Abstract
Objective
To assess the feasibility of developing a Combined Clinical and Pharmacogenetic Predictive Test, comprised of multiple single nucleotide polymorphisms (SNPs) that is associated with poor bronchodilator response (BDR).
Methods
We genotyped SNPs that tagged the whole genome of the parents and children in the Childhood Asthma Management Program (CAMP) and implemented an algorithm using a family-based association test that ranked SNPs by statistical power. The top eight SNPs that were associated with BDR comprised the Pharmacogenetic Predictive Test. The Clinical Predictive Test was comprised of baseline forced expiratory volume in 1 s (FEV1). We evaluated these predictive tests and a Combined Clinical and Pharmacogenetic Predictive Test in three distinct populations: the children of the CAMP trial and two additional clinical trial populations of asthma. Our outcome measure was poor BDR, defined as BDR of less than 20th percentile in each population. BDR was calculated as the percent difference between the prebronchodilator and postbronchodilator (two puffs of albuterol at 180 μg/puff) FEV1 value. To assess the predictive ability of the test, the corresponding area under the receiver operating characteristic curves (AUROCs) were calculated for each population.
Results
The AUROC values for the Clinical Predictive Test alone were not significantly different from 0.50, the AUROC of a random classifier. Our Combined Clinical and Pharmacogenetic Predictive Test comprised of genetic polymorphisms in addition to FEV1 predicted poor BDR with an AUROC of 0.65 in the CAMP children (n= 422) and 0.60 (n= 475) and 0.63 (n= 235) in the two independent populations. Both the Combined Clinical and Pharmacogenetic Predictive Test and the Pharmacogenetic Predictive Test were significantly more accurate than the Clinical Predictive Test (AUROC between 0.44 and 0.55) in each of the populations.
Conclusion
Our finding that genetic polymorphisms with a clinical trait are associated with BDR suggests that there is promise in using multiple genetic polymorphisms simultaneously to predict which asthmatics are likely to respond poorly to bronchodilators.
Keywords: asthma, bronchodilator response, personalized medicine, pharmacogenetic test, predictive medicine
Introduction
With the sequencing of the human genome [1,2], the falling costs of genomic technology, the availability of genome-wide association information [3], and the proliferation of studies linking genetic variants to treatment response [4], it seems almost certain that using individual genetic fingerprints to tailor medical regimens will be a reality in the near future [5]. Pharmacogenetics is the study of how genetic differences affect the variability in response to medications among individuals and many believe it will allow for ‘individualized therapy’ or ‘predictive medicine’ that is tailored to an individual to maximize the potential for therapeutic benefit while minimizing the risk of unwanted side effects. In 2001, Collins and McKusick [6] predicted that the pharmacogenomics approach for predicting drug responsiveness would be standard practice for many disorders and drugs by 2020. At present, only a handful of pharmacogenetic tests comprised of single nucleotide polymorphisms (SNPs) exist. These include CYP2C9 and VKORC1 testing for warfarin [7,8] or HLA-B*5701 testing for Abacavir toxicity [9]. However, few tests have been developed for medications for asthma.
Asthma affects up to 300 million people of all ages in the world and 15 million people in the United States [9,10]. The primary reliever medications used in asthma are rapid acting β2-agonists, which relax the bronchial smooth muscle by activating β2-adrenergic receptors. Beta agonists are used virtually by every patient with asthma because they are the most effective medications for the treatment of acute asthma, yet many of these patients may not benefit from them [11]. Clinical factors such as baseline lung function, defined as forced expiratory volume in 1 s (FEV1) before the administration of a bronchodilator, are known to be associated with consistent bronchodilator response (BDR) [12,13]. FEV1, a strong index of the degree of airways obstruction, is associated with asthma symptoms and health care utilization and is an important part of the National Asthma Education and Prevention Program guidelines for asthma [14]. Nevertheless, it is unknown whether multiple genetic factors could improve prediction of poor BDR. Several studies have shown associations between SNPs and BDR in patients with asthma [15–18]. The most frequently reported polymorphisms related to BDR are SNPs in the β2-adrenergic receptor (ADRB2) gene, corresponding to the receptor’s amino acid positions 16 (Arg16/Gly16) and 27 (Gln27/Glu27), but they seem to play only a small role in prediction of BDR [19]. The prediction of treatment responses in complex phenotypes of drug–response such as BDR is complicated because it is likely to be influenced by multiple coding and regulatory variants in multiple genes [20]. No predictive tests have been developed yet for complex drug treatment, but previous studies have evaluated whether multiple SNPs can be used simultaneously to predict the risk of diabetes [21–23].
The objectives of this study were: to assess the feasibility of developing a Combined Clinical and Pharmacogenetic Predictive Test, comprised of multiple SNPs that predicts poor BDR. We chose to study the outcome BDR because it is a direct measure of response to bronchodilators and we chose to use a dichotomous outcome measure rather than a continuous measure because a useful test in the clinical setting will differentiate the poorest responders from normal responders.
Methods
Study populations and test development
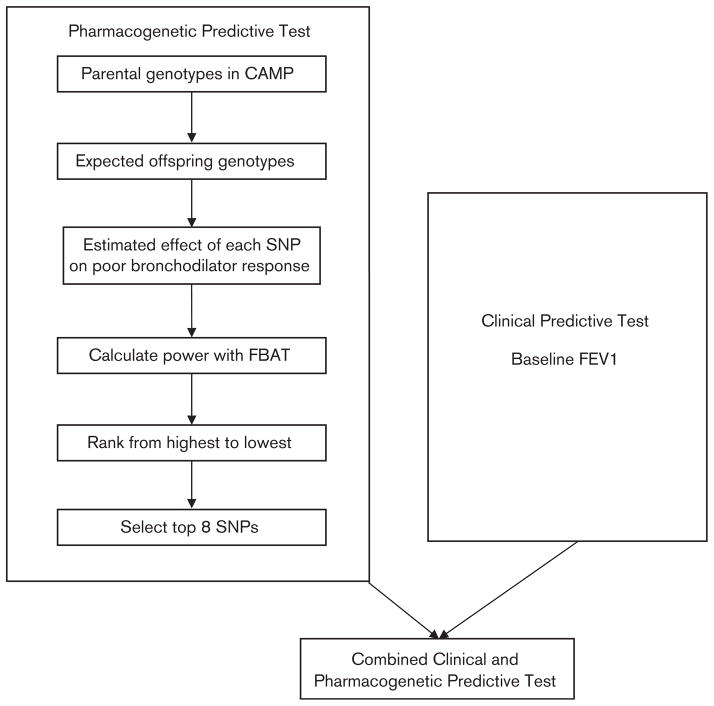
DNA samples from three clinical trials studying asthma medications were included in this study. We used whole genome data from the parents of 422 Caucasian probands in the Childhood Asthma Management Program (CAMP) to select the SNPs. The algorithm for development of our predictive tests is depicted in Fig. 1. To select the eight SNPs for our predictive test, we used the Pedigree Based Association Test (PBAT screen, Golden Helix, Inc., Bozeman, Montana, USA), which selects the top powered SNPs using only genetic data from the parents in CAMP [24–26]. The PBAT screen is a software package with a unique set of tools for complex family-based association tests at the genome-wide level, which calculated power estimates for each SNP [24–26]. Through the use of the conditional mean model, these power estimates are calculated in a way that is statistically independent of the offspring genetic data that was used as our first population to assess the predictive test [27,28]. First, the conditional mean model uses parental genotype data to infer offspring genotypes. This information is then used as the predictor variable in a regression model and the offspring phenotype is used as the response variable. The estimated slope from this regression equation is the genetic effect estimate that is then used, along with the specified genetic model and the allele frequency, to calculate the power that each SNP has to detect the association of interest. These power estimates were used to rank order the SNPs. The top eight SNPs were selected for our predictive test and were not in linkage disequilibrium with each other at r2 greater than 0.8. We included eight SNPs in our Pharmacogenetic Predictive Test because from a statistical standpoint, we wanted to balance the inclusion of as much genetic information as possible with the number of patients in our populations. If we had included more SNPs in our test, we would have risked overfitting our model such that our predictive accuracy could have been falsely high. We chose to include eight SNPs based on having 81 patients who had poor BDR in CAMP to have 10 patients per parameter. Previous analyses have suggested that with 10 events (or cases) per parameter, reseachers are at less risk of overestimation or underestimation [29]. Details of the eight SNPs are presented in Table 1. We accounted for whether each of the eight SNPs achieved the high power and P value by additive, dominant, or recessive model. These eight SNPs (listed in Table 1) along with prebronchodilator FEV1 comprised our Combined Clinical and Pharmacogenetic Predictive Test.
Fig. 1.
Algorithm for development of the predictive tests. CAMP, Childhood Asthma Management Program; FBAT, family-based association tests; FEV1, forced expiratory volume in 1 s; SNP, single nucleotide polymorphism.
Table 1.
Selection of single nucleotide polymorphisms for Pharmacogenetic Predictive Test
| Power rank from screen | SNP | Chromosome/location | Mode of inheritance | Informative families | Family-based association test, P value |
|---|---|---|---|---|---|
| 1 | rs10956264 | Chr8:126,899,990 | Recessive | 85 | 0.13 |
| 2 | rs1348482 | Chr8:126,872,910 | Recessive | 85 | 0.068 |
| 3 | rs2831845 | Chr21:28,772,450 | Dominant | 111 | 0.094 |
| 4 | rs17772026a | Chr4:8,048,550 | Recessive | 75 | 0.0029 |
| 5 | rs7841870 | Chr8:126,868,190 | Recessive | 85 | 0.068 |
| 6 | rs2223098 | Chr21:28,774,785 | Dominant | 111 | 0.083 |
| 7 | rs7155790 | Chr14:22,659,450 | Additive | 116 | 0.096 |
| 8 | rs12511017 | Chr4:36418770 | Dominant | 97 | 0.15 |
Genome-wide SNPs were screened using parental genotypes in CAMP to find those likely to predict poor bronchodilator response. The top eight SNPs from the screening step (ranked by power from most likely to least likely) are shown.
CAMP, Childhood Asthma Management Program; SNP, single nucleotide polymorphism.
Located in ABLIM2 gene (actin binding LIM protein family, member 2).
In three separate populations, we evaluated the performance of our three predictive tests of poor BDR: (i) a Clinical Predictive Test using FEV1 only, (ii) a Pharmacogenetic Predictive Test using the eight SNPs only and (iii) a Combined Clinical and Pharmacogenetic Predictive Test, which included FEV1 and the eight SNPs in predicting BDR. Our first test population comprised of the CAMP children, which included genetic information from 422 patients. We used the BDR at the baseline visit as our main outcome measure for all patients in all three treatment arms before patients had begun controller medications. The second population was a cohort of 235 patients from a trial conducted by the American Lung Association Asthma Clinical Research Centers, the Effectiveness of Low Dose Theophylline as Add-on Treatment in Asthma (LODO) trial; details of this trial had been previously published [30]. Our third population, which will be referred to as the Asthma Trial, was composed of 475 patients with asthma who were enrolled in an asthma medication trial conducted by Sepracor, Inc. [28]. This study was approved by the review committees of CAMP, LODO, and the Asthma Trial, respectively. In all the three populations, BDR was based on prebronchodilator and postbronchodilator measurements. After two puffs (180 μg/puff) of albuterol by metered dose inhaler with spacer were administered, at least 10min elapsed before the postbronchodilator spirometry was performed. BDR was calculated as the percent difference between the prebronchodilator and postbronchodilator (two puffs of albuterol at 180 μg/puff) FEV1 value [BDR=100× (post FEV1=pre FEV1/pre FEV1)].
Single nucleotide polymorphism genotyping
SNPs in CAMP were genotyped using the Infinium HumanHap550 genotyping at Illumina (San Diego, California, USA). Genotyping quality was evaluated using the program PLINK (V1.01 http://pngu.mgh.harvard.edu/purcell/plink). SNPs with low Illumina gencall scores, poor completion rates, or four or more parent-offspring genotyped inconsistencies were dropped. Using the Basic Local Alignment Search Tool (National Center for Biotechnology Information, National Library of Medicine, Bethesda, Maryland, USA), SNPs were further limited to those whose flanking sequences were reliably mapped to unique autosomal locations in the hg17 reference genome sequence. Mitochondrial and sex-linked markers were not included.
Statistical methodology
We chose BDR as our main outcome measure because it is a direct measure of response to bronchodilators. We chose to use a dichotomous outcome measure rather than a continuous measure because a useful test in the clinical setting will differentiate the poorest responders from normal responders. As we were limited to populations from clinical trials with different inclusion and exclusion criteria, the range of BDR was different in each population. Thus we defined poor response as less than 20 percentile in each cohort. We chose to use 20 percentile as a cut-point because at least 20% of patients who are taking β-agonists in asthma clinical trials experience exacerbations from asthma, suggesting poor response to β-agonists [31,32]. Using SAS Version 9.1 (SAS Institute, Cary, North Carolina, USA), logistic regression models for BDR were calculated using CAMP data corresponding to (i) the Clinical Predictive Test, (ii) the Pharmacogenetic Predictive Test and (iii) the Combined Clinical and Pharmacogenetic Predictive Test. Similarly, we modeled the association of each test in LODO and the Asthma Trial using separate logistic models for each trial. The models were used to calculate predicted probabilities of having poor BDR for patients in each population.
The performance of predictive models was evaluated by calculating receiver operating characteristic (ROC) curves. Predictive accuracy was measured as the area under the ROC curve (AUROC), and significance for this accuracy was obtained by comparing the classification ability of models obtained from random classification. The standard error for the AUROCs and for the difference between AUROCs of two curves was estimated using the nonparametric asymptotic method, which is commonly used for this purpose [33,34]. All ROC analyses were performed in R [35].
Results
Table 2 shows the baseline characteristics of the patients in the three asthma populations. The mean age of our three populations differed greatly. CAMP was a pediatric population with a mean age of 8.7 years and the mean ages of LODO and the Asthma Trial were 41 and 32 years, respectively. The mean BDR for the Asthma Trial was 40%, which is a reflection of the inclusion criteria of BDR greater than 15%. The 20th percentile for BDR was 3% for CAMP and 1.7% for LODO, whereas it was significantly higher for the Asthma Trial at 23%. In the CAMP population, 81 patients were classified as having poor BDR, in the Asthma Trial, 98 patients were classified as having poor response, and in LODO, 61 patients were poor responders.
Table 2.
Baseline characteristics of participants in the three Asthma Trials
| CAMP (n = 4) | LODO (n = 235) | Asthma Triala (n = 475) | P valueb | |
|---|---|---|---|---|
| Age in years (standard deviation) (range) | 8.7 (2.1) (5.2–13.2) | 40.8 (14.1) (15–76) | 32.0 (13.6) (12–80) | < 0.0001 |
| Race/ethnicity | < 0.0001 | |||
| Caucasian | 100% (422) | 71% (157) | 99% (467) | |
| Black | 0% (0) | 29% (63) | 0.8% (4) | |
| Latino | 0% (0) | 0% (0) | 0.4% (2) | |
| Sex | < 0.0001 | |||
| Male | 63% (266) | 26% (57) | 50% (238) | |
| Female | 37% (156) | 74% (163) | 50% (236) | |
| FEV1, mean (SD) | 1.6 (0.46) | 2.47 (0.75) | 2.2 (0.48) | < 0.0001 |
| Bronchodilator response, mean (SD) | 10.8% (10.3%) | 9.4% (11.5) | 40% (21%) | < 0.0001 |
| 20th percentile bronchodilator response | 4% | 1.7% | 23% | Not applicable |
CAMP, Childhood Asthma Management Program; FEV1, forced expiratory volume in 1 s; LODO, Effectiveness of Low Dose Theophylline as Add-on Treatment in Asthma Trial; SD, standard deviation.
Medication trial conducted by Sepracor, Inc.
P values calculated using t-tests for continuous variables and χ2 for categorical variables.
In CAMP, the AUROC for the Clinical Predictive Test (prebronchodilator FEV1) was 0.55, whereas the AUROC for the Combined Clinical and Pharmacogenetic Predictive Test was 0.65 (Fig. 2a), and the difference between these AUROCs was statistically significant (P=0.038). The AUROC results for LODO and the Asthma Trial were also significantly higher for the Combined Clinical and Pharmacogenetic Predictive Test compared with the Clinical Predictive Test (Fig. 2b and c). In all the three trials, the AUROCs for the Clinical Predictive Test were not significantly different than those of a random classifier, which would have no discriminatory value as a predictive test. The Pharmacogenetic Predictive Test, comprised of SNPs alone, consistently outperformed the clinical test (Table 3). The increased predictive accuracy of the Pharmacogenetic Predictive Test as compared with the clinical test is in improved specificity as shown in Table 3. We evaluated our tests stratified by race and found that the results were the same whether non-Caucasians were included or excluded from the analysis. The logistic regression models for each population are shown in Table 4. In Table 5, we present the results of sensitivity analyses, which predicted the lowest 10th or 30th percentile of poor BDR. The results are quite stable.
Fig. 2.
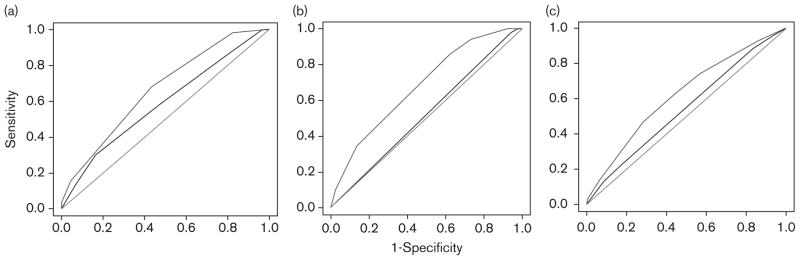
Receiver operating characteristic (ROC) curves of Clinical and Combined Clinical and Pharmacogenetic Predictive Tests. The middle lines correspond to the Clinical Predictive Test and the top lines correspond to the Combined Clinical and Pharmacogenetic Predictive Test. The bottom lines represent an area under the ROC (AUROC) of 0.50 which would represent a test that has no discriminatory value. (a) Childhood Asthma Management Program (CAMP). The AUROC is 0.55 for the Clinical Predictive Test and is significantly better at 0.65 for the Pharmacogenetic Predictive Test, P=0.038. (b) Effectiveness of Low-Dose Theophylline as Add-on Treatment in Asthma Trial. The AUROC is 0.44 for the Clinical Predictive Test and is significantly better at 0.63 for the Pharmacogenetic Predictive Test, P=0.0037. (c) Asthma Trial conducted by Sepracor. The AUROC is 0.52 for the Clinical Predictive Test and is significantly better at 0.61 for the Pharmacogenetic Predictive Test, P=0.033.
Table 3.
Accuracy of the Clinical, Pharmacogenetic, and Combined Clinical and Pharmacogenetic Predictive Test in three populations
| AUROC (standard error, P value) | Clinical Predictive Test FEV1 |
Pharmacogenetic Predictive Test 8 SNPs |
Combined Clinical and Pharmacogenetic Predictive Test FEV1 + 8 SNPs |
|---|---|---|---|
| CAMP | 0.55 (SE 0.042, P 0.12) Sensitivity: 96% Specificity: 4% |
0.64 (SE 0.037, P 0.00028) Sensitivity: 93% Specificity: 5% |
0.65 (SE 0.037, P 0.00053) Sensitivity: 78% Specificity: 12% |
| LODO | 0.44 (SE 0.045, P 0.90) Sensitivity: 100% Specificity: 0% |
0.60 (SE 0.047, P 0.013) Sensitivity: 86% Specificity: 13% |
0.63 (SE 0.043, P 0.0020) Sensitivity: 82% Specificity: 13% |
| Asthma Trial | 0.52 (SE 0.033, P 0.27) Sensitivity: 80% Specificity: 1% |
0.60 (SE 0.032, P 0.00085) Sensitivity: 64% Specificity: 24% |
0.60 (SE 0.032, P 0.00076) Sensitivity: 54% Specificity: 22% |
Standard errors (SE) are provided for each AUROC as well as the P value for the difference between each model and models obtained to random classification (AUROC 0.5). We also provide the sensitivity and specificity of each of the tests assuming a predicted probability of 0.70 as being the cut-off of a responder versus nonresponder. AUROC, area under the receiver operating characteristic curves; CAMP, Childhood Asthma Management Program; FEV1, forced expiratory volume in 1 s; LODO, Effectiveness of Low Dose Theophylline as Add-on Treatment in Asthma Trial; SNP, single nucleotide polymorphism.
Table 4.
Estimates and P values of logistic regression models for each of the Predictive Tests
| CAMP
|
LODO
|
Asthma Trial
|
||||
|---|---|---|---|---|---|---|
| Estimate | P value | Estimate | P value | Estimate | P value | |
| Clinical Predictive Test | ||||||
| FEV1 | − 0.63 | 0.02 | − 0.23 | 0.27 | − 0.11 | 0.65 |
| Pharmacogenetic Predictive Test | ||||||
| rs10956264 | − 0.14 | 0.78 | 1.31 | 0.038 | − 0.16 | 0.71 |
| rs1348482 | 0.32 | 0.041 | 5.18 | 0.96 | − 4.68 | 0.96 |
| rs2831845 | − 6.75 | 0.97 | 0.12 | 0.62 | 0.28 | 0.13 |
| rs17772026 | − 0.11 | 0.43 | 0.13 | 0.45 | − 0.12 | 0.34 |
| rs7841870 | 0.43 | 0.38 | − 6.44 | 0.96 | 4.85 | 0.96 |
| rs2223098 | 0.0089 | 0.95 | − 0.026 | 0.90 | 0.038 | 0.77 |
| rs7155790 | 0.12 | 0.55 | − 0.089 | 0.72 | 0.081 | 0.65 |
| rs12511017 | − 0.15 | 0.26 | − 0.22 | 0.21 | − 0.053 | 0.66 |
| Combined Clinical and Pharmacogenetic Predictive Test | ||||||
| FEV1 | − 0.60 | 0.033 | − 0.38 | 0.14 | − 0.23 | 0.36 |
| rs10956264 | 0.18 | 0.75 | 1.47 | 0.029 | − 0.16 | 0.70 |
| rs1348482 | 0.29 | 0.065 | 5.16 | 0.97 | − 4.79 | 0.96 |
| rs2831845 | − 6.75 | 0.97 | 0.09 | 0.73 | 0.28 | 0.12 |
| rs17772026 | − 0.11 | 0.44 | − 0.018 | 0.93 | − 0.12 | 0.33 |
| rs7841870 | 0.10 | 0.86 | − 6.58 | 0.96 | 4.96 | 0.96 |
| rs2223098 | −0.0050 | 0.97 | 0.0044 | 0.98 | 0.033 | 0.80 |
| rs7155790 | 0.13 | 0.52 | − 0.21 | 0.44 | 0.075 | 0.68 |
| rs12511017 | − 0.12 | 0.36 | − 0.23 | 0.25 | − 0.051 | 0.68 |
CAMP, Childhood Asthma Management Program; FEV1, forced expiratory volume in 1 s; LODO, Effectiveness of Low Dose Theophylline as Add-on Treatment in Asthma Trial.
Table 5.
This table shows the area under the receiver operating characteristic curve for the Combined Clinical and Pharmacogenetic Test in each clinical trial if we predict the lowest 10th, 20th, or 30th percentile of bronchodilator response
| Ft | CAMP | LODO | Asthma Trial |
|---|---|---|---|
| 10th percentile of BDR | 0.62 | 0.70 | 0.62 |
| 20th percentile of BDR | 0.65 | 0.63 | 0.60 |
| 30th percentile of BDR | 0.65 | 0.64 | 0.62 |
AUROC, area under the receiver operating characteristic curves; BDR, bronchodilator response; CAMP, Childhood Asthma Management Program; LODO, Effectiveness of Low Dose Theophylline as Add-on Treatment in Asthma Trial.
Discussion
We found that eight SNPs combined with FEV1 are associated with BDR in three separate populations. The addition of genetic information to clinical factors significantly increased the predictive accuracy of a predictive test. This proof of concept study suggests that there is promise in using multiple genetic polymorphisms simultaneously to predict poor BDR. Future development of Pharmacogenetic Predictive Tests that help clinicians determine which patients are poor responders to albuterol and should be treated with an alternative short-acting medication such as ipratropium could have great clinical benefits. This could be a first step in realizing the promise of the information gained from the Human Genome Project. Our study has several strengths. First, our study is one of the first to assess the association of multiple SNPs concurrently with complex drug treatment response in asthma. Previous studies have focused on examining associations between single SNPs and BDR. The results of the AUROCs are in the same range as previously published studies of a genetic test that predicts development of diabetes, which has an AUROC of 0.67 [36]. Second, our study is unique in that we studied three independent populations, suggesting these findings may be generalizable both to adults and children with varying levels of asthma severity. The populations are well described and carefully planned clinical trials with outcomes that were measured with trained research assistants. Third, our study suggests that using a whole genome approach in selecting SNPs based on linkage disequilibrium is a robust method for selecting SNPs and may be superior to a candidate gene approach. The vast majority of studies in the literature study polymorphisms related to the ADRB2 gene. In the CAMP children, the AUROC for eight SNPS in the ADRB2 gene in predicting poor BDR was 0.57, which is less than the test based on eight SNPs selected based on whole genome data, which was 0.64. The AUROC values for the Combined Clinical and Pharmacogenetic Predictive Test are quite stable regardless of whether the lowest 10th percentile or 30th percentile of BDR is being predicted, suggesting these eight SNPs are quite robust in predicting poor BDR.
Despite the strengths of our study, a few caveats deserve mention. The eight SNPs in our predictive test do not fully capture the genetic contribution to an individual’s BDR. The logistic regression models have small βestimates, the estimates have different directionality in each of the studies, and none of the individual SNPs are significantly associated with BDR in any of the studies. Nevertheless, our study suggests that these eight SNPs are associated with BDR in each of the clinical trials. We did not include more SNPs in our Pharmacogenetic Predictive Test to avoid problems with having too many predictors for a relatively small sample of cases and controls. Owing to the relatively small sample size of 422 children in our initial test population, CAMP, a Pharmacogenetic Predictive Test created with multiple variables poses the risk of overfitting the training data and not replicating in independent populations. However, we did find that the eight SNPs were associated with BDR in three separate populations. However, with a predictive accuracy between 0.61 and 0.65, our predictive test is not good enough to be used in clinical practice. A predictive accuracy of above 0.7 has been suggested as being the lowest acceptable threshold for stating that a predictive model has discriminatory value. We experimented with including more SNPs in our Pharmacogenetic Predictive Test and found that we reached a predictive accuracy of above 0.7 when more SNPs were included, but we did not present this data because we were concerned that this predictive accuracy was falsely high due to having too many predictors compared with the number of patients in our cohorts. A future solution would be to recruit a larger cohort of patients. Nevertheless, our study shows that the addition of genetic knowledge to a traditional clinical predictor can improve prediction of poor BDR.
Our three populations also had varying levels of lung function and BDR, yet our Combined Clinical and Pharmacogenetic Predictive Test was associated with which patients had the lowest 20th percentile of BDR in each of the populations. This ability to predict poor BDR in populations with varying levels of disease severity suggests using multiple polymorphisms to predict BDR may be generalizable to other populations of adults and children. Real-life populations and practices are comprised of asthma patients with varying degrees of lung function and BDR, and Pharmacogenetic Predictive Tests are more likely to be adopted if they predict poor BDR in many populations.
Although many investigators have suggested that genetics will be the cornerstone of future predictive medicine [6], progress to reach this end has been slow and some investigators have become skeptical. Our study suggests that personalized medicine to predict response to bronchodilators may be feasible, but focus for complex drug treatment response tests should be placed on assessment of multiple polymorphisms simultaneously. Advancement of the field of pharmacogenetics and predictive medicine will require the development and application of statistical techniques that evaluate dozens or hundreds of SNPs concurrently in relatively small sample sizes or study populations consisting of thousands of patients with asthma. In addition, functional studies to evaluate the role of these eight SNPs were beyond the scope of this study. One SNP was located in the ABLIM2, encoding actin binding LIM protein family, member 2, which is a novel gene to be associated with BDR [37]. The relationship of ABLIM2, which has the highest expression levels in muscle and neuronal tissue and may modulate transcription, to asthma or BDR is unclear [38]. Seven of the eight SNPs in our Pharmacogenetic Predictive Test are not located in genes, but this is not surprising given the finding that the original predictive SNPs for sensitivity to warfarin were actually SNPs in linkage disequilibrium for a promoter region SNP that alters VKORC1 transcription. Three of the SNPs in our test are located in close proximity to the TRIB1 gene, which is expressed in the lung. The TRIB1 product is a G-protein-coupled receptor induced protein and it is biologically plausible that TRIB1 could play a role in predicting BDR. Furthermore, studies are needed to elucidate interactions between SNPs because this study focused on the main effects of these eight SNPs. In summary, the addition of multiple genetic polymorphisms to clinical information is significantly associated with poor BDR in patients with asthma. A fundamental premise of pharmacogenetics is that genetic information will help predict whether a person will respond to a given medication. Our study suggests that this premise may be valid even for complex clinical drug responses such as the response to asthma medications. The expectation that the Human Genome Project and the HapMap Project will eventually lead to predictive medicine may be realistic.
Acknowledgments
This study was supported by NIH U01 HL65899, and P01 HL083069. The authors thank all CAMP patients for their ongoing participation in this study. They also acknowledge the CAMP investigators and research team, supported by NHLBI, for collection of CAMP Genetic Ancillary Study data. All study on data collected from the CAMP Genetic Ancillary Study was conducted at the Channing Laboratory of the Brigham and Women’s Hospital under appropriate CAMP policies and human patient’s protections. The CAMP Genetics Ancillary Study is supported by U01 HL075419, U01 HL65899, P01 HL083069, R01 HL086601, and T32 HL07427 from the National Heart, Lung and Blood Institute, National Institutes of Health. The authors acknowledge the American Lung Association (ALA) and the ALA’s Asthma Clinical Research Center’s investigators and research teams for use of LoDo data, with additional funding from HL071394 and HL074755 from the NHLBI, and Nemours Children’s Clinic. They also acknowledge Sepracor, Inc. for use of the Asthma Trial data.
References
- 1.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 2.Venter JC, Adams MD, Myers EW, Li PW, Mural MJ, Sutton GG, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 3.Christensen K, Murray JC. What genome-wide association studies can do for medicine. N Engl J Med. 2007;356:1094–1097. doi: 10.1056/NEJMp068126. [DOI] [PubMed] [Google Scholar]
- 4.Hunter DJ, Khoury MJ, Drazen JM. Letting the genome out of the bottle–will we get our wish? N Engl J Med. 2008;358:105–107. doi: 10.1056/NEJMp0708162. [DOI] [PubMed] [Google Scholar]
- 5.Tantisira K, Weiss S. The pharmacogenetics of asthma treatment. Curr Allergy Asthma Rep. 2009;9:10–17. doi: 10.1007/s11882-009-0002-9. [DOI] [PubMed] [Google Scholar]
- 6.Collins FS, McKusick VA. Implications of the human genome project for medical science. JAMA. 2001;285:540–544. doi: 10.1001/jama.285.5.540. [DOI] [PubMed] [Google Scholar]
- 7.Aithal GP, Day CP, Kesteven PJ, Daly AK. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1999;353:717–719. doi: 10.1016/S0140-6736(98)04474-2. [DOI] [PubMed] [Google Scholar]
- 8.D’Andrea G, D’Ambrosio RL, Di Perna P, Chetta M, Santacroce R, Brancaccio V, et al. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005;105:645–649. doi: 10.1182/blood-2004-06-2111. [DOI] [PubMed] [Google Scholar]
- 9.Vandekerckhove L, Blot S, Vogelaers D. Abacavir hypersensitivity. N Engl J Med. 2008;358:2514–2515. doi: 10.1056/NEJMc080541. [DOI] [PubMed] [Google Scholar]
- 10.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 11.Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br Med Bull. 2000;56:1054–1070. doi: 10.1258/0007142001903535. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, Litonjua AA, Tantisira KG, Fuhlbrigge AL, Szefler SJ, Strunk RC, et al. Clinical predictors and outcomes of consistent bronchodilator response in the childhood asthma management program. J Allergy Clin Immunol. 2008;122:921–928. e924. doi: 10.1016/j.jaci.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan DJ. Clinical pharmacokinetics of beta-agonists. Clin Pharmacokinet. 1990;18:270–294. doi: 10.2165/00003088-199018040-00002. [DOI] [PubMed] [Google Scholar]
- 14.Fuhlbrigge AL, Weiss ST, Kuntz KM, Paltiel AD. Forced expiratory volume in 1 s percentage improves the classification of severity among children with asthma. Pediatrics. 2006;118:e347–e355. doi: 10.1542/peds.2005-2962. [DOI] [PubMed] [Google Scholar]
- 15.Reihsaus E, Innis M, MacIntyre N, Liggett SB. Mutations in the gene encoding for the beta 2-adrenergic receptor in normal and asthmatic subjects. Am J Respir Cell Mol Biol. 1993;8:334–339. doi: 10.1165/ajrcmb/8.3.334. [DOI] [PubMed] [Google Scholar]
- 16.Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, et al. The effect of polymorphisms of the beta(2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000;162:75–80. doi: 10.1164/ajrccm.162.1.9907092. [DOI] [PubMed] [Google Scholar]
- 17.Lima JJ, Thomason DB, Mohamed MH, Eberle LV, Self TH, Johnson JA. Impact of genetic polymorphisms of the beta2-adrenergic receptor on albuterol bronchodilator pharmacodynamics. Clin Pharmacol Ther. 1999;65:519–525. doi: 10.1016/S0009-9236(99)70071-8. [DOI] [PubMed] [Google Scholar]
- 18.Martinez FD, Graves PE, Baldini M, Solomon S, Erickson R. Association between genetic polymorphisms of the beta2-adrenoceptor and response to albuterol in children with and without a history of wheezing. J Clin Invest. 1997;100:3184–3188. doi: 10.1172/JCI119874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litonjua AA. The significance of beta2-adrenergic receptor polymorphisms in asthma. Curr Opin Pulm Med. 2006;12:12–17. doi: 10.1097/01.mcp.0000198068.50457.95. [DOI] [PubMed] [Google Scholar]
- 20.Weiss ST, McLeod HL, Flockhart DA, Dolan ME, Benowitz NL, Johnson JA, et al. Creating and evaluating genetic tests predictive of drug response. Nat Rev Drug Discov. 2008;7:568–574. doi: 10.1038/nrd2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elston RC. The genetic dissection of multifactorial traits. Clin Exp Allergy. 1995;25 (Suppl 2):103–106. doi: 10.1111/j.1365-2222.1995.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 22.Yang Q, Khoury MJ, Botto L, Friedman JM, Flanders WD. Improving the prediction of complex diseases by testing for multiple disease-susceptibility genes. Am J Hum Genet. 2003;72:636–649. doi: 10.1086/367923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weedon MN, McCarthy MI, Hitman G, Walker M, Groves CJ, Zeggini E, et al. Combining information from common type 2 diabetes risk polymorphisms improves disease prediction. PLoS Med. 2006;3:e374. doi: 10.1371/journal.pmed.0030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lange C, DeMeo D, Silverman EK, Weiss ST, Laird NM. Using the noninformative families in family-based association tests: a powerful new testing strategy. Am J Hum Genet. 2003;73:801–811. doi: 10.1086/378591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange C, DeMeo DL, Laird NM. Power and design considerations for a general class of family-based association tests: quantitative traits. Am J Hum Genet. 2002;71:1330–1341. doi: 10.1086/344696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lange C, Laird NM. On a general class of conditional tests for familybased association studies in genetics: the asymptotic distribution, the conditional power, and optimality considerations. Genet Epidemiol. 2002;23:165–180. doi: 10.1002/gepi.209. [DOI] [PubMed] [Google Scholar]
- 27.Van Steen K, McQueen MB, Herbert A, Raby B, Lyon H, Demeo DL, et al. Genomic screening and replication using the same data set in family-based association testing. Nat Genet. 2005;37:683–691. doi: 10.1038/ng1582. [DOI] [PubMed] [Google Scholar]
- 28.Litonjua AA, Lasky-Su J, Schneiter K, Tantisira KG, Lazarus R, Klanderman B, et al. ARG1 is a novel bronchodilator response gene: screening and replication in four asthma cohorts. Am J Respir Crit Care Med. 2008;178:688–694. doi: 10.1164/rccm.200709-1363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 30.The Childhood Asthma Management Program Research Group. Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. Am J Respir Crit Care Med. 2007;175:235–242. doi: 10.1164/rccm.200603-416OC. [DOI] [PubMed] [Google Scholar]
- 31.American Lung Association Asthma Clinical Research Centers. Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 32.Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town GI. Asthma exacerbations during long term beta agonist use: influence of beta(2) adrenoceptor polymorphism. Thorax. 2000;55:762–767. doi: 10.1136/thorax.55.9.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 34.Lasko TA, Bhagwat JG, Zou KH, Ohno-Machado L. The use of receiver operating characteristic curves in biomedical informatics. J Biomed Inform. 2005;38:404–415. doi: 10.1016/j.jbi.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: 2007. [Google Scholar]
- 36.Lu Q, Elston RC. Using the optimal receiver operating characteristic curve to design a predictive genetic test, exemplified with type 2 diabetes. Am J Hum Genet. 2008;82:641–651. doi: 10.1016/j.ajhg.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Litonjua AA, Tantisira KG, Lasky-Su JA, Murphy A, Lazarus R, Klanderman B, et al. Genome-wide association analysis of bronchodilator response in asthma (abstract) Am J Respir Crit Care Med. 2008 [Google Scholar]
- 38.Barrientos T, Frank D, Kuwahara K, Bezprozvannaya S, Pipes GC, Bassel-Duby R, et al. Two novel members of the ABLIM protein family, ABLIM-2 and -3, associate with STARS and directly bind F-actin. J Biol Chem. 2007;282:8393–8403. doi: 10.1074/jbc.M607549200. [DOI] [PubMed] [Google Scholar]