus sylvatica f. purpurea and Populus balsamifera . Journal of Experimental Botany 46, 1177–1183. [Google Scholar]
- Hedfalk K, Tornroth-Horsefield S, Nyblom M, Johanson U, Kjellbom P, Neutze R. 2006. Aquaporin gating. Current Opinion in Structural Biology 16, 447–456. [DOI] [PubMed] [Google Scholar]
- Heinen RB, Ye Q, Chaumont F. 2009. Role of aquaporins in leaf physiology. Journal of Experimental Botany 60, 2971–2985. [DOI] [PubMed] [Google Scholar]
- Henzler T, Waterhouse RN, Smyth AJ, Carvajal M, Cooke DT, Schaffner AR, Steudle E, Clarkson DT. 1999. Diurnal variations in hydraulic conductivity and root pressure can be correlated with the expression of putative aquaporins in the roots of Lotus japonicus . Planta 210, 50–60. [DOI] [PubMed] [Google Scholar]
- Hill AE, Shachar-Hill B, Shachar-Hill Y. 2004. What are aquaporins for? Journal of Membrane Biology 197, 1–32. [DOI] [PubMed] [Google Scholar]
- Javot H, Maurel C. 2002. The role of aquaporins in root water uptake. Annals of Botany 90, 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HG, Sutherland R. 1991. Stomatal control of xylem embolism. Plant, Cell and Environment 14, 607–612. [Google Scholar]
- Jones HG, Tardieu F. 1998. Modelling water relations of horticultural crops: a review. Scientia Horticulturae 74, 21–46. [Google Scholar]
- Kamaluddin M, Zwiazek JJ. 2004. Effects of root medium pH on water transport in paper birch (Betula papyrifera) seedlings in relation to root temperature and abscisic acid treatments. Tree Physiology 24, 1173–1180. [DOI] [PubMed] [Google Scholar]
- Kammerloher W, Fischer U, Piechottka GP, Schaffner AR. 1994. Water channels in the plant plasma-membrane cloned by immunoselection from a mammalian expression system. The Plant Journal 6, 187–199. [DOI] [PubMed] [Google Scholar]
- Levin M, Resnick N, Rosianskey Y, Kolotilin I, Wininger S, Lemcoff JH, Cohen S, Galili G, Koltai H, Kapulnik Y. 2009. Transcriptional profiling of Arabidopsis thaliana plants’ response to low relative humidity suggests a shoot–root communication. Plant Science 177, 450–459. [Google Scholar]
- Lo Gullo MA, Nardini A, Salleo S, Tyree MT. 1998. Changes in root hydraulic conductance (K R) of Olea oleaster seedlings following drought stress and irrigation. New Phytologist 140, 25–31. [Google Scholar]
- Lopez D, Bronner G, Brunel N, et al. 2012. Insights into Populus XIP aquaporins: evolutionary expansion, protein functionality, and environmental regulation. Journal of Experimental Botany 63, 2217–2230. [DOI] [PubMed] [Google Scholar]
- Lopez F, Bousser A, Sissoëff I, Gaspar M, Lachaise B, Hoarau J, Mahé A. 2003. Diurnal regulation of water transport and aquaporin gene expression in maize roots: contribution of PIP2 proteins. Plant and Cell Physiology 44, 1384–1395. [DOI] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V. 2008. Plant aquaporins: membrane channels with multiple integrated functions. Annual Review of Plant Biology 59, 595–624. [DOI] [PubMed] [Google Scholar]
- McElrone AJ, Bichler J, Pockman WT, Addington RN, Linder CR, Jackson RB. 2007. Aquaporin-mediated changes in hydraulic conductivity of deep tree roots accessed via caves. Plant, Cell and Environment 30, 1411–1421. [DOI] [PubMed] [Google Scholar]
- Nejad AR, van Meeteren U. 2007. The role of abscisic acid in disturbed stomatal response characteristics of Tradescantia virginiana during growth at high relative air humidity. Journal of Experimental Botany 58, 627–636. [DOI] [PubMed] [Google Scholar]
- Plavcova L, Hacke UG. 2012. Phenotypic and developmental plasticity of xylem in hybrid poplar saplings subjected to experimental drought, nitrogen fertilization, and shading. Journal of Experimental Botany 63, 6481–6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postaire O, Tournaire-Roux C, Grondin A, Boursiac Y, Morillon R, Schaffner AR, Maurel C. 2010. A PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis . Plant Physiology 152, 1418–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge RG. 2011. A Java program for LRE-based real-time qPCR that enables large-scale absolute quantification. PLoS One 6, e17636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge RG, Stewart D. 2008. A kinetic-based sigmoidal model for the polymerase chain reaction and its application to high-capacity absolute quantitative real-time PCR. BMC Biotechnology 8, 47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge RG, Stewart D. 2010. Assessing the performance capabilities of LRE-based assays for absolute quantitative real-time PCR. PLoS One 5, e9731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai-Ishikawa J, Murai-Hatano M, Hayashi H, Ahamed A, Fukushi K, Matsumoto T, Kitagawa Y. 2011. Transpiration from shoots triggers diurnal changes in root aquaporin expression. Plant, Cell and Environment 34, 1150–1163. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nature Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchi F, Maciver B, Zeidel ML, Zwieniecki MA. 2009. Functional analysis of putative genes encoding the PIP2 water channel subfamily in Populus trichocarpa . Tree Physiology 29, 1467–1477. [DOI] [PubMed] [Google Scholar]
- Secchi F, Zwieniecki MA. 2010. Patterns of PIP gene expression in Populus trichocarpa during recovery from xylem embolism suggest a major role for the PIP1 aquaporin subfamily as moderators of refilling process. Plant, Cell and Environment 33, 1285–1297. [DOI] [PubMed] [Google Scholar]
- Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R. 2002. PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. The Plant Cell 14, 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS, Pockman WT. 1993. Limitation of transpiration by hydraulic conductance and xylem cavitation in Betula occidentalis . Plant, Cell and Environment 16, 279–287. [Google Scholar]
- Tyree MT, Velez V, Dalling JW. 1998. Growth dynamics of root and shoot hydraulic conductance in seedlings of five neotropical tree species: scaling to show possible adaptation to differing light regimes. Oecologia 114, 293–298. [DOI] [PubMed] [Google Scholar]
- Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD. 2009. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiology 149, 445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins O, Nahal H, Foong J, Provart NJ, Campbell MM. 2009. Expansion and diversification of the Populus R2R3-MYB family of transcription factors. Plant Physiology 149, 981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazny E, Borst JW, Muylaert M, Batoko H, Hemminga MA, Chaumont F. 2007. FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proceedings of the National Academy of Sciences, USA 104, 12359–12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.