Abstract
The involvement of ethylene response factor (ERF) transcription factor (TF) in the transcriptional regulation of ethylene biosynthesis genes during fruit ripening remains largely unclear. In this study, 15 ERF genes, designated as MaERF1–MaERF15, were isolated and characterized from banana fruit. These MaERFs were classified into seven of the 12 known ERF families. Subcellular localization showed that MaERF proteins of five different subfamilies preferentially localized to the nucleus. The 15 MaERF genes displayed differential expression patterns and levels in peel and pulp of banana fruit, in association with four different ripening treatments caused by natural, ethylene-induced, 1-methylcyclopropene (1-MCP)-delayed, and combined 1-MCP and ethylene treatments. MaERF9 was upregulated while MaERF11 was downregulated in peel and pulp of banana fruit during ripening or after treatment with ethylene. Furthermore, yeast-one hybrid (Y1H) and transient expression assays showed that the potential repressor MaERF11 bound to MaACS1 and MaACO1 promoters to suppress their activities and that MaERF9 activated MaACO1 promoter activity. Interestingly, protein–protein interaction analysis revealed that MaERF9 and -11 physically interacted with MaACO1. Taken together, these results suggest that MaERFs are involved in banana fruit ripening via transcriptional regulation of or interaction with ethylene biosynthesis genes.
Key words: Banana, ERF, ethylene biosynthesis, fruit ripening, interaction, transcriptional regulation.
Introduction
Ethylene is a simple gaseous plant hormone involved in many plant physiological processes, including fruit ripening (Guo and Ecker, 2004). Fruit ripening is a complex and genetically programmed process that results in marked changes in colour, flavour, aroma, texture, and nutritional value of the flesh (Giovannoni, 2004). These changes are the result of the coordinated activation of multiple genetic and biochemical pathways, which are influenced by internal and external cues regulated by many critical transcription factors (TFs) (Martel et al., 2011). Ethylene response factor (ERFs) are plant-specific and belong to the AP2/EREBP-type TFs which function as trans-acting factors
at the last step of ethylene signalling (El-Sharkawy et al., 2009; Lin et al., 2009; Klee and Giovannoni, 2011). Many ERF proteins are important in plant responses to developmental cues and stresses by specifically interacting with the GCC box (a core sequence of AGCCGCC) and/or dehydration-responsive elements/C-repeat (DRE/CRT, a core sequence of CCGAC) presented in the promoters of many ethylene-responsive genes, demonstrating that a transcriptional cascade is involved in ethylene signalling or biosynthesis (El-Sharkawy et al., 2009; Zhang et al., 2009; Yin et al., 2010). Although accumulating studies have shown that ERF proteins play an important role in fruit ripening (Lin et al., 2009; Klee and Giovannoni, 2011) and multiple ERF proteins have been characterized in many crops (Xu et al., 2011) and some fruits, such as tomato (Li et al., 2007; Zhang et al., 2009), apple (Wang et al., 2007), plum (El-Sharkawy et al., 2009), kiwifruit (Yin et al., 2010), and longan (Kuang et al., 2012), little information is available about the ERF proteins in transcriptional regulation of ethylene biosynthesis in relation to fruit ripening.
Ethylene synthesis starts from methionine, which is first converted to S-adenosylmethionine by S-adenosylmethionine synthetase. S-Adenosylmethionine is subsequently metabolized to the ethylene precursor 1-aminocyclopropane-1-carboxylate (ACC) by ACC synthase (ACS), a rate-limiting step in ethylene biosynthesis. ACC is then oxidized by ACC oxidase (ACO) to generate ethylene in a reaction that also produces CO2 and hydrogen cyanide (Yang and Hoffman, 1984). Previous studies have revealed that the expression of ACS and ACO is related to ethylene production in most cases, including fruit ripening (Alexander and Grierson, 2002). It has been well documented that ethylene biosynthesis is modulated by many factors or regulators at both transcriptional and post-transcriptional levels (Chae et al., 2003; Liu and Zhang, 2004; Wang et al., 2004; Joo et al., 2008; Zhang et al., 2009; Prasad et al., 2010; Wan et al., 2011). For instances, an E3 ubiquitin ligase ETO1, targets type-2 ACS enzymes for degradation, thereby inhibiting its activity (Chae et al., 2003). A member of the RING E3 ligase XBAT32 negatively modulates the abundance of ACS proteins and ethylene biosynthesis (Prasad et al., 2010). ACS protein stability is also regulated by protein phosphorylation and dephosphorylation during ethylene induction (Joo et al., 2008; Hahn and Harter, 2009). These reports suggest that modulation of ethylene biosynthesis at the protein level is crucial for ethylene production. In addition, transcriptional regulation mediated by TFs also plays an important role in ethylene biosynthesis. Transcription factors such as tomato RIN, LeERF2, tobacco TERF1, and rice OsDERF1, modulate the expression of ethylene biosynthesis genes whereas downstream stress-responsive genes have been reported recently (Ito et al., 2008; Zhang et al., 2009; Wan et al., 2011; Qin et al., 2012). These investigations demonstrate that transcriptional regulation is a pivotal mechanism in controlling ethylene synthesis. However, it is unclear whether and how ethylene biosynthesis is transcriptionally modulated during economic fruit ripening.
Increasing evidence suggests that the control of climacteric fruit ripening relies largely on the modulation of ethylene production and/or action, and autoregulation of ethylene biosynthesis at the transcriptional level and regulation in ACS and ACO genes is only one consequence of ethylene response in ripening fruits (Inaba et al., 2007; Zhang et al., 2009; Yin et al., 2010). Banana (Musa acuminata, AAA group), a typical climacteric fruit, is characterized by a peak in ethylene production that orchestrates ripening-associated processes (Clendennen and May, 1997). Some genes associated with ethylene biosynthesis and perception pathways have been isolated from banana fruit, including ACS, ACO, ethylene receptor, a CTR1 orthologue, and ethylene insensitive 3-like genes (Clendennen et al., 1997; Liu et al., 1999; Inaba et al., 2007; Mbéguié-A-Mbeguié et al., 2008). Like many other climacteric fruits, banana ethylene biosynthesis and perception genes are differentially regulated at the transcriptional level during fruit ripening (Do et al., 2005; Inaba et al., 2007; Mbéguié-A-Mbeguié et al., 2008; Choudhury et al., 2008). Very recently, a banana fruit Mcm1-agamous-deficiens-S (MADS) 5 TF was identified to bind to CArG-box sequence in the promoters of major ripening genes, including MaACS1 and MaACO1, to be involved in fruit ripening through directly regulating these ripening genes (Choudhury et al., 2012a). In addition, banana MaACS1 is reported to be phosphorylated by a Ser/Thr protein kinase during fruit ripening (Choudhury et al., 2012b). These reports provide important novel evidence of transcriptional and post-translational regulations of MaACS1 in relation to fruit ripening. However, the mechanism of transcriptional regulation of the key ethylene biosynthetic genes MaACS1 and MaACO1 during banana fruit ripening is still largely unknown. In the present study, 15 ERF TFs were isolated and characterized from banana fruit. Their expression patterns in association with four different ripening treatments were analysed. Moreover, the interactions of the ripening-related MaERFs with MaACS1 and MaACO1 were investigated.
Materials and methods
Plant materials and treatments
Pre-climacteric banana (M. acuminata AAA group, cv. Cavendish) fruit at 75–80% maturation were obtained from a local commercial plantation near Guangzhou, China. Four postharvest treatments were performed, including a control (natural ripening), ethylene-induced ripening (100 μl ethylene l–1, 18h), 1-methylcyclopropene (1-MCP)-delayed ripening (0.5 μl 1-MCP l–1, 18h), and a combination of 1-MCP with ethylene treatment (1-MCP + ethylene), as described previously (Shan et al., 2012). After each treatment, fruit were held at 22 °C and 90% relative humidity until production of climacteric ethylene and complete ripening. For each treatment, samples were taken based on the rate of ethylene production and fruit firmness changes during ripening as described by Shan et al. (2012). All of the samples were frozen in liquid nitrogen after sampling, and then stored at –80 °C for further use. All assessments were conducted using three biological replicates.
RNA extraction, gene isolation, and sequence analysis
Frozen tissues were ground in liquid nitrogen using a mortar and pestle. Total RNA was extracted using the hot borate method of Wan and Wilkins (1994). Potentially contaminating DNA was eliminated by treatment with DNAse I digestion using an RNAse-free kit (Promega Madison, WI, USA). The DNA-free total RNA was then used as template for reverse-transcription PCR. The first-strand cDNA of the product was subjected to PCR amplification.
According to gene annotation and bioinformatics analysis, 15 ERF genes, termed MaERF1–MaERF15, were isolated from a transcriptome database obtained using a high-throughput Solexa/Illumina sequencing platform (Beijing Genomics Institute, Shenzhen, China) and their sequences were verified by further cloning and sequencing. Four of the 15 ERF genes, MaERF1, -3, -9, and -12, were full-length sequences in the database, with complete start and stop codes, while the full-length sequences of the other 11 ERF genes were obtained by 3’- or 5’-rapid amplification of cDNA ends (RACE) using a RACE kit (TaKaRa Biotechnology, Dalian, China) according to the manufacturer’s instructions. The specific primers used for RACE are provided in Supplementary Table S1 (available at JXB online). Alignments were carried out using clustalx (version 1.83) and GeneDoc software, and a phylogenetic tree was constructed using the minimum-evolution method with mega5.
Quantitative real-time PCR analysis
Isolation of total RNA from the samples and synthesis of first-strand cDNA were performed as described above. The synthesized cDNA was diluted 1:40 with water, and 2 μl of the diluted cDNA was used as a template for quantitative real-time PCR (qPCR). Reactions were performed in a total volume of 20 μl, containing 1 μl each 10 μM primer and 10 μl SYBR Green PCR Supermix on CFX96 Real-Time PCR System (Bio-Rad Laboratories) according to the manufacturer’s instructions. The cycling program included an initial denaturation step at 94 °C for 5min, followed by 40 cycles of 94 °C for 10, 60 °C for 30 s, and 72 °C for 30 s. No-template controls for each primer pair were included in each run. The oligonucleotide primers for qPCR analysis were designed on the basis of the 3’-untranslated region using Primer 5.0 software. The sequences of all primers used for qPCR analysis are described in Supplementary Table S2. Ribosomal protein 2 and clathrin adaptor complex were selected as reference genes under different experimental conditions according to this group’s previous study on the selection of reliable reference genes for expression study by qPCR in banana fruit (Chen et al., 2011). Each assay using the gene-specific primers amplified a single product of the correct size with high efficiency (90–110%). All qPCRs were normalized using the cycle threshold (Ct) value corresponding to the reference gene. The relative expression levels of the targeted genes were calculated using the formula 2–ΔΔCt. The values represent the mean of three biological replicates.
Subcellular localization of MaERF proteins
The coding sequences of MaERFs without the stop codon were amplified by PCR (primers are listed in Supplementary Table S3), and then subcloned into the pBI221-GFP vector, in frame with the green fluorescent protein (GFP) sequence, resulting in 35S::gene–GFP vectors under the control of the cauliflower mosaic virus (CaMV) 35S promoter. The fusion constructs and the control GFP vector were used for transient assays using a modified polyethylene glycol (PEG) transfection method with tobacco BY-2 suspension culture cell protoplasts as described previously (Abel and Theologis, 1994). GFP fluorescence was observed with a fluorescence microscope (Axioskop 2 Plus, Zeiss). All transient expression assays were repeated at least three times.
Yeast one-hybrid (Y1H) assay
Yeast one-hybrid assay was carried out by using the Matchmaker Gold Yeast One-Hybrid System (Clontech). According to the reported sequences of the MaACS1 and MaACO1 promoters (Wang and Peng, 2001a,b), the MaACS1 and MaACO1 promoters were cloned into pAbAi (primers are listed in Supplementary Table S4). Plasmid was linearized and transformed into Y1H Gold strain. Positive yeast cells were then transformed with pGADT7-AD, which contained MaERF9 or MaERF11. The DNA–protein interaction was determined based on the growth ability of the cotransformants on SD/-Leu medium with aureobasidin A, according to the manufacturer’s protocol.
Transient expression assay and GUS activity analysis
The reporter construct was generated using full-length of MaACS1 and MaACO1 promoter sequences in the binary vector pBI101 cloned into the SalI and BamHI upstream sites of the GUS reporter gene. For the transient expression assay, tobacco (Nicotiana benthamiana) leaves were co-infiltrated with Agrobacterium GV3101 containing the reporter vector and the effector vector containing MaERF9 or -11 in the binary vector pCAMBIA 1300 using the SalI and BamHI sites as described by Yu et al. (2012). After 48h of growth in the chamber, the infected leaves were used to analyse the GUS activity. The plant proteins were extracted and their fluorescence was measured as described by Jefferson et al. (1987), using a fluorometer (VersaFluor Fluorometer, Bio-Rad, Hercules, CA, USA). The aliquot at time zero was used as the control. The protein concentration was determined using a protein assay kit (Bio-Rad). The primers used in the construction the effector and reporter vectors are listed in Supplementary Table S5.
Yeast two-hybrid (Y2H) assay
Y2H assays were performed using the Matchmaker Gold Yeast Two-Hybrid System (Clontech). The coding regions of MaERF9 and -11, MaACS1 and MaACO1 were cloned into pGADT7 and pGBKT7 to fuse with the activation domain (AD) and DNA-binding domain (DBD), respectively, to create different baits and preys (primers are shown in Supplementary Table S6). MaACS1 and MaACO1 did not show any transcriptional activation activity in yeast cells (data not shown). Different pairs of bait and prey constructs were then co-transformed into yeast strain Gold Y2H using the lithium acetate method. Yeast cells grew on a SD/–Leu/–Trp) according to the manufacturer’s protocol (Clontech) for 3 d. Transformed colonies were plated onto minimal medium quadruple dropout (SD medium with –Leu/–Trp/–His/–Ade) containing 125 μM aureobasidin A and 4mg ml–1 X-α-Gal at 30 °C to test for possible interactions between MaERF9/MaERF11 and MaACS1/MaACO1 by their growth status and α-galactosidase activities.
Bimolecular fluorescence complementation assay
To generate constructs for the bimolecular fluorescence complementation (BiFC) assays, the full-length coding sequences of MaERF9 and -11, MaACS1 and MaACO1 without their stop codons were subcloned into the pUC-pSPYNE or pUC-pSPYCE vectors. The expression of target genes alone was used as negative controls. The resulting constructs were used for transient assays using a modified PEG transfection of tobacco BY-2 suspension culture cell protoplasts as described above. The transformed protoplasts then grew in liquid MS medium containing 0.4M sucrose for 24–48h, and transfected cells were imaged using a fluorescence microscope with a yellow fluorescent protein (YFP) filter (Axioskop 2 Plus). The primers used in the BiFC assay are listed in Supplementary Table S7.
Statistical analysis
The experiment was arranged in a completely randomized design. Each sample time point for each treatment was comprised of three independent biological replicates. Data were plotted as means ± standard errors (SE) in figures. Least significant difference (LSD) at the 5% level was analysed by DPS software (version 3.01; Zhejiang University, Hangzhou, China). A complete linkage hierarchical clustering of 15 banana fruit MaERF genes was generated using the Pearson clustering algorithm according to their expression profiles.
Results
Sequence analysis of 15 MaERF genes
This study isolated 15 novel ERF full-length cDNAs from banana fruit, designated MaERF1 to MaERF15. The sizes of the deduced amino acid sequences differed substantially (e.g. MaERF3 codes 432 amino acids, while MaERF11 codes 183 amino acids) (Supplementary Fig. S1). The sequence similarities of the MaERFs varied from 18.0% (MaERF3 and MaERF6) to 76.7% (MaERF12 and MaERF13) (Supplementary Table S8). Alignments of the full-length deduced proteins of MaERFs and AtERFs showed conserved motifs, including a DNA-binding domain designated the ERF domain (ranging from 58 to 59 amino acids, Supplementary Figs. S1A, B and S2), which is a defining character of the ERF transcriptional factor gene family (Fujimoto et al., 2000; Nakano et al., 2006; Yin et al., 2010). In addition, MaERF10–MaERF15 also exhibited a repressor domain, the ERF-associated amphiphilic repression domain (EAR) located at the 3’-end of the sequence (Supplementary Fig. S1A, C).
Phylogenetic analysis of MaERFs, AtERFs, and other fruit ERFs, including tomato, apple, and longan, revealed that MaERF sequences were clustered into seven out of 12 groups of ERF proteins (Nakano et al., 2006) (Supplementary Fig. S3). MaERF1 and MaERF2 are classified as members of subfamily IXc, MaERF5 and -6 belong to subfamily X, and MaERF7 and -8 belong to subfamily VII. MaERF3, -4, and -9 are assigned to subfamilies I, IXb, and II ERFs respectively. MaERF10–MaERF15 belong to subfamily VIII. Overall, these data suggest that MaERF1–MaERF15 may exhibit diverse functions.
Nuclear localization of five MaERF proteins
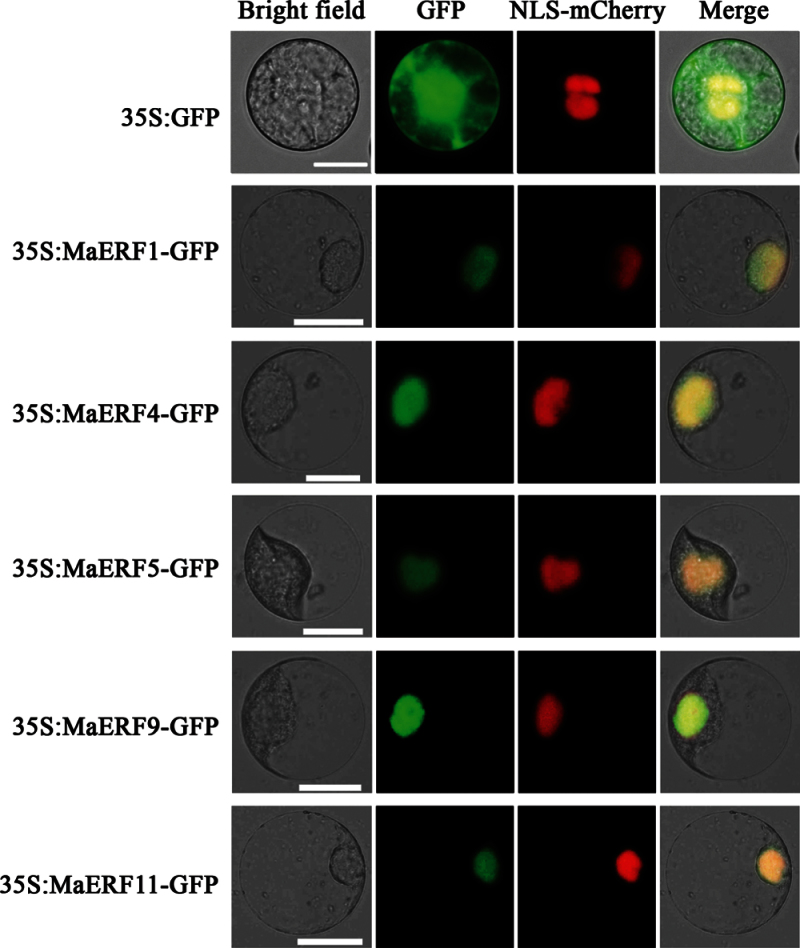
Potential nuclear localization signals (NLS) sequences were predicted for all banana ERFs based on the sequence analysis. To examine the subcellular localization of MaERFs in vivo, five MaERF genes from different subfamilies, including MaERF1 (subfamily IXc), MaERF4 (subfamily IXb), MaERF5 (subfamily X), MaERF9 (subfamily II), and MaERF11 (subfamily VIII) were selected, and then the full-length coding sequences of these five MaERF genes were fused in frame with the GFP gene. Transient expression of these constructs in tobacco BY-2 protoplasts showed that the fluorescence of all these MaERF proteins was localized exclusively in the nucleus (Fig. 1). By contrast, the GFP control showed ubiquitous distribution of GFP signal in the whole cells. Similar results were also obtained in tobacco leaves (Supplementary Fig. S4).
Fig. 1.
Subcellular localization of MaERFs in tobacco BY-2 protoplasts. Protoplasts were transiently transformed with MaERF–GFP constructs or GFP vector using a modified PEG method. GFP fluorescence was observed with a fluorescence microscope. VirD2NLS-mCherry was included in each transfection to serve as a control for successful transfection, as well as for nuclear localization. Images were taken in a dark field for green fluorescence, while the outline of the cell and the merged were photographed in a bright field. Bars, 25 μm (this figure is available in colour at JXB online).
Differential expression of MaERF genes in peel and pulp during fruit ripening
The data related with fruit ripening and softening, including changes in fruit firmness and ethylene production in banana fruit in four different ripening treatments caused by natural, ethylene-induced, 1-MCP-delayed, and combined 1-MCP and ethylene treatments, have been described in Shan et al. (2012). Ethylene treatment accelerated fruit ripening and promoted a climacteric rise, with a peak appearance at 3 d. Control natural fruit started the ethylene climacteric at 15 d and reached a peak at 21 d. In contrast, 1-MCP treatment delayed ethylene production, with a peak appearance at day 30. In addition, application of ethylene to the 1-MCP-treated fruit promoted ethylene evolution, with the ethylene production starting to increase at day 25 and then reaching a peak at day 28 (Shan et al., 2012).
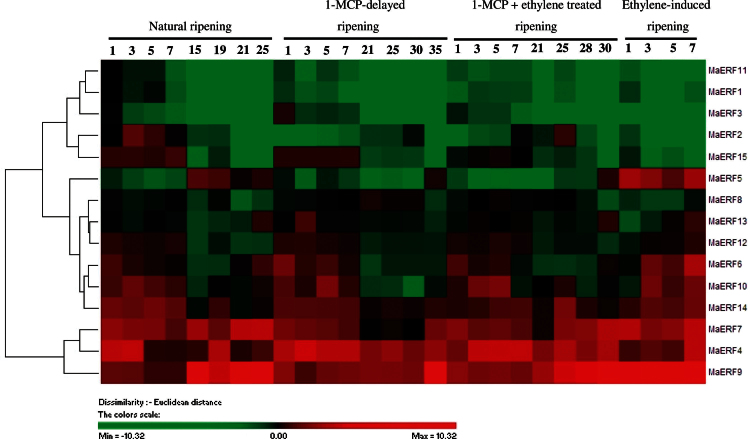
The expression patterns of MaERF1–MaERF15 in peel and pulp of banana fruit with the four different ripening treatments were investigated by qPCR. Figs. 2 and 3 showed complicated expression patterns of MaERF genes. In the peel, among the 15 MaERF genes, MaERF4, -7, and -9 showed increased transcript levels, followed by the increase in ethylene production at about 15, 1, 25, and 25 d of storage in natural, ethylene-induced, 1-MCP-delayed ripening, or combined 1-MCP and ethylene-treated fruit, respectively. A more marked increase was observed for MaERF9 transcript level (Fig. 2 and Supplementary Fig. S5). In contrast, MaERF1, -2, -3, -8, -11, and -15 transcript levels decreased after ethylene treatment, following the increase in ethylene production, with a more obvious decrease for MaERF1 and -11 transcript levels (Fig. 2 and Supplementary Fig. S5). In addition, MaERF5, -6, -10, -12, -13, and -14 were induced in ethylene-treated fruit, while their transcript levels in natural, 1-MCP-delayed ripening, or combined 1-MCP and ethylene-treated fruit decreased or changed only slightly following the increase in ethylene production (Fig. 2 and Supplementary Fig. S5).
Fig. 2.
Agglomerative hierarchical cluster analysis of transcript levels from 15 MaERF genes in peel of banana fruit with four different ripening characteristics caused by natural (control), ethylene-induced, 1-MCP-delayed, and combined 1-MCP and ethylene treatments. The cluster was generated using the Pearson clustering algorithm according to gene expression profile analysis by qPCR. Data are log2-transformed (or −ΔΔCt) value of gene expression of each time point compared to day 0 of control fruit. The scale indicates log2 variations: red, increase; green, decrease. Means ± SE from three repeats are provided in Supplementary Fig. S5. The physiology data related with fruit ripening and softening, including changes in fruit firmness and ethylene production in banana fruit with these four different ripening characteristics has been described in Shan et al. (2012) (this figure is available in colour at JXB online).
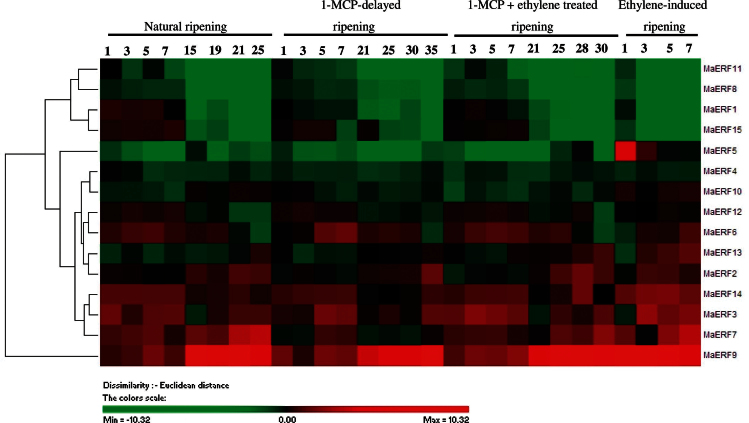
Fig. 3.
Agglomerative hierarchical cluster analysis of transcript levels from 15 MaERF genes in banana fruit pulp with four different ripening characteristics caused by natural (control), ethylene-induced, 1-MCP-delayed, and combined 1-MCP and ethylene treatments. The cluster was generated using the Pearson clustering algorithm according to gene expression profile analysis by qPCR. Data are log2-transformed (or −ΔΔCt) value of gene expression of each time point compared to day 0 of control fruit. The scale indicates log2 variations: red, increase; green, decrease. Means ± SE from three repeats are provided in Supplementary Fig. S6. The physiology data related with fruit ripening and softening, including changes in fruit firmness and ethylene production in banana fruit with these four different ripening characteristics has been described in Shan et al. (2012) (this figure is available in colour at JXB online).
In the pulp, transcript levels of MaERF3, -7, -9, and -14 were higher in ethylene-treated fruit, and their transcript levels in natural, ethylene-induced, 1-MCP-delayed ripening, or combined 1-MCP and ethylene-treated fruit clearly increased with ethylene evolution, with a more obvious accumulation for MaERF9 (Fig. 3 and Supplementary Fig. S6). MaERF2, -6, -10, -12, and -13 transcript levels slightly increased in ethylene-treated fruit, and their transcript levels in natural, 1-MCP-delayed ripening, or combined 1-MCP and ethylene-treated fruit decreased or changed only slightly during ripening (Fig. 3 and Supplementary Fig. S6). MaERF1, -4, -5, -8, -11, and -15 transcript levels decreased after ethylene treatment, following the increase in ethylene production, with more remarkable decrease for MaERF1, -11, and -15 (Fig. 3 and Supplementary Fig. S6). Based on these 15 MaERFs expression patterns during fruit ripening, two most ripening-related banana ERF genes, including ripening-induced MaERF9 and ripening-downregulated MaERF11, were selected and focused upon for the following analysis.
Interaction of MaERF9 and -11 with promoters of MaACS1 and MaACO1
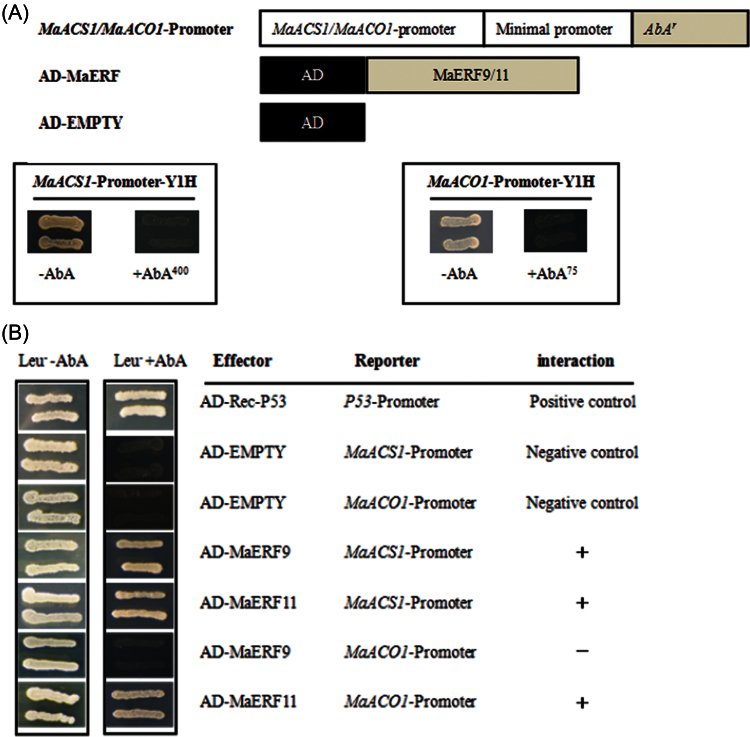
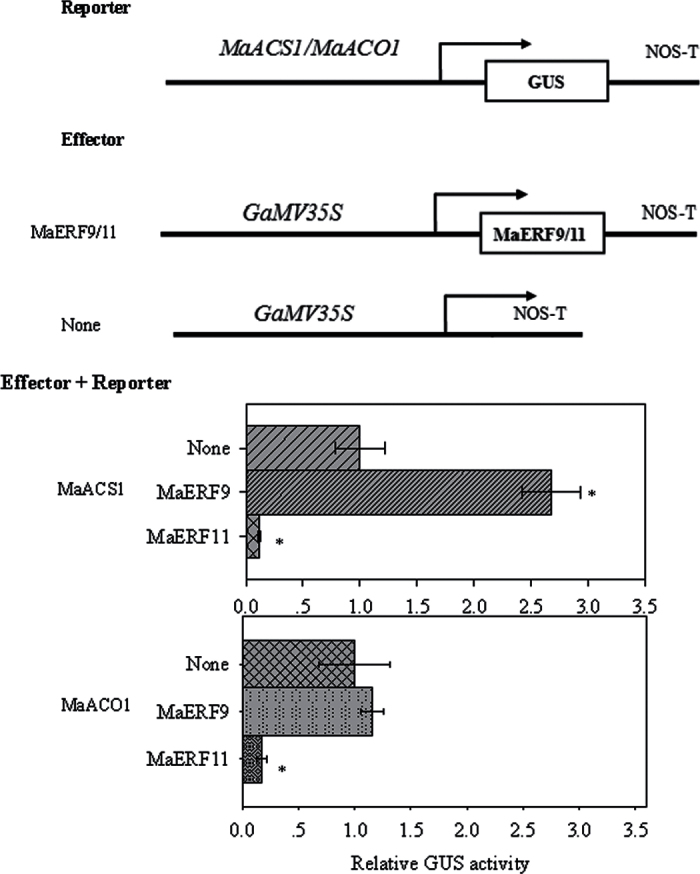
In previous studies, banana fruit MaACS1 and MaACO1 have been reported as key genes involved in ethylene biosynthesis. In peel and pulp tissues, MaACS1 and MaACO1 were ripening- and ethylene-induced genes during fruit ripening (Inaba et al., 2007). Similar to the previous report, this study also showed that MaACS1 and MaACO1 in peel and pulp tissues were ethylene-induced, and that their transcript levels increased with ethylene evolution during fruit ripening (data not shown). Recently, several TFs, such as tomato RIN, LeERF2, tobacco TERF1, and rice OsDERF1, have been reported to modulate the expression of ethylene biosynthesis genes (Ito et al., 2008; Zhang et al., 2009; Wan et al., 2011; Qin et al., 2012). Therefore, the current study decided to investigate whether ripening-induced MaERF9 and ripening-downregulated MaERF11 could regulate MaACS1 and MaACO1 in banana fruit ripening. A yeast one-hybrid assay based on the Matchmaker Gold Yeast One-Hybrid System was first performed for this purpose. The promoters of MaACS1 and MaACO1 were cloned in front of the reporter gene AUR1-C, an antibiotic resistance gene that confers aureobasidin A resistance in yeast. No basal activities of the MaACS1 and MaACO1 promoters were detected in yeast (Fig. 4A). After the Y1H reporter strains were transformed with plasmids carrying cassettes constitutively expressing MaERF9 and -11 effectors, yeast cells harbouring MaACS1 promoter grew well in the presence of aureobasidin A (Fig. 4B). While the yeast cells harbouring the MaACO1 promoter grew well when co-transformed with MaERF11 effector, but could not grew when co-transformed with MaERF9 (Fig. 4B). These observations indicate that MaERF11 can bind to MaACS1 and MaACO1 promoters, while MaERF9 only binds to MaACS1 promoter in yeast. Next, the tobacco transient cotransformational system was used to further confirm MaERF9 and -11 binding to the promoters of MaACS1 and MaACO1 in vivo. The full-length promoters of MaACS1 and MaACO1 were used to drive the GUS reporter gene, and the open reading frames of MaERF9 and -11 were overexpressed under the control of the 35S promoter. Each promoter–GUS construct was then co-transferred with either 35S:MaERF9 and 35S:MaERF11, respectively, through Agrobacterium transfection. The interaction of MaERF9 with the MaACS1 promoter resulted in a 3.27-fold enhancement of GUS activity, but no significant change was found with the MaACO1 promoter (Fig. 5). However, a marked decrease in GUS activity was observed to the interaction of MaERF11 with the MaACS1 or MaACO1 promoter (Fig. 5). These results not only indicate that MaERF11 can bind to MaACS1 and MaACO1 promoters, and MaERF9 binds to MaACS1 promoter, but also suggest that MaERF9 might directly activate the transcription of MaACS1 by interacting with the MaACS1 promoter. In addition, MaERF11 might act as transcription repressors of MaACS1 and MaACO1 by suppressing their promoter activities.
Fig. 4.
Yeast one-hybrid (Y1H) analysis of MaERF9 and -11 binding to MaACS1 and MaACO1 promoters. (A) No basal activities of MaACS1 and MaACO1 promoters were detected in yeast grown on SD medium lacking Leu in the presence of 400 and 75ng ml–1 aureobasidin A, respectively. (B) Yeast growth assays after the Y1H reporter strains were transformed with plasmids carrying cassettes constitutively expressing MaERF9 and -11 effectors. Interaction was determined based on the ability of transformed yeast to grow on SD medium lacking Leu in the presence of aureobasidin A.
Fig. 5.
Binding of MaACS1 and MaACO1 promoters to MaERF9 and -11 in vivo transient expression assay. The top panel shows the schematics of the GUS reporter vectors containing the MaACS1 and MaACO1 promoters and the MaERF9 and -11 effector vectors. The bottom panel shows the interactions of MaERF9 and -11 with MaACS1 and MaACO1 promoters. Agrobacterium tumefaciens containing the reporter and the effector vectors was injected into tobacco leaves to analyse the interactions of MaERF9 and -11 with MaACS1 and MaACO1 promoters through the activity of GUS. Asterisks indicate a significant difference at the 5% level compared to the leaves with no effector. Value are means ± SE of three replicates.
Physical interactions between MaERF9 and -11 and MaACS1 and MaACO1
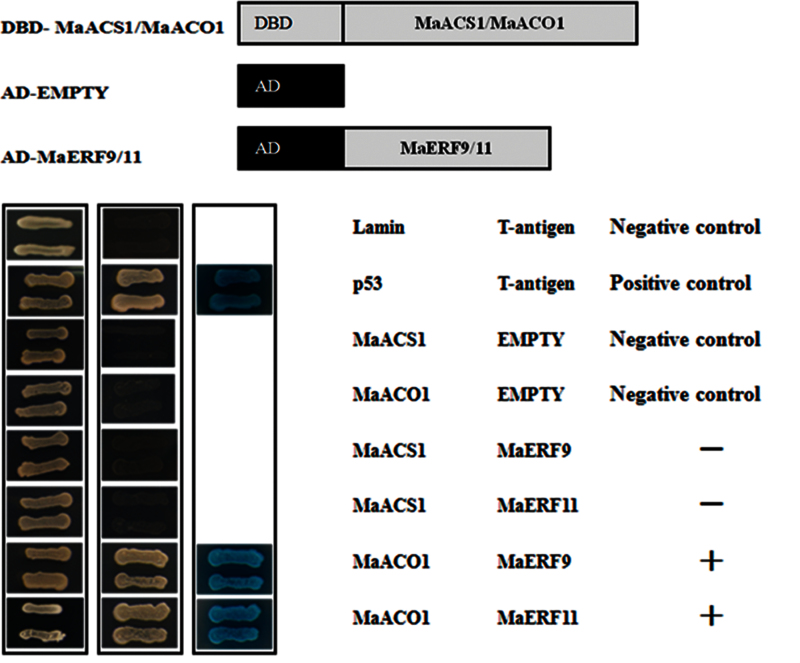
The interactions between MaERF9 and -11 and MaACS1/MaACO1 were investigated using the Matchmaker Gold Yeast Two-Hybrid System. MaACS1, MaACO1 and MaERF9 and -11 coding sequences were subcloned into pGADT7 and pGBKT7 vectors for the Y2H assay, respectively. As shown in Fig. 6, yeast cells co-transformed with a positive control (pGBKT7-53 + pGADT7-T) and MaERF9 or MaERF11 with MaACO1, could grow on selective medium (synthetic medium lacking tryptophan, leucine, histidine, and adenine) supplied with the toxic drug aureobasidin A, and turned blue in the presence of the chromagenic substrate X-α-Gal, indicating that MaERF9 or MaERF11 physically interacted with MaACO1. However, yeast cells harbouring MaERF9 or MaERF11 with MaACS1, and the negative controls, could not grow on the selective medium and did not turn blue under the same conditions (Fig. 6).
Fig. 6.
Physical interactions between MaERF9 and -11 and MaACS1 and MaACO1 detected in an Y2H assay. The coding regions of MaERF9 and -11 were cloned into the pGADT7 vector to create the AD-MaERF9 and -11 constructs, while the coding regions of MaACS1 and MaACO1 were cloned into the pGBKT7 vector to create the DBD-MaACS1/MaACO1 constructs. Gold Y2H yeast strains were co-transformed with DBD-MaACS1/MaACO1 and AD-MaERF9 and -11, respectively. The ability of yeast cells to grow on synthetic medium lacking tryptophan, leucine, histidine, and adenine but containing 125 μM aureobasidin A and to turn blue in the presence of the chromagenic substrate X-α-Gal was scored as a positive interaction. Yeast cells transformed with pGBKT7-53 + pGADT7-T, DBD–MaACS1 + pGADT7-T, DBD–MaACO1 + pGADT7-T, or pGBKT7-Lam + pGADT7-T were included as positive or negative controls, respectively (this figure is available in colour at JXB online).
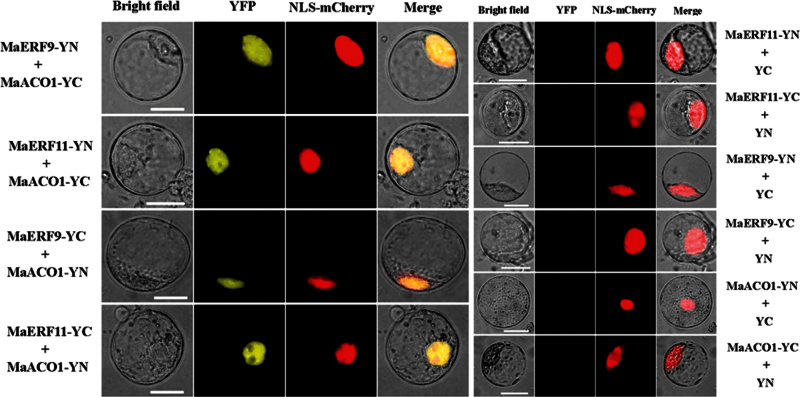
To further confirm the interactions between MaERF9 and -11 and MaACO1 observed in the Y2H assay, this study performed a BiFC assay in tobacco BY-2 protoplasts. MaERF9 or MaERF11 tagged with pSPYNE (split YFP N-terminal fragment expression) and MaACO1 tagged with pSPYCE (split YFP C-terminal fragment expression) were transiently co-expressed in tobacco BY-2 protoplasts following PEG transfection. A robust YFP fluorescent signal was detected in the nucleus of BY-2 cells which expressed MaERF9-pSPYNE or MaERF11-pSPYNE and MaACO1-pSPYCE (Fig. 7). No YFP fluorescent signal was observed either in the cells which expressed MaERF9 or -11-pSPYNE with only the pSPYCE or in those expressed MaACO1-pSPYCE with only the pSPYNE (Fig. 7). Similar results were also observed when MaERF9/MaERF11-pSPYCE were co-transfected with MaACO1-pSPYNE (Fig. 7). These results of Y2H and BiFC assays not only demonstrate in vitro and in vivo interactions between MaERF9 and -11 and MaACO1, but also the specific localization of the interacting proteins in the nucleus.
Fig. 7.
BiFC visualization of the MaERF9/MaERF11 and MaACO1 interactions in transiently co-expressed tobacco BY-2 protoplasts. MaERF9/MaERF11 and MaACO1 proteins were fused with the N (YN) and C (YC) termini of YFP, respectively. Expression of MaERF9/MaERF11 or MaACO1 alone was used as a negative control. VirD2NLS-mCherry was included in each transfection to serve as a control for successful transfection, as well as for nuclear localization. YFP fluorescence is yellow; the merged image is a digital merge of bright field and fluorescent images. Bars, 25 μm (this figure is available in colour at JXB online).
Discussion
ERFs are plant-specific TFs that bind to conserved motifs in promoter regions of target genes, thus regulating the expression of genes involved in the response to ethylene. ERFs comprise one of the largest TF families, with 122 members in Arabidopsis and 139 in rice (Nakano et al., 2006). Although sequence identity can be as low as 13% among these different ERFs, all ERFs exhibited a highly conserved AP2/ERF DNA-binding domain of 57–66 amino acids (Nakano et al., 2006; Yin et al., 2010). In this study, based on a transcriptome database, 15 banana fruit ERF genes, designated MaERF1 to MaERF15, were cloned. Similar to the previous reports (Nakano et al., 2006; Yin et al., 2010; Liu et al., 2011), alignment of the 15 MaERFs proteins showed that they shared a highly conserved ERF domain (Supplementary Fig. S1A, B), although they were highly diverse in size, ranging from 183 to 482 amino acids. The lowest similarity observed was 18% while the highest similarity observed was 76% (Supplementary Fig. S2 and Table S8). The 15 MaERFs fell into seven out of the 12 different groups of the previously characterized ERF proteins (Nakano et al., 2006; Yin et al., 2010) (Supplementary Fig. S3). In subfamily VIII, MaERF10 to MaERF15 also had an EAR repressor domain (Supplementary Fig. S1C), suggesting that these five members might act as transcription repressors (Ohta et al., 2001). Consistent with their role as TFs, all the 15 MaERFs have basic amino acid regions that potentially serve as NLS to target the proteins to the nucleus. Nuclear localization was further confirmed by transient expression of five MaERFs from different subfamilies in tobacco BY-2 protoplasts or leaves (Fig. 1 and Supplementary Fig. S4).
As one of the important TFs in the ethylene pathway, the involvement of the ERF gene family in the regulation of fruit ripening has been well studied. Subfamilies III and IV of ERFs have been reported to be associated with stress responses (Sakuma et al., 2006; Qin et al., 2008), while subfamilies VII, VIII, and IX are ethylene responsive (Tournier et al., 2003; Yang et al., 2005; Yin et al., 2010). Moreover, subfamily VII genes have been particularly associated with fruit ripening and the genes of tomato LeERF2 (Tournier et al., 2003), apple MdERF1 (Wang et al., 2007), plum PsERF2a/PsERF2b (El-Sharkawy et al., 2009), and kiwifruit AdERF4/AdERF6 (Yin et al., 2010) accumulate during this developmental process. However, no clear and consistent pattern in mRNA level changes were observed in the kiwifruit ERFs across the three subfamilies VII, VIII, and IX. The transcript levels of subfamily VII member AdERF5, subfamily VIII members AdERF7/AdERF8, and subfamily IX members AdERF11–13 decreased during ripening (Yin et al., 2010). In the present work, similar to the report of Yin et al. (2010), gene expression profiles in banana fruit with four different ripening treatments (natural ripening, ethylene-induced ripening, 1-MCP-delayed ripening, and 1-MCP and ethylene-treated ripening) revealed that banana MaERF genes were expressed in intricate patterns in peel and pulp during fruit ripening (Figs. 2 and 3 and Supplementary Figs. S5 and S6). Among the 15 banana ERF genes, subfamily VII member MaERF7 and subfamily II member MaERF9, were apparently upregulated by ethylene in peel and pulp, showing strong correlation to the increase in ethylene production associated with the ripening climacteric. The transcript levels in peel and pulp of subfamily IX member MaERF1, subfamily VII member MaERF8, and subfamily VIII members MaERF11 and -15 decreased after ethylene treatment, following the increase in ethylene production, with a more obvious decrease for MaERF11 than the other MaERFs (Figs. 2 and 3 and Supplementary Figs. S5 and S6). In addition, other banana ERF genes, changed slightly or showed different expression patterns in banana fruit peel and pulp with four different ripening treatments (Figs. 2 and 3 and Supplementary Figs. S5 and S6). It has been shown that most reported fruit ERFs are induced in natural ripening or by ethylene treatment (Thara et al., 1999; Tournier et al., 2003; Huang et al., 2004; Wang et al., 2007; El-Sharkawy et al., 2009). Recently, mRNA levels of some ERF genes, including seven kiwifruit ERFs, two petunia ERFs, and two longan ERFs, decreased during ripening or senescence (Yin et al., 2010; Liu et al., 2011; Kuang et al., 2012). However, the mechanism of these ripening-downregulated ERFs involved in ripening was not studied. Therefore, this study chose to investigate what was considered to be the two most ripening-related banana ERF genes (MaERF9 and -11) and their possible role in banana fruit ripening, including the mechanism of their action.
Recently, a few key transcriptional regulators involved in the transcriptional regulation of ACS and ACO genes were identified in tomato, such as RIN-MADS is a MADS-box transcription factor that binds to the promoter of ACS2, ACS4, and other ripening-associated genes (Qin et al., 2012). In tomato, HB-1, a homeodomain-zip homeobox TF, directly interacts with the promoter of ACO1, which is responsible for the activation of ACO1 expression during ripening (Lin et al., 2008). Ethylene response factor 2 (ERF2), on the other hand has been shown to interact with the GCC box of the ACO3 promoter (Zhang et al., 2009). A negative regulator of ethylene biosynthesis ERF gene AP2a has also been identified, as repression of AP2a expression resulting in an overproduction of ethylene (Chung et al., 2010; Karlova et al., 2011). Downregulation of ethylene biosynthesis by ethylene-induced ERFs would mean that ethylene turns down its own production during fruit ripening, similar to observations made on system 1 and system 2 ethylene biosynthesis (Van de Poel et al., 2012). Recently, banana fruit MADS5 TF is also identified to bind to CArG-box sequence in the promoters of major ripening genes, including MaACS1 and MaACO1 (Choudhury et al., 2012a). However, it remains unclear whether banana fruit ERF TF directly regulate the expression of MaACS1 and MaACO1 during fruit ripening. In the present investigation, in vitro and in vivo assays showed that MaERF9 and -11 could bind to the MaACS1 promoter and MaERF11 could bind to the MaACO1 promoter (Figs. 4 and 5), providing insight into the role of the ERF TF in the transcriptional regulation of ethylene synthesis through regulating MaACS1 and MaACO1. Importantly, it was observed that MaERF9 activated the promoter of MaACS1, while MaERF11 which includes an EAR repressor domain, repressed the promoters of MaACS1 and MaACO1 (Fig. 5). This suggests that MaERF9 and -11 may be, respectively, positive and negative regulators in the transcriptional regulation of ethylene production during fruit ripening. To date, no report has shown ERF protein interaction with ACS or ACO. The finding in this study that banana MaERF9 and -11 interacted with MaACO1 in vitro and in vivo (Figs. 6 and 7) established such interaction. It will be interesting to investigate whether the interaction of MaERF9 and -11 with MaACO1 is similar to that of DELLA with SCARECROW-LIKE 3 (SCL3) in Arabidopsis. In Arabidopsis seedlings, SCL3 is a direct target gene of DELLA, whose expression is induced by DELLA and repressed by gibberellin (GA), moreover, SCL3 autoregulates its own transcription by directly interacting with DELLA, indicating that SCL3 seems to act as an attenuator of DELLA proteins (Zhang et al., 2011). However, further studies are required to assess the biological significance of the interaction between MaERF and MaACO1.
In summary, 15 banana fruit MaERF genes were isolated and characterized. Gene expression profiles in banana fruit exposed to four different ripening treatments clearly revealed that MaERF genes are expressed differentially in peel and pulp tissues during fruit ripening. Yeast one-hybrid and transient expression assays showed that MaERFs bind MaACS1 and MaACO1 promoters and can activate or repress their activities during fruit ripening. MaERFs were shown to physically interact with MaACO1. Taken together, these results suggest that MaERFs may be involved in banana fruit ripening via transcriptional regulation of or interaction with ethylene biosynthesis genes. The study provides some new information in helping to understand the regulatory network of ERF TF in fruit ripening.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Table S1. Primers used for RACE.
Supplementary Table S2. Primers used for quantitative real-time PCR analysis.
Supplementary Table S3. Primers used for fusing GFP.
Supplementary Table S4. Primers used for Y1H assay.
Supplementary Table S5. Primers used for transient expression assay.
Supplementary Table S6. Primers used for Y2H analysis.
Supplementary Table S7. Primers used for BiFC assays.
Supplementary Table S8. Sequence similarities among the different MaERFs genes.
Supplementary Fig. S1. Schematic analysis of MaERFs with ERF domains and EAR repressor domains.
Supplementary Fig. S2. Amino acid sequence alignment of the MaERF proteins.
Supplementary Fig. S3. Phylogenetic tree of ERFs.
Supplementary Fig. S4. Subcellular localization of MaERFs in tobacco leaves.
Supplementary Fig. S5. Expression of MaERFs genes in banana fruit peel with four different ripening treatments.
Supplementary Fig. S6. Expression of MaERFs genes in banana fruit pulp with four different ripening treatments.
Acknowledgements
The authors thank Professor Jörg Kudla (Institut für Biologie und Biotechnologie der Pflanzen, Universität Münster), Professor Seiichiro Hasezawa (Department of Integrated Biosciences, the University of Tokyo), and Professor Xiao-ya Chen (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) for the generous gift of BiFC vectors, tobacco BY-2 suspension cells, and the transient expression vectors (pBI101 and pCAMBIA 1300), respectively. This work was supported in part by the Key Laboratory of Plant Resources Conservation and Sustainable Utilization (South China Botanical Garden, Chinese Academy of Sciences), the National Natural Science Foundation of China (grant no. 31272213), the National Public Benefit of Agricultural Research Foundation of China (grant no. 200903044-5), the China Agriculture Research System (grant no. CARS-32-02A), and the Guangdong Modern Agricultural Industry Technology System (grant no. LNSG2011-12).
References
- Abel S, Theologis A. 1994. Transient transformation of Arabidopsis leaf protoplasts: a versatile experimental system to study gene expression. The Plant Journal 5, 421–427. [DOI] [PubMed] [Google Scholar]
- Alexander L, Grierson D. 2002. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. Journal of Experimental Botany 53, 2039–2055. [DOI] [PubMed] [Google Scholar]
- Chae HS, Faure F, Kieber JJ. 2003. The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. The Plant Cell 15, 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhong HY, Kuang JF, Li JG, Lu WJ, Chen JY. 2011. Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta 234, 377–390. [DOI] [PubMed] [Google Scholar]
- Choudhury SR, Roy S, Saha PP, Singh SK, Sengupta DN. 2008. Characterization of differential ripening pattern in association with ethylene biosynthesis in the fruits of five naturally occurring banana cultivars and detection of a GCC-box-specific DNA-binding protein. Plant Cell Reports 27, 1235–1249. [DOI] [PubMed] [Google Scholar]
- Choudhury SR, Roy S, Nag A, Singh SK, Sengupta DN. 2012a. Characterization of an AGAMOUS-like MADS Box protein, a probable constituent of flowering and fruit ripening regulatory system in banana. PLoS ONE 7, e44361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury SR, Roy S, Sengupta DN. 2012b. A Ser/Thr protein kinase phosphorylates MA-ACS1 (Musa acuminata 1-aminocyclopropane-1-carboxylic acid synthase 1) during banana fruit ripening. Planta 236, 491–511. [DOI] [PubMed] [Google Scholar]
- Chung MY, Vrebalov J, Alba R, Lee J, McQuinn R, Chung JD, Klein P, Giovannoni JJ. 2010. A tomato (Solanum lycopersicum) APETALA2/ERF gene, SlAP2a, is a negative regulator of fruit ripening. The Plant Journal 64, 936–947. [DOI] [PubMed] [Google Scholar]
- Clendennen SK, May GD. 1997. Differential gene expression in ripening banana fruit. Plant Physiology 115, 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clendennen SK, Kipp PB, May GD. 1997. The role of ethylene in banana fruit ripening. In: Kanellis AK, Chang C, Kende H, Grierson G, eds, Biology and biochemistry of the plant hormone ethylene. Dordrecht: Kluwer Academic Publishers; pp 141–148. [Google Scholar]
- Do YY, Thay TS, Chang TW, Huang PL. 2005. Molecular cloning and characterization of a novel 1-aminocyclopropane-1-carboxylate oxidase gene involved in ripening of banana fruits. Journal of Agricultural and Food Chemistry 53, 8239–8247. [DOI] [PubMed] [Google Scholar]
- El-Sharkawy I, Sherif S, Mila I, Bouzayen M, Jayasankar S. 2009. Molecular characterization of seven genes encoding ethylene responsive transcriptional factors during plum fruit development and ripening. Journal of Experimental Botany 60, 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M. 2000. Arabidopsis ethylene-response element binding factors act as transcriptional activator or repressors of GCC box-mediated gene expression. The Plant Cell 12, 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ. 2004. Genetic regulation of fruit development and ripening. The Plant Cell 16, S170–S180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ecker JR. 2004. The ethylene signaling pathway: new insights. Current Opinion in Plant Biology 7, 40–49. [DOI] [PubMed] [Google Scholar]
- Hahn A, Harter K. 2009. MAP kinase cascades and ethylene – signalling, biosynthesis or both? Plant Physiology 149, 1207–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Zhang ZJ, Zhang XL, Zhang HB, Huang DF, Huang RF. 2004. Tomato TERF1 modulates ethylene response and enhances osmotic stress tolerance by activating expression of downstream genes. FEBS Letters 573, 110–116. [DOI] [PubMed] [Google Scholar]
- Inaba A, Liu XJ, Yokotani N, Yamane M, Lu WJ, Nakano R, Kubo Y. 2007. Differential feedback regulation of ethylene biosynthesis in pulp and peel tissues of banana fruit. Journal of Experimental Botany 58, 1047–1057. [DOI] [PubMed] [Google Scholar]
- Ito Y, Kitagawa M, Ihashi N, Yabe K, Kimbara J, Yasuda J, Ito H, Inakuma T, Hiroi S, Kasumi T. 2008. DNA-binding specificity, transcriptional activation potential, and the rin mutation effect for the tomato fruit-ripening regulator RIN. The Plant Journal 55, 212–223. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S, Liu Y, Lueth A, Zhang S. 2008. MAPK phosphorylation-induced stabilization of ACS6 protein is mediated by the non-catalytic C-terminal domain, which also contains the cis-determinant for rapid degradation by the 26S proteasome pathway. The Plant Journal 54, 129–140. [DOI] [PubMed] [Google Scholar]
- Karlova R, Rosin FM, Busscher-Lange J, Parapunova V, Do PT, Fernie AR, Fraser PD, Baxter C, Angenent GC, de Maagd RA. 2011. Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. The Plant Cell 23, 923–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ. 2011. Genetics and control of tomato fruit ripening and quality attributes. Annual Review of Genetics 45, 41–59. [DOI] [PubMed] [Google Scholar]
- Kuang JF, Chen JY, Luo M, Wu KQ, Sun W, Jiang YM, Lu WJ. 2012. Histone deacetylase HD2 interacts with ERF1 and is involved in longan fruit senescence. Journal of Experimental Botany 63, 441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Zhu BZ, Xu WT, Zhu HL, Chen AJ, Xie YH, Shao Y, Luo YB. 2007. LeERF1 positively modulated ethylene triple response on etiolated seedling, plant development and fruit ripening and softening in tomato. Plant Cell Reports 26, 1999–2008. [DOI] [PubMed] [Google Scholar]
- Lin Z, Hong Y, Yin M, Li C, Zhang K, Grierson D. 2008. A tomato HD-Zip homeobox protein, LeHB-1, plays an important role in floral organogenesis and ripening. The Plant Journal 55, 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ZF, Zhong SL, Grierson D. 2009. Recent advances in ethylene research. Journal of Experimental Botany 60, 3311–3336. [DOI] [PubMed] [Google Scholar]
- Liu JX, Li JY, Wang HN, Fu ZD, Liu J, Yu YX. 2011. Identification and expression analysis of ERF transcription factor genes in petunia during flower senescence and in response to hormone treatments. Journal of Experimental Botany 62, 825–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XJ, Shiomi S, Nakatsuka A, Kubo Y, Nakamura R, Inaba A. 1999. Characterization of ethylene biosynthesis associated with ripening in banana fruit. Plant Physiology 121, 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang S. 2004. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis . The Plant Cell 16, 3386–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel C, Vrebalov J, Tafelmeyer P, Giovannoni JJ. 2011. The tomato MADS-box transcription factor RIPENING INHIBITOR interacts with promoters involved in numerous ripening processes in a COLORLESS NONRIPENING-dependent manner. Plant Physiology 157, 1568–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbéguié-A-Mbéguié D, Hubert O, Fils-Lycaon B, Chillet M, Baurens FC. 2008. EIN3-like gene expression during fruit ripening of Cavendish banana (Musa acuminata cv. Grande Naine). Physiologia Plantarum 133, 435–448. [DOI] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H. 2006. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiology 140, 411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Matsui K, Hiratsu K, Shinshi H, Ohme-Takagi M. 2001. Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. The Plant Cell 13, 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad ME, Schofield A, Lyzenga W, Liu H, Stone SL. 2010. Arabidopsis RING E3 ligase XBAT32 regulates lateral root production through its role in ethylene biosynthesis. Plant Physiology 153, 1587–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Sakuma Y, Tran LSP, Maruyma K, Kidokoro S, Fujita Y, Fujita M, Umezawa T, Sawano Y, Miyazono KI. 2008. Arabidopsis DREB2A-interacting proteins function as RING E3 ligases and negatively regulate plant drought stress-responsive gene expression. The Plant Cell 20, 1693–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin GZ, Wang YY, Cao BH, Wang WH, Tian SP. 2012. Unraveling the regulatory network of the MADS box transcription factor RIN in fruit ripening. The Plant Journal 70, 243–255. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Maruyama K, Qin F, Osakabe Y, Shinozaki K, Yamaguchi-Shinozaki K. 2006. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proceedings of the National Academy of Sciences, USA 103, 18822–18827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan W, Kuang JF, Chen L, et al. 2012. Molecular characterization of banana NAC transcription factors and their interactions with ethylene signalling component EIL during fruit ripening. Journal of Experimental Botany 63, 5171–5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thara VK, Tang XY, Gu YQ, Martin GB, Zhou JM. 1999. Pseudomonas syringae pv tomato induces the expression of tomato EREBP-like genes Pti4 and Pti5 independent of ethylene, salicylate and jasmonate. The Plant Journal 20, 475–483. [DOI] [PubMed] [Google Scholar]
- Tournier B, Sanchez-Ballesta MT, Jones B, Pesquet E, Regad F, Latché A, Pech JC, Bouzayen M. 2003. New members of the tomato ERF family show specific expression pattern and diverse DNA-binding capacity to the GCC box element. FEBS Letters 550, 149–154. [DOI] [PubMed] [Google Scholar]
- Van de Poel B, Bulens I, Markoula A, et al. 2012. Targeted systems biology profiling of tomato fruit reveals coordination of the yang cycle and a distinct regulation of ethylene biosynthesis during postclimacteric ripening. Plant Physiology 160, 1498–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Wilkins TA. 1994. A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Analytical Biochemistry 223, 7–12. [DOI] [PubMed] [Google Scholar]
- Wan L, Zhang J, Zhang H, Zhang Z, Quan R, Zhou SR, Huang RF. 2011. Transcriptional activation of OsDERF1 in OsERF3 and OsAP2-39 negatively modulates ethylene synthesis and drought tolerance in rice. PLoS ONE 6, e25216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Tan DM, Takahashi A, Li TZ, Harada T. 2007. MdERFs, two ethylene-response factors involved in apple fruit ripening. Journal of Experimental Botany 58, 3743–3748. [DOI] [PubMed] [Google Scholar]
- Wang KL, Yoshida H, Lurin C, Ecker JR. 2004. Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature 428, 945–950. [DOI] [PubMed] [Google Scholar]
- Wang XL, Peng XX. 2001a. Cloning of promoter of fruit-specific ACC synthase gene and primary study on its function. Chinese Journal of Biotechnology 17, 293–296. [PubMed] [Google Scholar]
- Wang XL, Peng XX. 2001b. Cloning of banana fruit ripening-related ACO1 and primary study on its function. Chinese Journal of Biotechnology 17, 428–431. [PubMed] [Google Scholar]
- Xu ZS, Chen M, Li LC, Ma YZ. 2011. Functions and application of the AP2/ERF transcription factor family in crop improvement. Journal of Integrative Plant Biology 53, 570–585. [DOI] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. 1984. Ethylene biosynthesis and its regulation in higher plants. Annual Review of Plant Physiology 35, 155–189. [Google Scholar]
- Yang Z, Tian LN, Latoszek-Green M, Brown D, Wu KQ. 2005. Arabidopsis ERF4 is a transcriptional repressor capable of modulating ethylene and abscisic acid responses. Plant Molecular Biology 58, 585–596. [DOI] [PubMed] [Google Scholar]
- Yin XR, Allan AC, Chen KS, Ferguson IB. 2010. Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiology 153, 1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZX, Li JX, Yang CQ, Hu WL, Wang LJ, Chen XY. 2012. The jasmonate-responsive AP2/ERF transcription factors AaERF1 and AaERF2 positively regulate artemisinin biosynthesis in Artemisia annua L. Molecular Plant 5, 353–365. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Zhang HW, Quan RD, Wang XC, Huang RF. 2009. Transcriptional regulation of ethylene response factor LeERF2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiology 150, 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZL, Ogawa M, Fleet CM, Zentella R, Hu J, Heo JO, Lim J, Kamiya Y, Yamaguchi S, Sun TP. 2011. Scarecrow-like 3 promotes gibberellin signaling by antagonizing master growth repressor DELLA in Arabidopsis . Proceedings of the National Academy of Sciences, USA 108, 2160–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.