Abstract
Abstract
This article provides the scientific rationale and background information for the Canadian Hypertension Education Program’s 2012 recommendations for the management of hypertension. It also summarizes the key new recommendations and the theme for 2012, which is the prevention of hypertension. The full recommendations are available at www.hypertension.ca.
For 13 years, the Canadian Hypertension Education Program (CHEP) has been producing annually updated recommendations and clinical practice guidelines related to the detection, treatment and control of hypertension. In this article we provide background information on the updates generated at this year’s consensus conference, and we summarize the key new recommendations (Box 1). The 2012 recommendations have prevention as their theme. The full recommendations are available online at www.hypertension.ca.
Box 1.

Major changes to the 2012 clinical practice recommendations of the Canadian Hypertension Education Program
Clinical Vignette
You see a 68-year-old retired man in your office for follow-up of his blood pressure (BP). His past medical history includes myocardial infarction with systolic dysfunction, dyslipidemia and chronic kidney disease (estimated glomerular filtration rate [eGFR] 48 mL/min). He is otherwise well, he does not have diabetes, and he can walk 4 blocks without any limitations imposed by symptoms. His current medications include an angiotensin converting enzyme (ACE) inhibitor, a beta blocker, acetylsalicylic acid and a statin. On previous examination his BP was 154/92 mm Hg, and today it is 150/90 mm Hg. He is surprised by these BP readings, as his BP was considerably lower when he checked it at the supermarket. He asks whether his readings are elevated because he is nervous and is worried that he will need more medications, given that he is already taking “too many pills.”
You discuss with him the white-coat effect on blood pressure and the importance of achieving BP targets. You provide him with the Measure Blood Pressure At Home tool from the Hypertension Canada website (www.hypertension.ca) and ask him to measure his BP at home using his wife’s home BP monitor; this device has been endorsed by Hypertension Canada. You arrange for him to return in 2 weeks for additional follow-up.
Managing hypertension by the numbers
The prevalence of hypertension in Canada is predicted to reach 7 500 000 in 2012/2013; over 1000 people are diagnosed with hypertension daily in this country.1 These numbers are largely driven by the aging of the baby-boom generation2 and their sedentary lifestyle and unhealthy eating habits (in particular, their consumption of excess sodium). Since the initiation of the CHEP in 1999, the awareness, treatment and control of hypertension have greatly improved. The percentage of Canadians who report that they are aware they have hypertension but are not receiving treatment has fallen dramatically, whereas the percentage of Canadians with hypertension whose condition is treated and controlled has risen from 13% in 1992 to 66% in recent surveys.3,4 In association with the improvements in BP control, mortality rates for stroke, heart failure and heart attack have fallen faster in Canada in the past 10 years than in the previous decade.5 In the United States, it is estimated that health care costs related to newly diagnosed cases of hypertension will be $130.4 billion more in 2030 than they were in 2010,6 underscoring the importance of the theme for CHEP’s 2012 clinical practice recommendations: prevention.
The importance of prevention
Despite continuous advancements in reducing the prevalence of cardiovascular diseases, these diseases remain a major cause of disability and premature death and contribute substantially to the escalation of health care costs in Canada.7 Modifications in individuals’ exposures to behavioural, environmental and societal risk factors can prevent or delay the onset of chronic disease and resulting disabilities and represent a feasible and practical target for change at both clinical and population levels.8 High BP is the most common and important modifiable risk factor for a range of chronic diseases, including coronary artery disease, stroke, congestive heart failure, chronic kidney disease, peripheral arterial disease and dementia.8
The majority of Canadians will develop hypertension if they live an average lifespan.9 Therefore, even modest changes in BP have significant potential to reduce the current burden of chronic disease.
More emphasis on maintaining a healthy lifestyle (eating a diet high in fresh fruit and vegetables, with low-fat dairy products that are low in saturated fats and sodium [DASH diet], exercising regularly, attaining and maintaining a healthy body weight and abdominal girth, engaging in low-risk alcohol consumption and living in a smoke-free environment) and on preventing or delaying chronic diseases will improve the quality of life of Canadians while reducing the impact these conditions have on individuals, families, communities, the health care system and society. A supportive environment (e.g., a food supply with limited processed foods that contain saturated and trans fats, simple sugars and added sodium and built communities that encourage regular physical activity) is critical to the implementation of lifestyle recommendations. Reducing sodium additives in foods is an important environmental policy, and readers are encouraged to visit www.sodium101.ca to see how they can become more engaged.
2012 Update of CHEP’s recommendations
Home monitoring
Recommendation
If the average home BP is less than 135/85 mm Hg, it is advisable to either repeat home monitoring to confirm that the home BP is less than 135/85 mm Hg or perform 24-hour ambulatory BP monitoring (ABPM) to confirm that the mean 24-hour ABPM is less than 130/80 mm Hg and the mean awake ABPM is less than 135/85 mm Hg, before diagnosing white-coat hypertension.
BP varies across individuals, making its assessment challenging. New information on BP measurement has expanded the role of home BP monitoring in addition to ABPM to aid the diagnosis of white-coat and masked hypertension (Box 2). The greater the number of recommended measures taken, the greater the accuracy of the estimate of the “true” BP. Home BP monitoring allows a greater number of BP measurements to be recorded, is convenient for the patient and provides more precise and accurate reflections of true BP and a better prediction of cardiovascular outcomes by removing the white-coat effect.10 In a study of 163 participants who had office and ambulatory BP readings 6 weeks apart and home readings for at least 6 weeks, the variance in BP was found to be significantly lower with home and ambulatory BP measures than with office measures.11 Increasing the number of office BP readings can also reduce this variability, and automated office devices like the BpTRU blood pressure monitor (BpTRU Medical Devices, Coquitlam, B.C.) come close to eliminating the white-coat effect.12
Box 2.
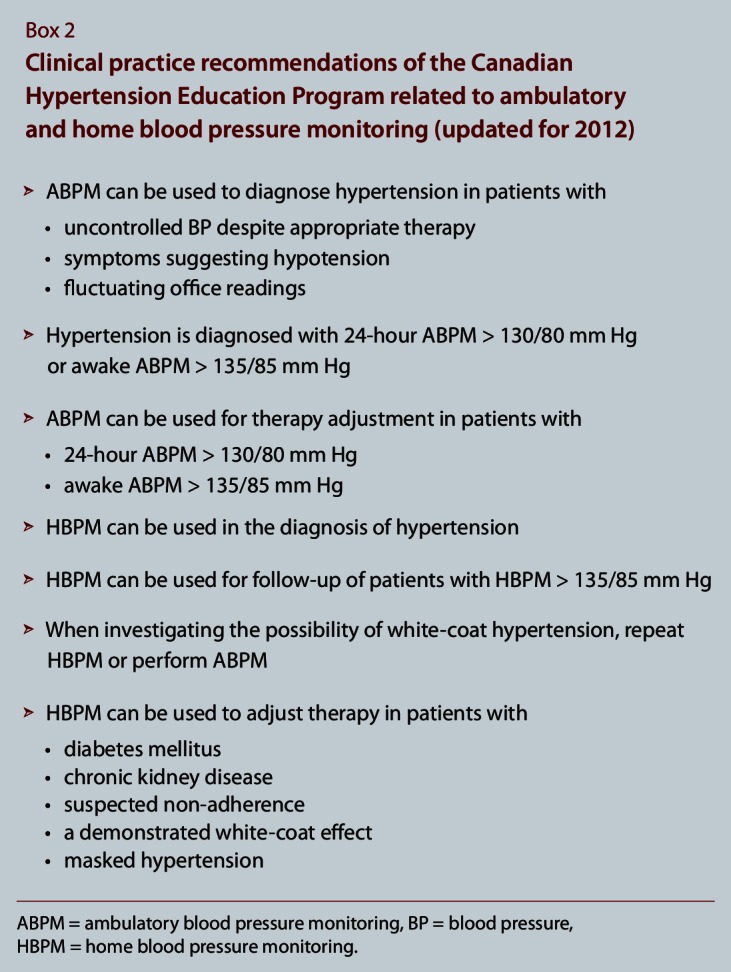
Clinical practice recommendations of the Canadian Hypertension Education Program related to ambulatory and home blood pressure monitoring (updated for 2012)
Clinical vignette revisited
The patient returns after 2 weeks with BP readings from home averaging 148/92 mm Hg. The CHEP recommendation for systolic pressure measured at home is less than 135 mm Hg, equivalent to a standardized office measurement of 140 mm Hg systolic. With a coefficient of variation of 5.5%, the standard deviation for a systolic pressure of 148 mm Hg is 8.1 mm Hg. Eighty percent of measurements will be between 1.28 standard deviations (137–158 mm Hg), and thus you can now safely rule out white-coat hypertension.
BP target for patients with non-diabetic chronic kidney disease
Recommendation
For patients with non-diabetic chronic kidney disease, the target BP is less than 140/90 mm Hg.
CHEP had previously recommended that individuals with non-diabetic chronic kidney disease be treated to a BP target of less than 130/80 mm Hg,13 in keeping with a meta-analysis demonstrating that achieving systolic pressures below 120 mm Hg was associated with better outcomes in patients with proteinuria.14 However, our critical reappraisal of the literature did not support treating this patient population to a more stringent BP target than that for the general population, even in the setting of significant proteinuria. As a consequence, this recommendation has been removed, and CHEP now recommends that patients with non-diabetic chronic kidney disease be treated to the same BP target as the general population (140/90 mm Hg), on the basis of current evidence. A summary of the relevant literature follows.
Modification of Diet in Renal Disease study
In the Modification of Diet in Renal Disease (MDRD) study of patients with chronic kidney disease and a low glomerular filtration rate (GFR), there was no difference between the groups with usual BP target (mean arterial pressure [MAP] 107 mm Hg; BP approximately 140/90 mm Hg) and low BP target (MAP 92 mm Hg; BP approximately 125/75 mm Hg) with respect to the slope of decline in GFR or secondary outcomes including kidney failure, death, a composite of kidney failure or death, and cardiovascular events.15 A long-term follow-up of the MDRD cohort demonstrated that individuals who had originally been randomly assigned to the group with the low BP target had a lower hazard of kidney failure.17 However, this difference was not apparent during the trial, and there was no information about the therapy these patients received, the BP targets or the BP achieved in each group after the trial was completed.16
In the original MDRD report, subgroup analyses also suggested that there was a significant interaction between baseline proteinuria and BP target (p = 0.02), and a re-analysis of the MDRD data showed that the rate of GFR decline appeared to increase above a MAP of 98 mm Hg in patients with between 0.25 g and 3 g of proteinuria per day.17 In patients with 3 g or more of proteinuria per day, the rate of GFR decline increased above a MAP of 92 mm Hg.17 It is important to note that this was a post-hoc subgroup analysis and that randomization was not stratified on the basis of level of proteinuria. In addition, the proteinuria categories were not prespecified, and there were no a priori power calculations done for the subgroups. The level of statistical significance was not adjusted for multiple testing, and baseline patient characteristics were not presented according to subgroup. Finally, the use of ACE inhibitors was higher in the patients assigned to the low BP target, and this may very well have influenced outcomes. In summary, although there was a suggestion that patients with higher degrees of proteinuria at baseline may benefit from a lower BP target, these results should have been interpreted as hypothesis generating, but they played a role in the initial adoption of a BP target of less than 125/75 mm Hg for patients with 1 g or more of proteinuria per day.
African American Study of Kidney Disease and Hypertension trial
The African American Study of Kidney Disease and Hypertension (AASK) study had a 2 × 3 factorial design with 2 different BP levels and 3 classes of antihypertensive medications (ACE inhibitor, calcium-channel blocker and beta blocker). The participants were African Americans with non-diabetic kidney disease. In the BP-lowering component of the AASK trial, there was no significant difference in renal outcomes (the chronic slope or the overall rate of decline in GFR per year) over the study period between individuals randomly assigned to the usual-BP goal (MAP 102–107 mm Hg) and those assigned to a low-BP goal (MAP 92 mm Hg).18 Patients assigned to the low-BP group experienced a 17% reduction in proteinuria, whereas those in the usual-BP goal experienced a 7% increase. There was no difference between the groups in the risk of kidney failure, the composite of kidney failure or death, the composite of a GFR event or death, or the combined end point of GFR event, kidney failure or death. There was also no difference in cardiovascular mortality or non-fatal cardiovascular events.
In the AASK trial there was also an interaction between baseline proteinuria (stratified as less than or equal to 300 mg per day or greater than 300 mg per day) and BP group assignment. This interaction was interpreted to mean that patients with higher levels of proteinuria might benefit from a more intensive BP target. However, the interaction appeared to be driven by the fact that patients with low levels of proteinuria who were randomly assigned to the low-BP group had worse outcomes than the usual-BP group. When the authors stratified the analysis according to level of proteinuria, the BP comparison “was not significantly different within either the lower (baseline urinary protein to creatinine ratio ≤ 0.22) or higher (baseline urinary protein to creatinine ratio > 0.22) proteinuria strata” for the secondary clinical composite outcome (doubling of serum creatinine level, kidney failure or death).19
A subsequent re-analysis of the AASK trial, which included a cohort follow-up period, used the secondary clinical composite outcome from the initial report as the primary outcome of interest.20 In this analysis, a significant reduction (26%) in the relative hazard of the outcome was reported (p = 0.04) in patients randomly assigned to the low-BP target who had more than 300 mg of proteinuria per day (protein to creatinine ratio greater than 0.22). The explanation for the discrepant findings in the 2 reports is unclear. In the original study, a mixed-effects model was used to model the relationship between BP target and outcomes. It appears that the re-analysis adjusted for the same fixed effects, but no mention was made of random effects being incorporated into the statistical models. Finally, although this re-analysis was focused on renal outcomes, no mention of the primary renal outcome in the original trial was made. Regardless, this analysis represented a subgroup analysis of a secondary end point in the original trial. Similar to the re-analysis of the MDRD data, this was a post-hoc subgroup analysis and randomization was not stratified on the basis of level of proteinuria. The proteinuria categories were not specified before the start of the trial but rather were set before the start of the analysis. There were no a priori power calculations for the subgroups, and the level of statistical significance was not adjusted for multiple testing. Although there was a suggestion that patients with more than 300 mg of proteinuria per day at baseline may derive benefit from a lower BP target and that those with less proteinuria may experience worse outcomes, these results should again be interpreted with caution.
Blood Pressure Control for Renoprotection in Patients with Chronic Renal Disease trial
The Blood Pressure Control for Renoprotection in Patients with Chronic Renal Disease (REIN-2) trial is the only randomized trial to specifically look at the impact of BP targets on outcomes in individuals with proteinuric, non-diabetic renal disease.21 Investigators randomly assigned patients with more than 1 g of proteinuria per day to conventional BP control (target diastolic BP < 90 mm Hg) or intensified BP control (target BP < 130/80 mm Hg). All patients were treated with ramipril, and the group with intensive BP control received 5–10 mg of felodipine per day to achieve targets. Additional agents were used as needed. The study was stopped owing to futility after a median of 19 months of follow-up. There was no difference in the adjusted hazard of reaching kidney failure between the conventional and intensive control groups (hazard ratio 1.00, 95% confidence interval 0.61–1.64).
This study has been criticized because of the use of a dihydropyridine calcium-channel blocker in the group with the intensive BP control, the small difference in achieved BP between groups and the limited amount of follow-up. In addition, because of the sample size there was limited power to rule out a clinically important difference. However, the REIN-2 trial was the only randomized study to specifically look at the impact of BP targets on outcomes in individuals with proteinuric, non-diabetic renal disease.
Overall, the evidence to support a more stringent BP target in patients with chronic kidney disease is of low quality, and the randomized trials conducted to date suggest that there is no benefit to reducing BP to targets less than 140/90 mm Hg. Despite observational evidence suggesting that more intensive BP control may be beneficial in individuals with more than 300 mg or more than 1 g per day of proteinuria, the lone randomized trial examining this issue had negative results. Although an important benefit cannot be ruled out, the current evidence base does not support a more intensive BP target in this group.
Various guidelines have recommended a target BP of less than 130/80 mm Hg for patients with chronic kidney disease without diabetes.22,23 In recent years there has been a retrenchment from this lower target. The National Institute for Health and Clinical Excellence guidelines for chronic kidney disease maintained the target of less than 130/80 mm Hg but only when the production of urine protein was 1 g per day or greater; otherwise, the target was less than 140/90 mm Hg.24
BP target for patients with diabetes mellitus
Recommendation
Patients with diabetes mellitus should be treated to attain BP of less than 130/80 mm Hg.
This recommendation for patients with diabetes, including those with chronic kidney disease, remains unchanged. For people with diabetes and hypertension, the diastolic target of 80 mm Hg was established in response to the findings of the Hypertension Optimal Treatment trial,25 but the systolic target continues to be a topic of discussion. The results of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial were reported in 2010 and were reviewed and debated again at the 2012 CHEP consensus conference. This trial compared the outcomes in an intensive-treatment arm (with a systolic target of less than 120 mm Hg) with the outcomes in a standard-treatment arm (with a target of less than 140 mm Hg). Overall the study was neutral for the main outcome measure (the risk of major cardiovascular events), which argues in favour of moving the systolic target to less than 140 mm Hg. However, the study did not test a target of less than 130 mm Hg, which is the current recommendation for people with diabetes. Furthermore, the study had been powered for an event rate double the 2.09% found in the standard-treatment arm, so the study may have been underpowered. This argues in favour of leaving the systolic recommendation at the current target of less than 130 mm Hg. Patients in the intensive-treatment arm had a significantly lower risk of stroke (47% less) associated with more hypotension, bradycardia and hyperkalemia; this finding argues for a target BP of less than 120 mm Hg if the goal is to prevent stroke.
Overall, the ACCORD trial raised questions about the relative benefits and risks of achieving lower systolic BP targets. It may be that lowering BP in patients with diabetes has more complex effects than previously thought and the target for the systolic BP may depend on what end point we are trying to prevent. Furthermore, intensive BP reduction in patients with hypertension and diabetes may result in a trade-off between stroke reduction and an increased risk of drug-related adverse effects.
Clinical vignette revisited
The patient’s BP is currently still above target. You consider which medications to add, given his history of heart failure and previous myocardial infarction.
Treatment of patients with hypertension and systolic dysfunction
Recommendation
Aldosterone antagonists (mineralocorticoid-receptor antagonists) may be added for patients with hypertension and systolic dysfunction.
As part of an ongoing effort to harmonize related recommendations between guidelines groups, CHEP and the Canadian heart failure guidelines group developed a harmonized recommendation for managing patients with systolic heart failure and hypertension.26 The RALES,27 EPHESUS28 and EMPHASIS-HF29 studies all demonstrated reductions in the rates of death and hospital admission for heart failure in patients with systolic dysfunction who were receiving mineralocorticoid-receptor antagonists. Over 60% of the patients in these studies had a diagnosis of hypertension, and BP was controlled in most patients at study entry. On the basis of the results of these studies, an aldosterone antagonist is recommended for patients with a history of hypertension and recent admission to hospital for a cardiovascular condition, acute myocardial infarction, elevated B-type natriuretic peptide or N-terminal pro B-type natriuretic peptide levels, or New York Heart Association class II to IV symptoms. Because of the risk of hyperkalemia, when an aldosterone antagonist is added to inhibitors of the renin–angiotensin–aldosterone system, appropriate monitoring for hyperkalemia is recommended. The harmonized recommendation does not specify the period within which potassium should be measured after this therapy is started; it depends on the risk for an individual patient (e.g., potassium should be measured within 1 week for individuals at very high risk). The Canadian heart failure guidelines recommend that eplerenone not be used in patients with an eGFR of less than 30 mL·min–1·1.73 m–2 or a serum potassium level of more than 5.0 mmol/L.26 The incidence of hyperkalemia in the EPHESUS and EMPHASIS-HF studies was significantly higher in patients treated with eplerenone than in patients who received a placebo (Table 1).
Table 1.
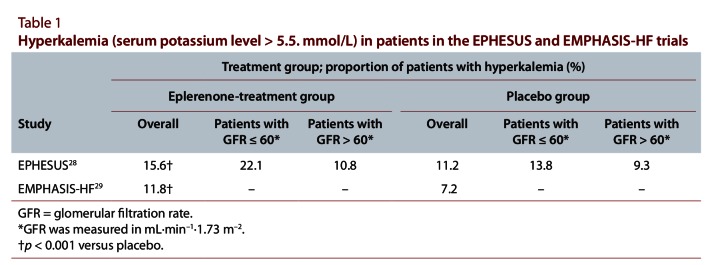
Hyperkalemia in patients in the EPHESUS and EMPHASIS-HF trials
The adverse event rate for gynecomastia was 0.7% for patients receiving eplerenone in the EMPHASIS-HF trial, which was not significantly different from that in the placebo arm in either study.30 In the RALES study, gynecomastia was reported in 10% of men receiving an average dose of 26 mg spironolactone per day, whereas it was 1% in the placebo arm.27
Resolution of clinical vignette
After discussion with the patient, you start him on spironolactone. You arrange electrolyte testing for potassium for the following week and counsel him about the potential for gynecomastia. His potassium level is 4.6 mmol/L a week later, and his follow-up office BP is 138/88 mm Hg. You also provide him with patient education on lifestyle adjustments to reduce his sodium intake, weight and waist circumference. The DASH diet is not recommended for this patient because of its high potassium content.31
Acknowledgments
This clinical practice review is based on CHEP’s annual clinical practice recommendations consensus conference, which was held at the annual congress of Hypertension Canada, 1 Oct. 2011.
Biographies
Sheldon W. Tobe, MD, is chair of the Canadian Hypertension Education Program and associate professor in the Division of Nephrology, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Ontario.
Luc Poirier, MSc, is chair of the Recommendations Task Force for the Canadian Hypertension Education Program and a senior pharmacist in the Hypertension Unit and Pharmacy Department, Centre hospitalier universitaire de Québec, Université Laval, Sainte-Foy, Québec.
Guy Tremblay, MD, is professor of medicine, Centre hospitalier universitaire de Québec, Hôpital du Saint-Sacrement, and cardiovascular consultant, Direction de la santé publique de la Capitale-Nationale, Sainte-Foy, Québec.
Patrice Lindsay, PhD, is director of performance and standards, Canadian Stroke Network, an adjunct professor at the Institute of Health Policy, Management and Evaluation, University of Toronto, and a member of the Canadian Task Force on Preventive Health Care, Toronto, Ontario.
Debra Reid, PhD, is a registered dietitian and national manager of the Strengthening the Forces health promotion program in the Department of National Defence, Ottawa, Ontario.
Norman R.C. Campbell, MD, is a professor of medicine, of community health sciences and of physiology and pharmacology at the University of Calgary, a member of the Libin Cardiovascular Institute of Alberta, and CIHR Canada Chair in Hypertension Prevention and Control in Calgary, Alberta.
Nadia Khan, MD, is an associate professor of medicine and clinician scientist at the Centre for Health Evaluation and Outcomes Sciences, Division of General Internal Medicine, University of British Columbia, Vancouver, British Columbia.
Robert R. Quinn, MD, PhD, is an adjunct assistant professor in the Division of Nephrology, University of Calgary, Calgary, Alberta.
Doreen Rabi is an assistant professor in the Departments of Medicine, Community Health and Cardiovascular Sciences at the University of Calgary, Calgary, Alberta.
Footnotes
Competing interests: Sheldon Tobe has received unrestricted grant support from Servier and has participated in contract research with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Merck, Novartis, Pfizer, Sanofi-aventis and Takeda. Luc Poirier has served on the advisory boards of Novartis Canada and Janssen Canada and has participated in research protocols with Takeda, Novartis and Merck. Norman Campbell received travel support in 2010 from Boehringer Ingelheim to attend meetings on hypertension.
Funding source: The Canadian Hypertension Education Program’s consensus conference and guidelines were funded by Hypertension Canada, which receives funds from multiple industry sources and the Public Health Agency of Canada.
Contributors: All of the authors listed have contributed substantially to the conception and design of this article, have helped to revise it and have contributed important intellectual content. All of the authors have approved the final version of the manuscript. The first draft of this manuscript was written by Sheldon Tobe, who is acting as guarantor and is responsible for the integrity of the work as a whole, from inception through to publication.
References
- 1.Robitaille Cynthia, Dai Sulan, Waters Chris, Loukine Lidia, Bancej Christina, Quach Susan, Ellison Joellyn, Campbell Norman, Tu Karen, Reimer Kim, Walker Robin, Smith Mark, Blais Claudia, Quan Hude. Diagnosed hypertension in Canada: incidence, prevalence and associated mortality. CMAJ. 2011 Nov 21;184(1):e49–e56. doi: 10.1503/cmaj.101863. http://pubmedcentralcanada.ca/pmcc/articles/pmid/22105752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odden Michelle C, Coxson Pamela G, Moran Andrew, Lightwood James M, Goldman Lee, Bibbins-Domingo Kirsten. The impact of the aging population on coronary heart disease in the United States. Am J Med. 2011;124(9):827–833. doi: 10.1016/j.amjmed.2011.04.010. http://pubmedcentralcanada.ca/pmcc/articles/pmid/21722862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkins Kathryn, Campbell Norman R C, Joffres Michel R, McAlister Finlay A, Nichol Marianne, Quach Susan, Johansen Helen L, Tremblay Mark S. Blood pressure in Canadian adults. Health Rep. 2010;21(1):37–46. http://www.scholaruniverse.com/ncbi-linkout?id=20426225. [PubMed] [Google Scholar]
- 4.McAlister F A, Feldman R D, Wyard K, Brant R, Campbell NR CHEP Outcomes Research Task Force. The impact of the Canadian Hypertension Education Programme in its first decade. Eur Heart J. 2009;30(12):1434–1439. doi: 10.1093/eurheartj/ehp192. http://eurheartj.oxfordjournals.org/cgi/doi/10.1093/eurheartj/ehp192. [DOI] [PubMed] [Google Scholar]
- 5.Campbell Norm R C, Brant Rollin, Johansen Helen, Walker Robin L, Wielgosz Andreas, Onysko Jay, Gao Ru-Nie, Sambell Christie, Phillips Stephen, McAlister Finlay A. Canadian Hypertension Education Program Outcomes Research Task Force. Increases in antihypertensive prescriptions and reductions in cardiovascular events in Canada. Hypertension. 2008 Dec 29;53(2):128–134. doi: 10.1161/HYPERTENSIONAHA.108.119784. http://www.scholaruniverse.com/ncbi-linkout?id=19114646. [DOI] [PubMed] [Google Scholar]
- 6.Heidenreich Paul A, Trogdon Justin G, Khavjou Olga A, Butler Javed, Dracup Kathleen, Ezekowitz Michael D, Finkelstein Eric A, Hong Yuling, Johnston S C, Khera Amit, Lloyd-Jones Donald M, Nelson Sue A, Nichol Graham, Orenstein Diane, Wilson Peter W F, Woo Y J. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. http://www.scholaruniverse.com/ncbi-linkout?id=21262990. [DOI] [PubMed] [Google Scholar]
- 7.Arthur H M, Suskin N, Bayley M, Fortin M, Howlett J, Heckman G, Lewanczuk R. The Canadian Heart Health Strategy and Action Plan: cardiac rehabilitation as an exemplar of chronic disease management. Can J Cardiol. 2010;26(1):37–41. doi: 10.1016/S0828-282X(10)70336-6. http://linkinghub.elsevier.com/retrieve/pii/S0828282X10703366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kearney Patricia M, Whelton Megan, Reynolds Kristi, Muntner Paul, Whelton Paul K, He Jiang. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. http://www.scholaruniverse.com/ncbi-linkout?id=15652604. [DOI] [PubMed] [Google Scholar]
- 9.Vasan Ramachandran S, Beiser Alexa, Seshadri Sudha, Larson Martin G, Kannel William B, D'Agostino Ralph B, Levy Daniel. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA. 2002;287(8):1003–1010. doi: 10.1001/jama.287.8.1003. http://www.scholaruniverse.com/ncbi-linkout?id=11866648. [DOI] [PubMed] [Google Scholar]
- 10.Bobrie Guillaume, Chatellier Gilles, Genes Nathalie, Clerson Pierre, Vaur Laurent, Vaisse Bernard, Menard Joël, Mallion Jean-Michel. Cardiovascular prognosis of “masked hypertension” detected by blood pressure self-measurement in elderly treated hypertensive patients. JAMA. 2004 Mar 17;291(11):1342–1349. doi: 10.1001/jama.291.11.1342. http://www.nlm.nih.gov/medlineplus/highbloodpressure.html. [DOI] [PubMed] [Google Scholar]
- 11.Warren Roderick E, Marshall Tom, Padfield Paul L, Chrubasik Sigrun. Variability of office, 24-hour ambulatory, and self-monitored blood pressure measurements. Br J Gen Pract. 2010;60(578):675–680. doi: 10.3399/bjgp10X515403. http://pubmedcentralcanada.ca/pmcc/articles/pmid/20849695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers Martin G, Godwin Marshall, Dawes Martin, Kiss Alexander, Tobe Sheldon W, Grant F C, Kaczorowski Janusz. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ. 2011 Feb 7;d342 doi: 10.1136/bmj.d286. http://pubmedcentralcanada.ca/pmcc/articles/pmid/21300709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabi Doreen M, Daskalopoulou Stella S, Padwal Raj S, Khan Nadia A, Grover Steven A, Hackam Daniel G, Myers Martin G, McKay Donald W, Quinn Robert R, Hemmelgarn Brenda R, Cloutier Lyne, Bolli Peter, Hill Michael D, Wilson Thomas, Penner Brian, Burgess Ellen, Lamarre-Cliché Maxime, McLean Donna, Schiffrin Ernesto L, Honos George, Mann Karen, Tremblay Guy, Milot Alain, Chockalingam Arun, Rabkin Simon W, Dawes Martin, Touyz Rhian M, Burns Kevin D, Ruzicka Marcel, Campbell Norman R C, Vallée Michel, Prasad G V R, Lebel Marcel, Campbell Tavis S, Lindsay M P, Herman Robert J, Larochelle Pierre, Feldman Ross D, Arnold J M O, Moe Gordon W, Howlett Jonathan G, Trudeau Luc, Bacon Simon L, Petrella Robert J, Lewanczuk Richard, Stone James A, Drouin Denis, Boulanger Jean-Martin, Sharma Mukul, Hamet Pavel, Fodor George, Dresser George K, Carruthers S G, Pylypchuk George, Gilbert Richard E, Leiter Lawrence A, Jones Charlotte, Ogilvie Richard I, Woo Vincent, McFarlane Philip A, Hegele Robert A, Poirier Luc, Tobe Sheldon W Canadian Hypertension Education Program. The 2011 Canadian Hypertension Education Program recommendations for the management of hypertension: blood pressure measurement, diagnosis, assessment of risk, and therapy. Can J Cardiol. 2011;27(4):415–433. doi: 10.1016/j.cjca.2011.03.015. http://www.scholaruniverse.com/ncbi-linkout?id=21801975. [DOI] [PubMed] [Google Scholar]
- 14.Jafar Tazeen H, Stark Paul C, Schmid Christopher H, Landa Marcia, Maschio Giuseppe, de Jong Paul E, de Zeeuw Dick, Shahinfar Shahnaz, Toto Robert, Levey Andrew S. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139(4):244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. http://www.scholaruniverse.com/ncbi-linkout?id=12965979. [DOI] [PubMed] [Google Scholar]
- 15.Klahr S. Primary and secondary results of the Modification of Diet in Renal Disease study. Miner Electrolyte Metab. 1996;22(1–3):138–142. [PubMed] [Google Scholar]
- 16.Sarnak Mark J, Greene Tom, Wang Xuelei, Beck Gerald, Kusek John W, Collins Allan J, Levey Andrew S. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease Study. Ann Intern Med. 2005;142(5):342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. http://www.scholaruniverse.com/ncbi-linkout?id=15738453. [DOI] [PubMed] [Google Scholar]
- 17.Peterson J C, Adler S, Burkart J M, Greene T, Hebert L A, Hunsicker L G, King A J, Klahr S, Massry S G, Seifter J L. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995 Nov 15;123(10):754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. http://www.scholaruniverse.com/ncbi-linkout?id=7574193. [DOI] [PubMed] [Google Scholar]
- 18.Wright Jackson T, Jr, Bakris George, Greene Tom, Agodoa Larry Y, Appel Lawrence J, Charleston Jeanne, Cheek DeAnna, Douglas-Baltimore Janice G, Gassman Jennifer, Glassock Richard, Hebert Lee, Jamerson Kenneth, Lewis Julia, Phillips Robert A, Toto Robert D, Middleton John P, Rostand Stephen G. African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002 Nov 20;288(19):2421–2431. doi: 10.1001/jama.288.19.2421. http://jama.ama-assn.org/cgi/doi/10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 19.Gassman Jennifer J, Greene Tom, Wright Jackson T, Jr, Agodoa Lawrence, Bakris George, Beck Gerald J, Douglas Janice, Jamerson Ken, Lewis Julia, Kutner Michael, Randall Otelio S, Wang Shin-Ru. Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK) J Am Soc Nephrol. 2003;14(7 Suppl 2) doi: 10.1097/01.asn.0000070080.21680.cb. http://pubmedcentralcanada.ca/pmcc/articles/pmid/12819322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appel Lawrence J, Wright Jackson T, Jr, Greene Tom, Agodoa Lawrence Y, Astor Brad C, Bakris George L, Cleveland William H, Charleston Jeanne, Contreras Gabriel, Faulkner Marquetta L, Gabbai Francis B, Gassman Jennifer J, Hebert Lee A, Jamerson Kenneth A, Kopple Joel D, Kusek John W, Lash James P, Lea Janice P, Lewis Julia B, Lipkowitz Michael S, Massry Shaul G, Miller Edgar R, Norris Keith, Phillips Robert A, Pogue Velvie A, Randall Otelio S, Rostand Stephen G, Smogorzewski Miroslaw J, Toto Robert D, Wang Xuelei AASK Collaborative Research Group. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010 Sep 2;363(10):918–929. doi: 10.1056/NEJMoa0910975. http://www.nejm.org/doi/abs/10.1056/NEJMoa0910975?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggenenti Piero, Perna Annalisa, Loriga Giacomina, Ganeva Maria, Ene-Iordache Bogdan, Turturro Marta, Lesti Maria, Perticucci Elena, Chakarski Ivan N, Leonardis Daniela, Garini Giovanni, Sessa Adalberto, Basile Carlo, Alpa Mirella, Scanziani Renzo, Sorba Gianbattista, Zoccali Carmine, Remuzzi Giuseppe. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365(9463):939–946. doi: 10.1016/S0140-6736(05)71082-5. http://linkinghub.elsevier.com/retrieve/pii/S0140673605710825. [DOI] [PubMed] [Google Scholar]
- 22.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty A M, Kjeldsen S E, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder R E, Struijker Boudier H A J, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen S D, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano J L, Kjeldsen S E, Erdine S, Narkiewicz K, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Cifkova R, Dominiczak A, Fagard R, Heagerty A M, Laurent S, Lindholm L H, Mancia G, Manolis A, Nilsson P M, Redon J, Schmieder R E, Struijker-Boudier H A J, Viigimaa M, Filippatos G, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Kiowski W, Lip G, Mallion J M, Manolis A J, Nilsson P M, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Viigimaa M, Waeber B, Williams B, Zamorano J L. 2007 guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25(6):1105–1187. doi: 10.1093/eurheartj/ehm236. http://www.scholaruniverse.com/ncbi-linkout?id=17562668. [DOI] [PubMed] [Google Scholar]
- 23.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Part 7: Stratification of risk for progression of kidney disease and development of cardiovascular disease. Am J Kidney Dis. 2002;39(2 Suppl 1) [PubMed] [Google Scholar]
- 24.National Collaborating Centre for Chronic Conditions. NICE Clinical Guideline 73. London (UK): Royal College of Physicians; 2008. Chronic kidney disease: early identification and management of chronic kidney disease in adults in primary and secondary care. [PubMed] [Google Scholar]
- 25.Hansson Lennart, Zanchetti Alberto, Carruthers S George, Dahlöf Björn, Elmfeldt Dag, Julius Stevo, Ménard Joël, Rahn Karl H, Wedel Hans, Westerling Sten HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351(9118):1755–1762. doi: 10.1016/S0140-6736(98)04311-6. http://linkinghub.elsevier.com/retrieve/pii/S0140673698043116. [DOI] [PubMed] [Google Scholar]
- 26.McKelvie Robert S, Moe Gordon W, Cheung Anson, Costigan Jeannine, Ducharme Anique, Estrella-Holder Estrellita, Ezekowitz Justin A, Floras John, Giannetti Nadia, Grzeslo Adam, Harkness Karen, Heckman George A, Howlett Jonathan G, Kouz Simon, Leblanc Kori, Mann Elizabeth, Meara Eileen, Rajda Miroslav, Rao Vivek, Simon Jessica, Swiggum Elizabeth, Zieroth Shelley, Arnold J, Malcolm O, Ashton Tom, D'Astous Michel, Dorian Paul, Haddad Haissam, Isaac Debra L, Leblanc Marie-Hélène, Liu Peter, Sussex Bruce, Ross Heather J. The 2011 Canadian Cardiovascular Society heart failure management guidelines update: focus on sleep apnea, renal dysfunction, mechanical circulatory support, and palliative care. Can J Cardiol. 2011;27(3):319–338. doi: 10.1016/j.cjca.2011.03.011. http://linkinghub.elsevier.com/retrieve/pii/S0828282X11002212. [DOI] [PubMed] [Google Scholar]
- 27.Pitt B, Zannad F, Remme W J, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999 Sep 2;341(10):709–717. doi: 10.1056/NEJM199909023411001. http://www.nejm.org/doi/abs/10.1056/NEJM199909023411001?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov. [DOI] [PubMed] [Google Scholar]
- 28.Pitt Bertram, Bakris George, Ruilope Luis M, DiCarlo Lorenzo, Mukherjee Robin EPHESUS Investigators. Serum potassium and clinical outcomes in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy. Circulation. 2008;118(16):2008–118. doi: 10.1161/CIRCULATIONAHA.108.778811. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=18824643. [DOI] [PubMed] [Google Scholar]
- 29.Zannad Faiez, McMurray John J V, Krum Henry, van Veldhuisen Dirk J, Swedberg Karl, Shi Harry, Vincent John, Pocock Stuart J, Pitt Bertram. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21. doi: 10.1056/NEJMoa1009492. http://www.nejm.org/doi/abs/10.1056/NEJMoa1009492?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov. [DOI] [PubMed] [Google Scholar]
- 30.Pitt B. Effect of aldosterone blockade in patients with systolic left ventricular dysfunction: implications of the RALES and EPHESUS studies. Mol Cell Endocrinol. 2004;217(1-2):53–58. doi: 10.1016/j.mce.2003.10.009. http://linkinghub.elsevier.com/retrieve/pii/S0303720703003800. [DOI] [PubMed] [Google Scholar]
- 31.Sacks F M, Svetkey L P, Vollmer W M, Appel L J, Bray G A, Harsha D, Obarzanek E, Conlin P R, Miller E R, 3rd, Simons-Morton D G, Karanja N, Lin P H. DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001 Jan 4;344(1):3–10. doi: 10.1056/NEJM200101043440101. http://www.nejm.org/doi/abs/10.1056/NEJM200101043440101?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov. [DOI] [PubMed] [Google Scholar]


