Abstract
Background
Spinal dural arteriovenous fistulas (SDAVFs) are the most common spinal vascular malformations and can be a significant cause of myelopathy although they are under diagnosed. Surgical or embolization treatment of SDAVFs improved significantly in the last decade. However, a high percentage of patients are still left with severe disability.
Objective
To describe the correlation between time to diagnosis and the rehabilitation outcomes of eight patients with SDAVFs.
Design
Retrospective chart study of all SDAVF patients in 20 years.
Setting
A tertiary university rehabilitation center.
Main outcome measures
The lower extremities motor score (LEMS), Functional Independence Measure (FIM), Spinal Cord Independence Measure (SCIM) and Walking Scale for Spinal Cord Injury (WISC II). Overall prognosis was evaluated using the Aminoff-Logue scale (ALS).
Results
There were seven men and one woman with mean age of 61.3 ± 15 (30–72) and mean time until the diagnosis of SDAVF of 265.5 ± 245 days (4–730). At the end of rehabilitation period, five of the eight patients remained wheelchair dependent. Strong correlation was found between LEMS, FIM, SCIM, and WISC II scores and the functional level according to the ALS scale. A significant correlation was found between time to diagnosis and the height of the SDAVF, the clinical and rehabilitation outcomes. Patients with high SDAVF which were diagnosed late had the poorest prognosis.
Conclusions
The potential for functional ambulation in patients with SDAVF is related to the time of intervention. This finding emphasizes the important of early diagnosis and early intervention in SDAVF.
Keywords: Spinal cord injuries, Vascular, Paraplegia, Tetraplegia, Spinal dural arteriovenous fistula, Microsurgery, Endoscopic embolization, Rehabilitation, Physical
Introduction
Spinal arteriovenous dural fistulas (SDAVFs) represents approximately 75–80% of all spinal vascular malformations and the majority of the affected patients are males older than 50 years of age.1 Progression to full-blown myelopathy or paraplegia is slow, and patients may initially present with acute lower extremity dysesthesias and intermittent radicular pain mimicking peripheral nerve lesions.2 There may be also bowel or bladder incontinence and impotence. These vague symptoms may complicate and delay the diagnosis. These patients have usually had symptoms for several months and undergoing unnecessary procedures and surgery prior to diagnosis.3 A high number of patients are left with severe limitation of independence and, according to one study, 50% remained severely disabled 3 years following symptom onset.4
SDAVF is one of the causes of vascular-related spinal cord injury (SCI). Other more common causes are aortic dissection, post-surgical ischemia, vascular embolism, and systemic hypotension.5 The mechanism of SCI in SDAVF is increased venous pressure in the coronal venous plexus due to shunting of arterialized blood in the affected region in the spinal cord often leading to edema and ischemic injury.6 Two treatment options are available: microsurgical interruption of the fistula and endovascular embolization.7 The optimal treatment of SDAVFs remains controversial, and there is an ongoing debate as to whether primary endovascular or primary microsurgical treatment is the optimal management for these lesions.8,9 More recently some authors have begun to favor a multidisciplinary combined approach.10,11
The prognosis of SDAVF is unpredictable. Evaluation of long-term outcome showed an enormous heterogeneity with clinical improvement ranging from 25 to 100% of the cases after treatment.12 The eventual outcome may depend on several factors, such as the duration of symptoms, the degree of disability before treatment, and the success of the initial procedure to close the fistula.13 It is generally thought that a better prognosis will be obtained in younger patients with less severe symptoms. In addition, a few studies showed a correlation between a poor pre-operative neurological status and a lack of improvement.14
In this article we described eight patients with SDAVF treated in one rehabilitation center and determined the correlation between clinical parameters and rehabilitation outcomes. Although patients with SDAVF have progressive myelopathy and are treated in rehabilitation centers, there are few data on their rehabilitation outcomes.
Methods
Population
We conducted a retrospective analysis of all patients diagnosed with SDAVF in a single rehabilitation center in 20 years. Patients were accepted for rehabilitation from a large neurosurgery and intervention radiology tertiary center where they underwent initial treatment. Demographic and clinical characteristics collected included age, gender, neurological level of SDAVF, time to diagnosis, time in acute department, and length of stay (LOS) in rehabilitation. Radiological data included magnetic resonance imaging (MRI) imaging and spinal angiography results. The diagnosis of SDAVF had been made by spinal MRI (Fig. 1) which showed swelling and edema of the spinal cord and dilatation of the perimedullary veins. Selective multilevel spinal catheter angiography confirmed the diagnosis and determined the exact feeder location and vascular anatomy of the fistula (Fig. 2). The retrospective chart review was approved by the Hadassah Medical Center IRB committee.
Figure 1.
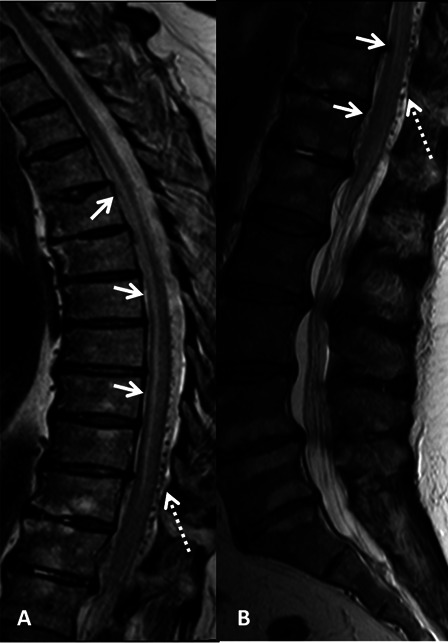
T2 weighted MR image of the thoracic (A) and lumbar (B) spine of patient 2 in the sagittal plane demonstrating diffuse swelling and increased T2 signal of the cord representing cord edema (short arrows). Tortuous pathological intradural vessels are seen consistent with SDAVF (dashed arrows).
Figure 2.
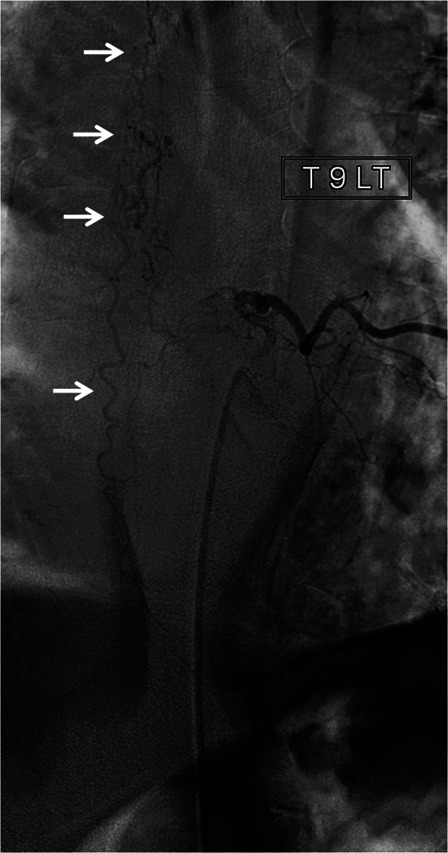
Selective spinal angiogram through injections of the left T9 radicular artery of patient 6 demonstrating the connection between the artery and a large, long serpiginous draining vein (arrows).
Rehabilitation program
In our rehabilitation facility, patients with SDAVF were treated by interdisciplinary approach for 3 hours per day including physiotherapy, occupational therapy, and rehabilitation nursing. All patients received psychological and social worker support. Some of the patients were also treated by robotic body weight-supported treadmill training using the Lokomat™ system.
Outcome measures
Muscle strength of the five key muscles of the lower extremities were measured using the British Medical Council scale, and the lower extremities motor score (LEMS) was calculated with a range of 0–50.15 Activities of daily living (ADL) independence was measured using both the general Functional Independence Measure (FIM) scale16 and more specific for SCI, the Spinal Cord Independence Measure (SCIM) scale.17 The FIM is an ordinal scale that assesses the severity of motor and neuropsychological disability; it consists of 18 items classified into six domains: four motor and two cognitive. Each item envisages seven levels of independent performance (seven equating to total independence and one equating to total dependence or inaccessible). The minimum score of the total FIM is 18, and the maximum score is 126, which is equivalent to total functional independence. The SCIM is a disability scale developed specifically for patients with SCI and was found to be a reliable and sensitive tool in measuring functional changes in SCI patients. The total SCIM score ranges from 0 to 100 including the following areas of function: self-care (sub score 0–20), respiration and sphincter management (0–40), and mobility (0–40). Each area is scored according to its proportional weight in the patients’ general activity. A new version of the SCIM exists; however, since our study is a 20-year follow up we chose to use the original version in these patients for comparison. The patients’ walking status was evaluated using the Walking Scale for Spinal Cord Injury II (WISCI II) score.18 The WISCI II is a 20-item scale measuring the walking status of a patient based on the requirements of assistance and/or bracing and/or walking aids. Grade 0 means that the patient has both neither standing nor walking abilities and grade of 20 means that the patient does not need an assistive device, brace, or assistance with walking for at least 10 meters. The prognosis of patients with SDAVF was evaluated using the Aminoff-Logue scale (ALS, Table 1).4 This scale consists of six grades of gait, between 0 normal to 5 confined to a wheelchair and four grades of micturition between 0 normal to 3 total incontinence or persistent retention. All functional studies were performed before and following the study.
Table 1.
Modified ALS for the assessment of myelopathy after FAS
| Gait | |
| 0 | Normal |
| 1 | Leg weakness, abnormal gait or stance, but no restriction of activity |
| 2 | Restricted activity |
| 3 | Requiring one stick for walking |
| 4 | Requiring two sticks, crutches, or walker |
| 5 | Confined to wheelchair |
| Micturition | |
| 0 | Normal |
| 1 | Hesitancy, frequency, urgency |
| 2 | Occasional urinary incontinence or retention |
| 3 | Total incontinence or persistent retention |
Statistics
A Pearson product-moment correlation coefficient (Pearson's r) was computed to assess the relationship between the outcomes measurements and the clinical parameters and between the outcomes measurement themselves. Data analyses were conducted using SAS v.9.2.19.
Results
Patients population
In the last 20 years, eight patients with SDAVF were treated in our inpatient rehabilitation unit. Demographic and functional outcomes details of these eight patients are summarized in Table 2. There were seven men and one woman. Mean age was 61.3 ± 15 years (30–72 years). The mean time until the diagnosis of SDAVF from the first symptom recognized by the patient was 265.5 ± 245 days (4–730 days). The mean LOS in the acute department was 25.5 ± 8.5 days (14–36 days) and the mean LOS in rehabilitation was 71 ± 31.5 days (32–113 days). As seen in Table 2, three patients were treated by embolization, three were treated by surgery alone, and two patients were treated by both interventions. It should be noted that in one patient, embolization was prematurely terminated because of a severe allergic reaction and that two patients were treated by surgery in early years when embolization was not available in Israel.
Table 2.
Clinical parameters of eight patients with SDAVF
| No. | Age/sex | Time to diagnosis days | Level of SDAVF | LOS acute care days | LOS in rehab days | Treatment | ALS gait | ALS micturition |
|---|---|---|---|---|---|---|---|---|
| 1 | 61/M | 730 | T9–T11 | 34 | 72 | Embolization X 2 | 5 | 3 |
| 2 | 69/F | 4 | L2–L4 | 21 | 105 | Embolization + laminectomy + resection | 5 | 3 |
| 3 | 76/M | 180 | L1–L2 | 15 | 72 | Decompressive laminectomy | 5 | 3 |
| 4 | 48/M | 365 | T6 | 28 | 72 | Embolization | 4 | 2 |
| 5 | 67/M | 150 | T9 | 36 | 113 | Decompression + embolization | 5 | 2 |
| 6 | 68/M | 60 | T7–T9 | 23 | 42 | Embolization | 3 | 0 |
| 7 | 30/M | 120 | T5–T7 | 14 | 32 | Laminectomy + resection | 4 | 2 |
| 8 | 72/M | 485 | T10–T12 | 33 | 32 | Laminectomy + biopsy | 5 | 3 |
SDAVF, spinal dural arteriovenous fistula; LOS, length of stay; ALS, Aminoff-Logue scale.
The diagnosis of SDAVF was delayed in most of the patients in our series requiring an average of 8 months and reaching almost 2 years in one patient. Many alternative diagnoses were proposed such as spinal stenosis, myeloradiculitis, peripheral neuropathy, and more. In three of our patients axonal motor neuropathy was found by nerve conduction studies. Cerebrospinal fluid studies were performed in five out of eight patients. The main findings were elevated RBC in two and high protein levels in three of them. Positive oligoclonal bands typical for inflammatory conditions were found in two patients. Some of the patients were treated because of these false diagnoses by surgical procedures, epidural injections, intravenous and oral steroids, and plasmapheresis.
Rehabilitation outcomes
At the end of the rehabilitation period, five patients remained in wheelchairs with ALS grading of 5, two patients had ALS grading of 4, and only one patient was able to ambulate with a cane (ALS of 3, Table 2). Two out of the three patients treated by embolization improved, one out of the three surgical-treated patients, and one out of two patients treated by combined approach improved. The mean muscle strength in the lower extremities measured by the LEMS improved from 13.37 + 12.85 (out of 50) at the entrance to rehabilitation to 22.75 + 12.83 at discharge (P = 0.014). However, LEMS remained below 30 in six out of eight patients reflecting the severe weakness in the legs of these patients at the end of rehabilitation (Fig. 3A). Mean FIM and SCIM scores improved from 86.5 + 12.7 and 41.12 + 17.7 at rehabilitation entry, respectively, to 109.6 + 8.7 and 64.75 + 19.4 at discharge (P < 0.001 for both scales). At the end of rehabilitation, four out of eight patients had FIM score of 105 or below and SCIM score of 52 or below (Figs. 3B and 3C) meaning that they achieved some independence; however, they still need constant assistance in ADL functions. The mean WISCI II score improved during the rehabilitation period from 1.125 + 2.03 to 7.785 + 7.16 (P = 0.015 (though in five out of eight patients WISCI II was three and below (Fig. 3D) reflecting the requirements of assistance and/or bracing and/or walking aids in ambulation.
Figure 3.
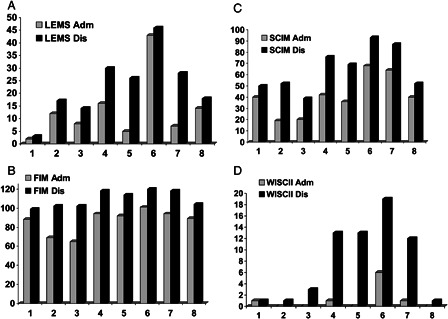
Clinical and functional outcomes of eight patients with SDAVF at admission (Adm) and discharge (Dis). (A) LEMS; (B) FIM; (C) SCIM; (D) WISC II. Numbers indicate patients.
Correlation between rehabilitation outcomes and clinical parameters
To evaluate whether there was any correlation between the demographic and clinical parameters and the functional outcomes as well as among the various functional outcomes themselves, we used the Pearson correlation coefficient (r). We found a strong correlation between muscle strength in the lower limbs at admission (LEMS at admission) and the discharge status of the patients including neurological recovery as measured by LEMS as well as functional recovery measured by FIM, SCIM, and WISCI II scores (Table 3). At discharge from rehabilitation there was also a strong correlation between lower values of the LEMS, low level of ambulation, and independence in ADL functions (FIM, SCIM, and WISC II) as well as the functional level according to the ALS scale (Table 3). A significant correlation was found between the height of the SDAVF and the clinical outcomes; higher SDAVF had worse prognosis (Table 4). Strong correlation was also found between the final outcome and time to diagnosis, except for patient 2 who had an acute presentation of SDAVF observed in 5–15% of the patients.19 If the SDAVF was diagnosed early and treated appropriately, there was a higher chance for better prognosis. There was moderate correlation between the age of the patients and functional outcome, older patients had worse prognosis than younger patients. No significant correlation was found between the final outcomes and the LOS in either acute care or in rehabilitation except for moderate correlation between ALS and LOS in rehabilitation (Table 4).
Table 3.
The correlation between clinical and functional parameters of eight patients with SDAVF at discharge from rehabilitation using the Pearson correlation coefficient (r)
| LEMS_Dis | FIM_Dis | SCIM_Dis | WISCI II_Dis | ALS Dis | |
|---|---|---|---|---|---|
| LEMS_Adm | 0.800 | 0.522 | 0.575 | 0.595 | −0.816 |
| LEMS_Dis | 1.00000 | 0.90636 | 0.87017 | 0.90885 | −0.86859 |
| FIM_Dis | 0.90636 | 1.00000 | 0.94325 | 0.95721 | −0.81609 |
| SCIM_Dis | 0.87017 | 0.94325 | 1.00000 | 0.91144 | −0.87523 |
| WISCI II_Dis | 0.90885 | 0.95721 | 0.91144 | 1.00000 | −0.83140 |
| ALS Dis | −0.86859 | −0.81609 | −0.87523 | −0.83140 | 1.00000 |
ALS, Aminoff-Logue scale; FIM, Functional Independence Measure; SCIM, Spinal Cord Independence Measure; WISCI II, Walking Scale for Spinal Cord Injury; LEMS, Lower extremities muscle score; Adm, admission; Dis, discharge.
Table 4.
The correlation between demographic parameters and the clinical and functional parameters of eight patients with SDAVF at discharge from rehabilitation using the Pearson correlation coefficient (r)
| ALS Dis | FIM Dis | SCIM Dis | WISC II Dis | LEMS Dis | |
|---|---|---|---|---|---|
| Level of SDAVF | 0.69437 | −0.85629 | −0.82531 | −0.79416 | −0.60880 |
| Time to diagnosis* | 0.51666 | −0.69167 | −0.55504 | −0.72403 | −0.75144 |
| Age | 0.39084 | −0.54747 | −0.59909 | −0.37939 | −0.22230 |
| Time in acute Dep | 0.30876 | −0.14110 | −0.12611 | −0.08498 | −0.19740 |
| Time in Rehab Dep | 0.50060 | −0.26226 | −0.35003 | −0.15501 | −0.27825 |
ALS, Aminoff-Logue scale; FIM, Functional Independence Measure; SCIM, Spinal Cord Independence Measure; WISC II, Walking Scale for Spinal Cord Injury; LEMS, Lower extremities muscle score; Dis, discharge; Dep, department; Rehab, rehabilitation.
*Patient 2 was excluded due to hyperacute course.
Discussion
To our knowledge, this is the first description of the rehabilitation outcomes of patients with SDAVF treated in a rehabilitation center. Our data showed that in spite of prolonged rehabilitation treatment, most of the patients with SDAVF (five out of eight), remained in a wheelchair and had low level of independence (ALS 5). According to our data the level of functioning was strongly correlated with the improvement of muscle strength according to the LEMS and with the improvement in gait according to the WISCI II. Patients with lower SDAVF had better prognosis, whereas patients in whom the SDAVF was discovered and treated late had poorer prognosis.
Although SDAVF is a rare syndrome it remains a major cause of disability and handicap. The usage of MRI and selective angiography has significantly improved the ability to characterize SDAVFs, however, these lesions remain inefficiently diagnosed. Even today, the true incidence of SDAVF is unknown and often there is a significant delay in the diagnosis as in our cases.20 The time between the onset of symptoms and diagnosis has been reported to be 12–44 months, with a mean duration of 22.9 months.3 This delay in diagnosis is mainly due to a frequently non-specific clinical presentation. Presenting symptoms of motor weakness, gait disturbances, and paresthesias commonly lead clinicians to consider and rule out many other disorders before considering SDAVFs. Common misdiagnoses include degenerative disc disease, spinal cord tumors, peripheral vascular disease, neuromuscular diseases, or neuropathy.2 The long-term clinical significance of the delay in diagnosis has yet to be fully elucidated.13,21 As shown in our study, it is likely that many patients would benefit from more prompt diagnosis and early intervention.
The prevalence of SDAVF in rehabilitation centers for patients with SCI is unknown. In one study, Jellema et al.22 found 11 cases of SDAVF out of 1429 patients admitted to SCI ward of a rehabilitation center in 24 years. In another study examining the rehabilitation outcome of vascular-related SCI,23 5 (17%) patients out of 30 patients had SDAVF in 10 years. It seems that the majority of patients with SDAVF do not require inpatient rehabilitation treatment. Only patients with the most severe motor and functional disabilities are treated in rehabilitation center, such as the patients described in this paper. Therefore they are representative of the patients with SDAVF treated in rehabilitation units.
The prognosis of SDAVF is unpredictable. The eventual outcome may depend on several factors, such as the duration of symptoms, the degree of disability before treatment, and the success of the initial procedure to close the fistula.13 It is generally thought that prognosis is better in younger patients with less severe symptoms. Another important factor that determined the final outcome is the time to diagnosis. It was shown that better outcome has been related to shorter delay between symptom onset and initiation of endovascular or surgical treatment.24 This was also found in most of our patients, in that there was a strong correlation between shorter time to intervention and better prognosis. If intervention is delayed, as in most of our patients, even prolonged time in rehabilitation does not change the grave prognosis.
In addition, few studies showed a correlation between a poor pre-operative neurological status and a lack of improvement;13,14,21 however, others fail to find such correlation.25 In our study, we found a strong correlation between the neurological impairment on admission as shown by the LEMS and the neurological and functional outcomes at discharge. The patients with less severe symptoms before intervention had better prognosis following rehabilitation. These findings emphasized the utmost importance of early diagnosis of SDAVF and the initiation of specific intervention, surgical, or embolization as soon as possible.
Treatment options for SDAVF include microsurgery and endovascular embolization. There is still controversy regarding the relative advantages of each one. Although recently many authors favor the endovascular approach, some still believe that surgery is still superior.24,26 Lately, a combined multidisciplinary approach was employed.27 A meta-analysis of 16 surgical studies and 10 endovascular therapy studies8 demonstrated that embolization resulted in initial occlusion of the fistula in 46% of cases with a morbidity of less than 4% and an absence of mortality, whereas surgery resulted in successful occlusion of the SDAVF in 98% of the cases with a morbidity of less than 2% and no mortality. In the surgical cases, clinical improvement was found in 55% and stabilization of the clinical condition in 34% of the patients, respectively. In our study, no significant difference was found between patients treated with endovascular, surgical, or combined approach.
Conclusions
The potential for recovery of functional ambulation in patients with SDAVF is related to the time of intervention. Patients in whom endovascular or surgical treatment is delayed have poor prognosis despite of prolonged rehabilitation treatment. This finding emphasizes the important of early diagnosis and early intervention in SDAVF.
References
- 1.Rodesch G, Lasjaunias P. Spinal cord arteriovenous shunts: from imaging to management. Eur J Radiol 2003;46(3):221–32 Review [DOI] [PubMed] [Google Scholar]
- 2.Jellema K, Tijssen CC, Van Gijn J. A cognitive myelopathy that mimcs a peripheral nerve disorder. Brain 2006;129(Pt 12):3150–64 [DOI] [PubMed] [Google Scholar]
- 3.Narvid J, Hetts SW, Larsen D, Neuhaus J, Singh TP, McSwain H, et al. Spinal dural arteriovenous fistulae: clinical features and long-term results. Neurosurgery 2008;62(1):159–66 [DOI] [PubMed] [Google Scholar]
- 4.Aminoff MJ, Logue V. The prognosis of patients with spinal vascular malformations. Brain 1974;97(1):211–8 [DOI] [PubMed] [Google Scholar]
- 5.McKinley W, Gibbs J, McKinley S. Incidence and outcome of vascular-related SCI. J Spinal Cord Med 2007;30(4):404–5 [Google Scholar]
- 6.Bederson JB, Spetzler RF. Pathophysiology of type I spinal dural arterio-venous malformations. BNI Q 1996;12(2):23–31 [Google Scholar]
- 7.Aghakhani N, Parker F, David P, Lasjaunias P, Tadie M. Curable cause of paraplegia. Spinal dural arteriovenous fistulae. Stroke 2008;39(10):2756–9 [DOI] [PubMed] [Google Scholar]
- 8.Steinmetz MP, Chow MM, Krishnaney AA, Andrews-Hinders D, Benzel EC, Masaryk TJ, et al. Outcome after the treatment of spinal dural arteriovenous fistulae: a contemporary single-institution series and meta-analysis. Neurosurgery 2004;55(1):77–87 [DOI] [PubMed] [Google Scholar]
- 9.Hessler C, Regelsberger J, Grzyska U, Illies T, Zeumer H, Westphal M. Therapeutic clues in spinal dural arteriovenous fistulas – a 30 year experience of 156 cases. Cent Eur Neurosurg 2010;71(1):8–12 [DOI] [PubMed] [Google Scholar]
- 10.Song JK, Viñuela F, Gobin YP, Duckwiler GR, Murayama Y, Kureshi I, et al. Surgical and endovascular treatment of spinal dural arteriovenous fistulas: long-term disability assessment and prognostic factors. J Neurosurg 2001;942 Suppl:199–204 [DOI] [PubMed] [Google Scholar]
- 11.Van Dijk JM, Ter Brugge KG, Willinsky RA, Farb RI, Wallace MC. Multidisciplinary management of spinal dural arteriovenous fistulas: clinical presentation and long-term follow-up in 49 patients. Stroke 2002;33(6):1578–83 [DOI] [PubMed] [Google Scholar]
- 12.Prieto R, Pascual JM, Gutiérrez R, Santos E. Recovery from paraplegia after the treatment of spinal dural arteriovenous fistula: case report and review of the literature. Acta Neurochir (Wien) 2009;151(11):1385–9 [DOI] [PubMed] [Google Scholar]
- 13.Cenzato M, Versari P, Righi C, Simionato F, Casali C, Giovanelli M. Spinal dural arteriovenous fistulae: analysis of outcome in relation to pretreatment indicators. Neurosurgery 2004;55(4):815–22 [DOI] [PubMed] [Google Scholar]
- 14.Cecchi PC, Musumeci A, Faccioli F, Bricolo A. Surgical treatment of spinal dural arterio-venous fistulae: long-term results and analysis of prognostic factors. Acta Neurochir (Wien) 2008;150(6):563–70 [DOI] [PubMed] [Google Scholar]
- 15.Shin JC, Yoo JH, Jung TH, Goo HR. Comparison of lower extremity motor score parameters for patients with motor incomplete spinal cord injury using gait parameters. Spinal Cord 2011;49(4):529–33 [DOI] [PubMed] [Google Scholar]
- 16.Keith RA, Granger CV, Hamilton BB, Sherwin FS. The Functional Independence Measure: a new tool for rehabilitation. Advances in Clinical Rehabiltation 1987;1:6–18 [PubMed] [Google Scholar]
- 17.Catz A, Itzkovich M, Agranov E, Ring H, Tamir A. SCIM – spinal cord independence measure: a new disability scale for patients with spinal cord lesions. Spinal Cord 1997;35(12):850–6 [DOI] [PubMed] [Google Scholar]
- 18.Ditunno JF, Jr, Barbeau H, Dobkin BH, Elashoff R, Harkema S, Marino RJ. Validity of the Walking Scale for spinal cord injury and other domains of function in a multicenter clinical trial, Neurorehabil Neural Repair. Neurorehabil Neural Repair 2007;21(6):539–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khurana VG, Perez-Terzic CM, Petersen RC, Krauss WE. Singing paraplegia: a distinctive manifestation of a spinal dural arteriovenous fistula. Neurology 2002;58(8):1279–81 [DOI] [PubMed] [Google Scholar]
- 20.Atkinson JL, Miller GM, Krauss WE, Marsh WR, Piepgras DG, Atkinson PP, et al. Clinical and radiographic features of dural arteriovenous fistula, a treatable cause of myelopathy. Mayo Clin Proc 2001;76(11):1120–30 [DOI] [PubMed] [Google Scholar]
- 21.Eskandar EN, Borges LF, Budzik RF, Putman CM, Ogilvy CS. Spinal dural arteriovenous fistulas: experience with endovascular and surgical therapy. J Neurosurg 2002;962 Suppl.:162–7 [DOI] [PubMed] [Google Scholar]
- 22.Jellema K, Tijssen CC, Sluzewski M, van Asbeck FW, Koudstaal PJ, van Gijn J. Spinal dural arteriovenous fistulas – an underdiagnosed disease. A review of patients admitted to the spinal unit of a rehabilitation center. J Neurol 2006;253(2):159–62 [DOI] [PubMed] [Google Scholar]
- 23.McKinley W, Sinha A, Ketchum J, Deng X. Comparison of rehabilitation outcomes following vascular-related and traumatic spinal cord injury. J Spinal Cord Med 2011;34(4):410–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Symon L, Kuyama H, Kendall B. Dural arteriovenous malformations of the spine. Clinical features and surgical results in 55 cases. J Neurosurg 1984;60(2):238–47 [DOI] [PubMed] [Google Scholar]
- 25.Jellema K, Tijssen CC, van Rooij WJ, Sluzewski M, Koudstall PJ, Algra A, et al. Spinal dural arteriovenous fistulas: long-term follow-up of 44 treated patients. Neurology 2004;62(10):1839–41 [DOI] [PubMed] [Google Scholar]
- 26.Niimi Y, Berenstein A, Setton A, Neophytides A. Embolization of spinal dural arteriovenous fistulae: results and follow-up. Neurosurgery 1997;40(4):675–82 [DOI] [PubMed] [Google Scholar]
- 27.Sherif C, Gruber A, Bavinzski G, Standhardt H, Widhalm G, Gibson D, et al. Long-term outcome of a multidisciplinary concept of spinal dural arteriovenous fistulae treatment. Neuroradiology 2008;50(1):67–74 [DOI] [PubMed] [Google Scholar]


