Abstract
Objective
In people with spinal cord injury (SCI), active-resisted stance using electrical stimulation of the quadriceps delivered a therapeutic stress to the femur (∼150% of body weight) and attenuated bone mineral density (BMD) decline. In standard densitometry protocols, BMD is averaged over the entire bone cross-section. An asymmetric adaptation to mechanical load may be masked by non-responding regions. The purpose of this study was to test a novel method to assess regional BMD of the femur in individuals with SCI. We hypothesize that there will be regional bone-sparing changes as a result of active-resisted stance.
Design
Mixed cross-sectional and longitudinal.
Setting
Research laboratory.
Participants
Twelve individuals with SCI and twelve non-SCI controls.
Intervention
Individuals with SCI experienced active-resisted stance or passive stance for up to 3 years.
Outcome measures
Peripheral quantitative computed tomography images from were partitioned so that femur anatomic quadrants could be separately analyzed.
Results
Over 1.5 years, the slope of BMD decline over time was slower at all quadrants for the active-resisted stance limbs. At >2 years of training, BMD was significantly higher for the active-resisted stance group than for the passive stance group (P = 0.007). BMD was preferentially spared in the posterior quadrants of the femur with active-resisted stance.
Conclusions
A regional measurement technique revealed asymmetric femur BMD changes between passive stance and active-resisted stance. Future studies are now underway to better understand other regional changes in BMD after SCI.
Keywords: Spinal cord injuries, Bone mineral density, Fractures, Muscle, Osteoblasts, Osteoporosis, Paralysis, Rehabilitation
Introduction
Prescriptive doses of activity after spinal cord injury (SCI) seek to enhance patients' health and well-being by reintroducing physiological levels of stress to paralyzed tissues. The musculoskeletal system can respond favorably to mechanical loads of proper magnitude, orientation, and periodicity.1,2 Although small-magnitude oscillatory inputs (vibration) trigger robust adaptations,2–4 bone generally exhibits a positive dose–response relationship with peak strain magnitude.5–9 Osteogenic levels of compressive load can be achieved via electrical stimulation of paralyzed muscle.10,11 However, interventions that do not deliver mechanical loads at an appropriate dose level (magnitude, duration, etc.) may fail to trigger desired adaptations of bone tissues. Determining the optimal dose of musculoskeletal stress to prevent bone loss is an important step to enhancing the health of individuals with SCI.
We recently discovered a dose–response relationship for femur bone mineral density (BMD) under three long-term mechanical loading conditions.12 Limbs that are resisted during quadriceps electrical stimulation in stance (active-resistive stance) typically experienced femur compressive loads of ∼150% of body weight (%BW), as estimated by a biomechanical model.13 Limbs that performed passive stance without electrical stimulation typically experienced modeled loads of ∼40% BW per limb. Finally, limbs that performed neither standing nor electrical stimulation received habitual loads estimated to be 0% BW. Over greater than 3 years of training, distal femur BMD for the active-resisted stance limbs significantly exceeded BMD of the passive stance and the non-standing limbs.12 Although there was a trend suggesting a difference between passive standing and non-standing limbs, no significant difference emerged, indicating that BMD loss was not attenuated for subjects performing passive stance.
Although passive stance may have positive effects upon several other secondary complications of SCI (pressure ulcers, bowel motility, kidney and bladder function, psychological state, etc.)14,15 no studies, to our knowledge, definitively support that it mitigates BMD loss.16–20 However, the regional sites used to analyze bone density may have influenced these previous findings. We previously determined that certain areas of bone respond to mechanical loading after SCI in an asymmetric fashion.21 For example, portions of the tibia cross-section responded well to electrical stimulation loading, while other portions did not differ from untrained limbs. In standard densitometry analysis protocols, BMD is obtained as an average from the entire bone cross-section. High-responding regions may be masked if non-responding regions are averaged into the analysis.
If active resisted or passive stance yields localized cross-sectional changes, then subtle changes in BMD within certain regions of the femur could be missed during standard peripheral quantitative computed tomography (pQCT) imaging. Accordingly, the purpose of this study is to partition pQCT scans of the distal femur in order to test if mechanical loads in stance attenuate bone loss asymmetrically across selected anatomical regions. We hypothesize that bone-sparing benefits of stance will emerge via this regional evaluation of femur anatomic quadrants. We also hypothesize that active-resisted stance will be superior to passive stance for all regions analyzed in those with SCI.
Methods
Subjects
The protocol was approved by our institution's Human Subjects Office Institutional Review Board. All subjects provided written informed consent before participating. Twelve individuals with motor complete (AIS-A and B)22 SCI participated in this study. Demographic data are shown in Table 1. An additional 12 individuals without SCI served as a normative control condition. Exclusion criteria were a history of bone pathology (i.e. bone metabolic disease, cancer, etc.), thyroid disorder, previous fracture at the scan sites, pregnancy, and medications known to affect bone metabolism. Individuals without an SCI underwent a single bilateral assessment with pQCT. Bilateral values were averaged across limbs for each subject. Subjects with SCI underwent between one and six bilateral pQCT scans.
Table 1.
Subject demographics
| Subject | Gender | SCI level | AIS | Age | SCI years | Time bins* | Loading dose |
|---|---|---|---|---|---|---|---|
| 1 | M | T7 | A | 27 | 0.30 | 2–7 | Active |
| 2 | M | T4 | A | 16 | 0.38 | 1–5 | Active |
| 3 | M | T8 | A | 20 | 0.24 | 1,3,5 | Active |
| 4 | M | T10 | A | 37 | 0.22 | 1,3 | Active |
| 5 | M | T10 | A | 26 | 0.99 | 4–6 | Active |
| 6 | F | C5–6 | A | 26 | 1.50 | 6 | Active |
| 7 | M | T6 | A | 28 | 2.05 | 7 | Active |
| 8 | M | T12 | A | 39 | 0.21 | 1,2,4,5 | Passive |
| 9 | M | T8 | B | 43 | 0.33 | 2–4,6 | Passive |
| 10 | M | T8 | A | 38 | 0.53 | 3,4,7 | Passive |
| 11 | M | T11 | A | 34 | 0.68 | 3–5 | Passive |
| 12 | M | T4 | A | 44 | 0.61 | 3,4 | Passive |
| 13 | M | 30 | |||||
| 14 | M | 24 | |||||
| 15 | M | 24 | |||||
| 16 | F | 22 | |||||
| 17 | M | 24 | |||||
| 18 | M | 42 | |||||
| 19 | M | 27 | |||||
| 20 | M | 23 | |||||
| 21 | M | 24 | |||||
| 22 | M | 30 | |||||
| 23 | F | 48 | |||||
| 24 | F | 31 |
*Time bins: bin 1 = 0–0.25 years; bin 2 = 0.25–0.50 years; bin 3 = 0.50–0.75 years; bin 4 = 0.75–1 year; bin 5 = 1–1.5 years; bin 6 = 1.5–2 years; and bin 7 = >2 years.
Numerals in bold denote scan sessions that were re-analyzed to determine reliability of the quadrant partitioning method.
Active resistive standing protocol
This study used pQCT images acquired for a previous investigation.12 Briefly, SCI subjects 1–7 (Table 1) performed unilateral quadriceps stimulation in supported stance (active-resisted stance loading) with the knee in 20° of flexion. The standing system is illustrated in Fig. 1A. Subjects performed unilateral quadriceps stimulation (20z, 60 contractions, supramaximal intensity; Fig. 1B) on an average of 3 days per week for up to 3 years. This protocol was designed to offer a substantial physiological challenge to the quadriceps (fatigue; Fig. 1B) in order to trigger adaptive hypertrophy (Fig. 1C). The quadriceps training protocol required approximately 30 minutes per bout. We developed a biomechanical model that estimated the compression and shear loads experienced by the femur during active-resisted stance training.13 Compressive loads during training were approximately 150%BW, a dose of load that we previously reported could attenuate BMD loss in the tibia compared to untrained limbs after SCI.10,11,21
Figure 1.
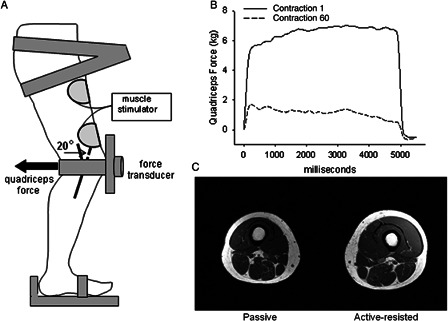
(A) Schematic representation of the standing system. Quadriceps force during muscle stimulation (active-resisted stance) is transmitted to the distal femur as compression and shear. (B) Representative example of quadriceps force during active-resisted stance training. Substantial fatigue developed over the course of 60 contractions. (C) Magnetic resonance image showing training-induced hypertrophy in a subject who performed unilateral active-resisted stance training for 6 months (subject 1, Table 1).
Passive standing protocol
A second group of five subjects with SCI stood in a standing frame or a standing wheelchair without applying quadriceps electrical stimulation (Table 1, passive stance loading cohort). Subjects were requested to stand for 30 minutes, to match the duration of standing performed by subjects in the active-resisted stance cohort. We previously determined that modeled femur compressive loads during passive stance at all knee angles approximate 40%BW.13 Subjects stood on an average of 3 days per week.
In the active-resisted stance group, the limb that did not receive electrical stimulation did perform passive stance during training sessions. Data from the unstimulated limbs of active-resisted stance subjects were therefore added to the passive standing group.
pQCT scan procedure
pQCT measurements were performed with a Stratec XCT 3000 densitometer (Stratec Medical, Pforzheim, Germany). This device is calibrated with respect to fat (fat density = 0 g/cm3). Voxel size was 0.4 mm3, scanner speed was 25 m/s, and slice thickness was 2.2 m.
Using a tape measure, femur and tibia length were measured using bony landmarks.23,24 An investigator passed the limb through the pQCT gantry and secured the subject's foot onto a footplate. A radiology technician performed a scout view of the tibio-femoral joint and placed a reference line at the distal limit of the lateral femoral condyle. Using this reference line, the scanner obtained an image at 12% of femur length (measured from the distal end). The subject was then repositioned for a scan of the contra-lateral limb.
pQCT analysis procedures
An investigator delineated four quadrants of the femur cross-section for individual analysis. First, using the pQCT scanner's image analysis software, a 20-sided polygon was fit to the periosteal border of the femur. A line was fit to opposing nodes of the polygon to bisect the region into two halves. The polygon was rotated until this bisecting line was parallel, per visual inspection, to the medial–lateral axis of the femur. A second line was anchored to the corners of the polygon to partition the polygon into quadrants of equal area. The investigator then defined a region of interest that followed the polygon dividing lines and the femur periosteal border within each quadrant (Fig. 2). Each femur quadrant was analyzed with a threshold of −100 g/cm3 to define the periosteal border21 and a threshold of 400 g/cm3 with a 3 × 3 voxel filter to exclude cortical and subcortical voxels. Because the cortical shell is very thin at this site (and is therefore subject to the partial-volume effect),25 we report only trabecular BMD for each quadrant.
Figure 2.
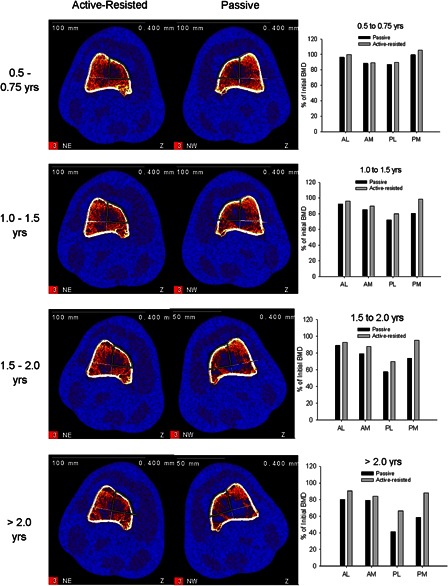
Longitudinal pQCT images from a subject who performed unilateral active-resisted stance training for > 2 years (subject 1, Table 1). This individual's contra-lateral limb performed passive stance and experienced extensive deterioration of the trabecular lattice. The plots at right depict the relative BMD decline for each limb compared to this subject's baseline BMD values.
Quadrant reliability analysis
The position of the quadrant bisecting lines determined which voxels entered the BMD analysis for each quadrant, and therefore may have influenced the measured BMD. To determine the effect of quadrant placement on BMD, we examined the repeatability of the quadrant placement method. We selected 15% of the scans for each sub-cohort (active, passive, and non-SCI) and repeated the quadrant placement procedure in a blinded fashion. The investigator then repeated the BMD analysis without receiving knowledge of results. For each individual quadrant, we obtained the coefficient of variation (CV) between the first and second BMD analyses. The reliability of the quadrant placement method was estimated as the mean of all CV values for the 52 re-analyzed quadrants. The concordance between the first and second analyses was also estimated using an intra-class correlation (ICC(3,1)).26
Statistical analysis
Quadrants were defined according to anatomic reference points: postero-medial (PM), postero-lateral (PL), antero-medial (AM), and antero-lateral (AL). BMD for each quadrant was normalized to the mean non-SCI BMD for that quadrant. To facilitate longitudinal comparisons among cohorts, we partitioned the dataset into seven time bins based on time post-SCI: 0–0.25, 0.25–0.50, 0.50–0.75, 0.75–1, 1–1.5, 1.5–2, and >2 years. Mean (SD) BMD was computed for all subjects present in each time bin (see Table 1 for subject representation across time bins). For passive stance subjects with bilateral data, one limb was randomly selected for analysis. This same limb was analyzed at all time points for which the subject contributed BMD data.
We used a two-way (group × quadrant) analysis of variance (ANOVA) to compare BMD differences among the active-resisted stance and passive stance limbs at each bone quadrant. Pairwise multiple comparisons (Tukey) were used when indicated. Significance was set to alpha <0.05.
Results
For the 52 re-analyzed quadrants (among 13 subjects), mean CV of BMD values obtained via the quadrant placement procedure was 0.89%. The ICC(3,1) value was 0.997, indicating near-perfect concordance for re-analyzed quadrants. Both of these tests indicate that the quadrant placement procedure was reliable and contributed very little variation to the measured BMD values.
Mean (s.d.) BMD values for all cohorts, time bins, and quadrants are shown in Table 2. Fig. 2 depicts longitudinal data for subject 1, who performed 3 years of unilateral active-resisted stance training. His contra-lateral limb received no electrical stimulation (passive stance). The electrical stimulation protocol provided a sufficient physiological challenge to trigger muscle hypertrophy:27 This subject's active-resisted stance limb quadriceps muscle cross-sectional area was 24% higher than the passive stance limb (Fig. 1). The progressive destruction of the trabecular lattice in the passive stance limb is visible in the pQCT images in Fig. 2. BMD differences between limbs were largest for the posterior quadrants. For example, at >2.0 years post-SCI, active-resisted stance BMD was 25.4% and 29.5% higher than passive stance BMD at PL and PM, respectively, within the same individual (Fig. 2). AL and AM BMD differed between limbs by only 10.7% and 5.2%.
Table 2.
BMD data
| Cohort | Bin/(n)* | AL† | AM† | PL† | PM† |
|---|---|---|---|---|---|
| Non-SCI | N/A | 225.37 | 189.33 | 232.94 | 224.12 |
| (23.90) | (25.12) | (25.98) | (30.28) | ||
| Active | 1 (5) | 233.24 | 188.26 | 219.66 | 224.02 |
| (11.49) | (9.08) | (15.72) | (10.07) | ||
| 2 (23) | 229.45 | 195.50 | 226.80 | 238.35 | |
| (15.06) | (1.41) | (7.07) | (18.17) | ||
| 3 (3) | 225.05 | 184.78 | 218.50 | 229.38 | |
| (8.16) | (14.91) | (5.89) | (10.76) | ||
| 4 (3) | 231.77 | 192.57 | 209.20 | 226.07 | |
| (6.10) | (8.53) | (3.10) | (7.18) | ||
| 5 (3) | 206.10 | 170.97 | 192.60 | 209.83 | |
| (30.64) | (18.50) | (29.18) | (24.83) | ||
| Passive | 1 (5) | 233.24 | 188.26 | 219.66 | 224.02 |
| (11.49) | (9.08) | (15.72) | (10.07) | ||
| 2 (3) | 181.73 | 140.67 | 175.03 | 189.80 | |
| (19.76) | (14.65) | (9.91) | (9.75) | ||
| 3 (6) | 189.42 | 149.87 | 193.85 | 203.12 | |
| (29.45) | (36.14) | (28.37) | (33.39) | ||
| 4 (6) | 177.70 | 138.98 | 174.22 | 189.47 | |
| (36.96) | (39.86) | (41.84) | (40.40) | ||
| 5 (5) | 189.90 | 156.08 | 170.00 | 193.52 | |
| (9.14) | (7.20) | (23.90) | (16.51) |
*Time bins: bin 1 = 0–0.25 years; bin 2 = 0.25–0.50 years; bin 3 = 0.50–0.75 years; bin 4 = 0.75–1 year; bin 5 = 1–1.5 years; bin 6 = 1.5–2 years; and bin 7 = >2 years. The number of subjects per bin appears in parentheses.
†Quadrants: AL = antero-lateral; AM = antero-medial; PL = postero-lateral; PM = postero-medial.
BMD values are g/cm2.
Group mean BMD values for each femur quadrant are shown in Fig 3. We computed the slope of BMD decline across time for each quadrant in the active-resisted stance limbs. Over the first 1.5 years of training, the slope of decline was highest for the PL quadrant (slope = −2.662) and lowest for the PM quadrant (slope = −1.287). No quadrant of the active-resisted limbs declined below 82.7% of non-SCI BMD at 1.5 years. The slope of BMD decline was greater for the passive stance limbs, ranging from a minimum of −3.357 for PM and a maximum of −4.738 for the AL quadrant. All four passive stance quadrants declined below 83.5% of non-SCI BMD by 1.5 years.
Figure 3.
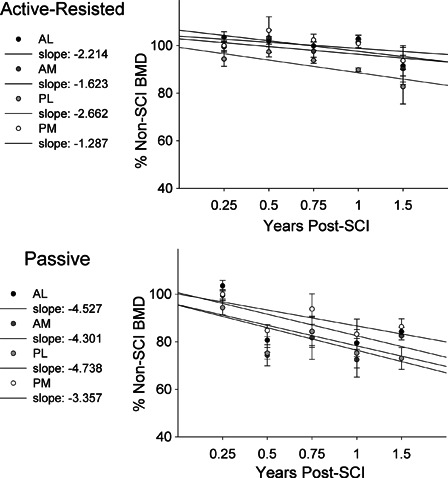
Longitudinal BMD for distal femur quadrants, normalized to the non-SCI BMD value for each quadrant. Values are mean (SE). The slope of longitudinal BMD decline for the quadrants is listed.
We analyzed long-term outcomes (1.5 to >2.0 years) of the two doses of load using statistical comparisons among quadrants and dose levels. A two-way ANOVA indicated that normalized BMD for the active-resisted stance limbs was higher than for passive stance limbs (P = 0.001). Follow-up tests revealed no significant differences between these cohorts at individual quadrants.
Discussion
The purpose of this study was to test a novel ‘quadrant’ method to assess BMD of the femur in individuals with SCI. We hypothesized that bone-sparing benefits of therapeutic stress would emerge in certain regions of the femur. This study supports that active-resisted stance is superior to passive stance for preservation of BMD at all regions of the femur after SCI. While results from a subject followed longitudinally suggest that bone was preferentially preserved in the posterior quadrants with active-resisted stance, the group data suggest that bone adaptations to active-resisted stance were symmetric; however, increased variation with a small sample prohibits a complete comparison of all quadrants between these two training groups.
Regional BMD differences
The present study illustrates that refined pQCT analysis techniques may reveal BMD adaptations in high-responding regions that are obscured by averaging with BMD from non-responding regions. The importance of this finding is that BMD studies of human subjects must strive to minimize subject exposure to ionizing radiation. While high-resolution CT-based imaging is the gold standard,12 pQCT offers the advantage of lower radiation exposure. By using refined analysis techniques, investigators may gain insight into subtle bone adaptations without resorting to CT imaging. Moreover, without the masking influence of non-responding regions during conventional analysis, small regional adaptations may be detectable during longitudinal training protocols, as shown by one subject who received both passive stance and active-resisted stance (Fig. 2).
The slope of BMD decline for all quadrants in the active-resisted stance group was roughly half as large as the comparable passive stance slope values (Fig. 3). When examining long-term effects of training (>1.5 years), active-resisted stance BMD differed significantly from passive stance BMD (P = 0.001), but no particular quadrant demonstrated a significant difference from passive stance. Thus active-resisted stance training appeared to trigger bone adaptations symmetrically across the femur cross section in these two fairly small cohorts of subjects. However, this trend was not likewise supported by the single active-resisted stance subject shown in Fig. 2, who demonstrated asymmetric preservation of BMD in the posterior quadrants. It appears that the pattern of bone preservation during active-resisted stance may be modified by subject anatomy or other individual traits.
Prior to the study we were interested to learn whether the line of action of the electrically stimulated quadriceps differentially loaded the anterior and posterior portions of the femur. We previously observed that soleus loads transmitted to the tibia via the Achilles tendon triggered BMD-sparing adaptations only in the posterior half of the tibia cross-section.21 Quadriceps forces transmitted through the patellar tendon could (in theory) preferentially load the anterior aspect of the femur. However, we viewed this suggestion with caution because the transmission of quadriceps forces is likely to depend on a complex interplay of patella–femoral and tibia–femoral contact forces. In addition, it is not uncommon for concurrent reflex-mediated activation of the antagonist muscles (hamstrings) to occur when using surface electrodes during electrical stimulation. A degree of hamstrings hypertrophy can be seen in the active-resisted limb of the subject depicted in Fig. 1.
Similarly, a sound argument could be made that standing with anterior support of the knees will cause preferential sparing of bone in the anterior quadrants of the femur. Active-resisted stance and passive stance subjects both experienced contact forces at the anterior surface of the knee (Fig. 1). We believe it is likely that such postural contact forces were an important source of mechanical loads in the two stance groups. Despite this bilateral source of load, it is clear that active-resisted stance offered a superior bone-sparing stimulus in the subject who received both conditions (passive and active-resisted; Fig. 2).
Alternative modifiers of BMD
Results from the single subject who performed active-resisted stance training on one limb and passive stance training on the other limb illustrate the dose–response nature of femur BMD adaptations to varied mechanical loads. Under identical genetic, nutritional, and hormonal conditions, BMD for all femur quadrants was higher at all time points for the active-resisted stance limb than for the passive stance limb (Fig. 2). It is important to note that the active-resisted stance training was physiologically rigorous, using maximal stimulation to elicit strong quadriceps contractions (Fig. 1). This subject rapidly demonstrated hypertrophy of the stimulated leg (within 6 months of training) and most effectively reduced BMD decline at the PM quadrant (29.5% difference between limbs at >2.0 years). It appears that an important outcome of active-resisted stance training was to ‘rescue’ BMD of the PM quadrant. We believe that the dose of active muscular load was likely a critical determinant of this subject's bone-sparing response.
Despite the critical nature of the dose of muscular load, we are aware that other modes of mechanical input are important stimuli for bone maintenance. For example, electrical stimulation at 20z yielded an unfused tetanic contraction of the quadriceps.28 The unfused nature of the force profile may introduce a vibratory stimulus to the limb. Animal studies have demonstrated that vibratory input at low loads in the frequency range of skeletal muscle contraction29 readily modulates bone anabolic mechanisms.2,3 Differences in BMD between the active-resisted stance and passive stance groups may have been caused by the oscillation of the muscle driven by the electrical stimulation frequency.
In addition, low-level muscle stimulation that does not trigger muscular overload or hypertrophy may still modulate genetic, neural, vascular, and endocrine signaling pathways. Activation of paralyzed muscle may alter gene expression in pathways for myokines such as myostatin,30 insulin-like growth factor 131 and fibroblast growth factor 2,32 which may alter the activity of osteocytes and osteoprogenitor cells.33,34 Furthermore, the presence of receptors for calcitonin gene-related peptide,35–37 neuropeptide Y38 and substance P39 in bone suggests that these neuroendocrine factors may play a role in bone adaptation. Future investigations of bone adaptation may explore the possible effects of muscle stimulation training upon adipose tissue, which is also known to regulate differentiation of bone marrow stem cells.40 It is theoretically possible that BMD gains observed after muscle stimulation training are a consequence of increased stem cell differentiation to osteocytes at the expense of adipocytes. Work is underway in our laboratory to discern the possible separate and/or synergistic effects of several mechanisms known to modulate bone signaling in people with paralysis.
Conclusions
Active-resisted stance yielded the lowest rate of BMD decline across time. A novel region-based pQCT analysis technique revealed that the posterior regions of the femur benefitted most from active-resisted stance training in one subject, but that training effects were more symmetric across the cohort. The identification of high-responding regions may allow more rapid detection of training effects in longitudinal studies. It may also allow detection of subtle training effects in interventions that may only yield small bone responses, such as passive stance. Further research is needed to determine the extent to which various regions of bone change as a result of therapeutic stress in people with SCI.
Acknowledgements
This work was supported by awards from the National Institutes of Health (R01NR010285, R01HD062507), the US Department of Veterans Affairs, the Craig H. Neilsen Foundation, and by the Christopher Reeve Paralysis Foundation to RKS.
References
- 1.Lanyon LE. Using functional loading to influence bone mass and architecture: objectives, mechanisms, and relationship with estrogen of the mechanically adaptive process in bone. Bone 1996;18:37S–43S [DOI] [PubMed] [Google Scholar]
- 2.Garman R, Gaudette G, Donahue LR, Rubin C, Judex S. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res 2007;25:732–40 [DOI] [PubMed] [Google Scholar]
- 3.Garman R, Rubin C, Judex S. Small oscillatory accelerations, independent of matrix deformations, increase osteoblast activity and enhance bone morphology. PLoS One 2007;2:e653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J 2001;15:2225–9 [DOI] [PubMed] [Google Scholar]
- 5.Heinonen A, Sievanen H, Kannus P, Oja P, Vuori I. Site-specific skeletal response to long-term weight training seems to be attributable to principal loading modality: a pQCT study of female weightlifters. Calcif Tissue Int 2002;70:469–74 [DOI] [PubMed] [Google Scholar]
- 6.Lee KC, Maxwell A, Lanyon LE. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone 2002;31:407–12 [DOI] [PubMed] [Google Scholar]
- 7.Hsieh YF, Robling AG, Ambrosius WT, Burr DB, Turner CH. Mechanical loading of diaphyseal bone in vivo: the strain threshold for an osteogenic response varies with location. J Bone Miner Res 2001;16:2291–7 [DOI] [PubMed] [Google Scholar]
- 8.De Souza RL, Matsuura M, Eckstein F, Rawlinson SC, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone 2005;37:810–8 [DOI] [PubMed] [Google Scholar]
- 9.Mosley JR, March BM, Lynch J, Lanyon LE. Strain magnitude related changes in whole bone architecture in growing rats. Bone 1997;20:191–8 [DOI] [PubMed] [Google Scholar]
- 10.Shields RK, Dudley-Javoroski S. Musculoskeletal plasticity after acute spinal cord injury: effects of long-term neuromuscular electrical stimulation training. J Neurophysiol 2006;95:2380–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley-Javoroski S, Shields RK. Dose estimation and surveillance of mechanical loading interventions for bone loss after spinal cord injury. Phys Ther 2008;88:387–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley-Javoroski S, Saha PK, Liang G, Li C, Gao Z, Shields RK. High dose compressive loads attenuate bone mineral loss in humans with spinal cord injury. Osteoporosis Int 2012;23:2335–46 doi:10.1007/s00198-011-1879-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frey Law L, Shields RK. Femoral loads during passive, active, and active-resistive stance after spinal cord injury: a mathematical model. Clin Biomech 2004;19:313–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eng JJ, Levins SM, Townson AF, Mah-Jones D, Bremner J, Huston G. Use of prolonged standing for individuals with spinal cord injuries. Phys Ther 2001;81:1392–9 [DOI] [PubMed] [Google Scholar]
- 15.Walter JS, Sola PG, Sacks J, Lucero Y, Langbein E, Weaver F. Indications for a home standing program for individuals with spinal cord injury. J Spinal Cord Med 1999;22:152–8 [DOI] [PubMed] [Google Scholar]
- 16.Eser P, Frotzler A, Zehnder Y, Schiessl H, Denoth J. Assessment of anthropometric, systemic, and lifestyle factors influencing bone status in the legs of spinal cord injured individuals. Osteoporos Int 2005;16:26–34 [DOI] [PubMed] [Google Scholar]
- 17.Ben M, Harvey L, Denis S, Glinsky J, Goehl G, Chee S, et al. Does 12 weeks of regular standing prevent loss of ankle mobility and bone mineral density in people with recent spinal cord injuries? Aust J Physiother 2005;51:251–6 [DOI] [PubMed] [Google Scholar]
- 18.Zehnder Y, Luthi M, Michel D, Knecht H, Perrelet R, Neto I, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int 2004;15:180–9 [DOI] [PubMed] [Google Scholar]
- 19.Needham-Shropshire BM, Broton JG, Klose KJ, Lebwohl N, Guest RS, Jacobs PL. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system: part 3. Lack of effect on bone mineral density. Arch Phys Med Rehabil 1997;78:799–803 [DOI] [PubMed] [Google Scholar]
- 20.Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone 2000;27:305–9 [DOI] [PubMed] [Google Scholar]
- 21.Dudley-Javoroski S, Shields RK. Asymmetric bone adaptations to soleus mechanical loading after spinal cord injury. J Musculoskelet Neuronal Interact 2008;8:227–38 [PMC free article] [PubMed] [Google Scholar]
- 22.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med 2011;34(6):535–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudley-Javoroski S, Shields RK. Longitudinal changes in femur bone mineral density after spinal cord injury: effects of slice placement and peel method. Osteoporos Int 2009;21:985–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shields RK, Dudley-Javoroski S, Boaldin KM, Corey TA, Fog DB, Ruen JM. Peripheral quantitative computed tomography: measurement sensitivity in persons with and without spinal cord injury. Arch Phys Med Rehabil 2006;87:1376–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hangartner TN, Gilsanz V. Evaluation of cortical bone by computed tomography. J Bone Miner Res 1996;11:1518–25 [DOI] [PubMed] [Google Scholar]
- 26.Portney LG, Watkins MP. Foundations of clinical research: applications to practice. 3rd ed Upper Saddle River, New Jersey: Pearson Education, Inc; 2009 [Google Scholar]
- 27.Dudley-Javoroski S, McMullen T, Peranich LM, Borgwardt MR, Shields RK. Reliability and responsiveness of musculoskeletal ultrasound in subjects with and without spinal cord injury. Ultrasound Med Biol 2010;36:1594–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudley-Javoroski S, Littmann AE, Chang SH, McHenry CL, Shields RK. Enhancing muscle force and femur compressive loads via feedback-controlled stimulation of paralyzed quadriceps in humans. Arch Phys Med Rehabil 2011;92:242–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang RP, Rubin CT, McLeod KJ. Changes in postural muscle dynamics as a function of age. J Gerontol A Biol Sci Med Sci 1999;54:B352–7 [DOI] [PubMed] [Google Scholar]
- 30.Adams CM, Suneja M, Dudley-Javoroski S, Shields RK. Altered mRNA expression after long-term soleus electrical stimulation training in humans with paralysis. Muscle Nerve 2011;43:65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest 2002;110:771–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwaniec UT, Magee KA, Mitova-Caneva NG, Wronski TJ. Bone anabolic effects of subcutaneous treatment with basic fibroblast growth factor alone and in combination with estrogen in osteopenic ovariectomized rats. Bone 2003;33:380–6 [DOI] [PubMed] [Google Scholar]
- 33.Hamrick MW. A role for myokines in muscle-bone interactions. Ex Sport Sci Rev 2011;39:43–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elkasrawy MN, Hamrick MW. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact 2010;10:56–63 [PMC free article] [PubMed] [Google Scholar]
- 35.Imai S, Tokunaga Y, Maeda T, Kikkawa M, Hukuda S. Calcitonin gene-related peptide, substance P, and tyrosine hydroxylase-immunoreactive innervation of rat bone marrows: an immunohistochemical and ultrastructural investigation on possible efferent and afferent mechanisms. J Orthop Res 1997;15:133–40 [DOI] [PubMed] [Google Scholar]
- 36.Cherruau M, Facchinetti P, Baroukh B, Saffar JL. Chemical sympathectomy impairs bone resorption in rats: a role for the sympathetic system on bone metabolism. Bone 1999;25:545–51 [DOI] [PubMed] [Google Scholar]
- 37.Imai S, Rauvala H, Konttinen YT, Tokunaga T, Maeda T, Hukuda S, et al. Efferent targets of osseous CGRP-immunoreactive nerve fiber before and after bone destruction in adjuvant arthritic rat: an ultramorphological study on their terminal-target relations. J Bone Miner Res 1997;12:1018–27 [DOI] [PubMed] [Google Scholar]
- 38.Bjurholm A. Neuroendocrine peptides in bone. Int Orthop 1991;15:325–9 [DOI] [PubMed] [Google Scholar]
- 39.Bjurholm A, Kreicbergs A, Brodin E, Schultzberg M. Substance P- and CGRP-immunoreactive nerves in bone. Peptides 1988;9:165–71 [DOI] [PubMed] [Google Scholar]
- 40.Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res 2011;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]


