Abstract
Succinic semialdehyde dehydrogenase (SSADH) deficiency is an autosomal-recessively inherited disorder of γ-aminobutyrate (GABA) catabolism characterized by ataxia and epilepsy. Since SSADH is responsible for GABA break-down downstream of GABA transaminase, patients manifest high extracellular levels of GABA, as well as the GABAB receptor (GABABR) agonist γ-hydroxybutyrate (GHB). SSADH knockout (KO) mice display absence seizures, which progress into lethal tonic-clonic seizures at around 3 weeks of age. It is hypothesized that desensitization of GABABRs plays an important role in the disease, although detailed studies of pre- and postsynaptic GABABRs are not available. We performed patch-clamp recordings from layer 2/3 pyramidal neurons in neocortical brain slices of wild-type (WT) and SSADH KO mice. Electrical stimulation of GABAergic fibers during wash in of the GABABR agonist baclofen revealed no difference in presynaptic GABABR mediated inhibition of GABA release between WT and SSADH KO mice. In contrast, a significant decrease in postsynaptic baclofen-induced potassium currents was seen in SSADH KO mice. This reduction was unlikely to be caused by accumulation of potassium, GABA or GHB in the brain slices, or an altered expression of regulators of G-protein signaling (RGS) proteins. Finally, adenosine-induced potassium currents were also reduced in SSADH KO mice, which could suggest heterologous desensitization of the G-protein dependent effectors, leading to a reduction in G-protein coupled inwardly rectifying potassium (GIRK) channel responses. Our findings indicate that high GABA and GHB levels desensitize postsynaptic, but not certain presynaptic, GABABRs, promoting a decrease in GIRK channel function. These changes could contribute to the development of seizures in SSADH KO mice and potentially also in affected patients.
List of keywords: GABA, GHB, GABAB, GIRK, heterologous desensitization, SSADH, neocortex, epilepsy, patch-clamp
Introduction
Succinic semialdehyde dehydrogenase (SSADH, Aldh5a1) deficiency is an autosomal-recessively inherited disorder of γ-aminobutyric acid (GABA) catabolism characterized clinically by intellectual disability, autism spectrum, sleep disturbances and epileptic seizures (Knerr et al., 2007). A mouse model of SSADH deficiency, SSADH knockout (KO) mice, has become an important tool to investigate the pathophysiology of this disease and possible treatment approaches (Gibson et al., 2005, Hogema et al., 2001, Nylen et al., 2008). SSADH is responsible for the oxidation of succinic semialdehyde (SSA) to succinic acid and participates in the break-down of GABA downstream of GABA transaminase (Turner and Whittle, 1983). Comparable to human patients, SSADH deficient mice manifest accumulation of GABA and γ-hydroxybutyrate (GHB) in the brain (Hogema et al., 2001, Jansen et al., 2008). Both GABA and GHB are agonists of metabotropic GABABRs, which are recognized as a therapeutic target in various brain pathologies (for review see Bowery, 2006).
Dysfunction of GABABR mediated inhibition has been suggested to contribute to the pathophysiology of SSADH deficiency. Accordingly, Buzzi and colleagues found a significant decrease of [3H]CGP-54626A binding in brain slices of SSADH KO mice. Moreover, they observed that electrically evoked GABABR mediated slow IPSPs (inhibitory postsynaptic potentials) in CA1 of the hippocampus were downregulated (Buzzi et al., 2006). Since GABA release does not appear to be affected in SSADH KO mice (Drasbek et al., 2008), this raises the possibility that postsynaptic GABABRs or downstream effector systems are altered in SSADH deficiency.
GABABRs are expressed on glutamatergic and GABAergic presynaptic terminals and on the somatodendritic region of target neurons. Presynaptic GABABRs influence neurotransmitter release by inhibiting calcium influx, while postsynaptic GABABRs influence excitability of neurons mainly by causing postsynaptic hyperpolarization via potassium efflux through G-protein-coupled inwardly-rectifying potassium (GIRK) channels that can shunt excitatory input (Nicoll, 2004).
GABABRs are members of the family of G-protein coupled 7-transmembrane domain receptors (GPCRs) (Kaupmann et al., 1997) and functional GABABRs are obligatory heterodimers composed of GABAB(1a,b) and GABAB(2) subunits that cross-stabilize each other (Brown et al., 2003, Kaupmann et al., 1997, Prosser et al., 2001, Schuler et al., 2001). GABABRs are coupled to G-proteins of the Gi/o subfamily and activation of GABAB receptors by agonists (e.g. GABA, GHB, or baclofen) results in phosphorylation of Gα (the α subunit of the G-protein complex) followed by the liberation of the Gβγ-subunits and opening of the GIRK channels (Bettler et al., 2004). Furthermore, GABAB2 subunits are responsible for binding and activation of Gα (i/o subtypes), and for the correct trafficking of the GABAB1 subunit to the cell surface (Calver et al., 2001, Margeta-Mitrovic, Jan and Jan, 2000, Margeta-Mitrovic, Jan and Jan, 2001, Robbins et al., 2001). GABABR heterodimers are proposed to be atypical GPCRs, as phosphorylation does not cause obligate internalization-dependent downregulation of the receptors on the neuron surface. Instead, during agonist application, GABABRs can undergo rapid desensitization, which is explained by uncoupling of the GABAB heterodimers from Gα-GIRK complexes rather than by receptor internalization (Labouebe et al., 2007). While high levels of agonists can influence GABAB receptors and effectors, little is known about the GIRK channel responses in SSADH KO mice, which show high GABA and GHB levels in the brain.
Here, brain slice electrophysiology was used to study possible alterations in the function of presynaptic and postsynaptic GABABRs of neocortical layer 2/3 pyramidal neurons in SSADH KO mice. While presynaptic GABABR mediated inhibition of GABA release appeared to function similarly in wild-type (WT) and KO mice, pyramidal neurons of SSADH KO mice demonstrated a significant loss of postsynaptic responsiveness to baclofen and adenosine.
Materials and Methods
Mouse breeding
Wild-type and SSADH knockout mice were obtained from heterozygous breeding in a university animal facility with a 12/12-hour light/dark cycle and food and water ad libitum. SSADH KO mice develop absence seizures that progress into lethal status epilepticus, leading to 100% mortality at postnatal day 18-22 (P18-22). Therefore, WT and KO mice were used for experiments from P14 to P18 (Cortez et al., 2004, Hogema et al., 2001).
Brain slice electrophysiology
Mice were used in accordance with university guidelines, and European Union legislation regarding laboratory animals. Mice of either sex were anesthetized deeply with isoflurane, decapitated, and the brains were dissected out and transferred to ice-cold artificial cerebrospinal fluid (ACSF) composed of (in mM): 126 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4, 26 NaCO3, 10 D-glucose (osmolality 305-315 mosmol·kg-1), pH 7.4 when bubbled with carbogen (5% CO2, 95% O2). 350 μm thick coronal slices were cut on a Vibratome 3000 Plus (Vibratome Company, St. Louis, MO). To improve brain slice quality, 3 mM kynurenic acid, 0.2 mM ascorbic acid, and 0.2 mM pyruvic acid were added during slicing and storage. Slices were allowed to rest for at least 1 hour before recording.
For recordings of IPSCs and postsynaptic GIRK currents, slices were placed in a chamber and perfused with 33-34°C bubbled ACSF at 2-3 ml·min-1. Neocortical pyramidal cells were visualized by a custom-built infrared microscope (Versascope, E. Marton, CA) equipped with a 40× water immersion objective (Olympus, Ballerup, Denmark) and a CCD100 camera (DAGE-MTI, Michigan City, IN). Layer 2/3 pyramidal cells were identified under infrared video microscopy displaying large pyramidal-shaped soma with a prominent dendrite projecting to layer 1 and confirmed as regular spiking neurons in most experiments (Drasbek, Hoestgaard-Jensen and Jensen, 2007). Patch pipettes were pulled from borosilicate glass (O.D. = 1.5 mm, I.D. = 0.8 mm; Garner Glass Company, Claremont, CA) on a DMZ Universal Puller (Zeitz Instruments, Munich, Germany). For GABAA IPSC recordings, pipette resistances were 3-5 MΩ when filled with a solution containing (in mM): 140 CsCl, 2 MgCl2, 0.05 EGTA, 10 HEPES, adjusted to pH 7.2 with CsOH (280-290 mosmol·kg-1). For recording of postsynaptic GABABR-mediated GIRK currents, patch pipettes contained (in mM): 130 KOH, 10 KCl, 0.3 EGTA, 10 HEPES, 0.3 Na3GTP, 2 MgATP and 5 disodium creatine-phoshate, adjusted to pH 7.3 with methanesulfonic acid (280–290 mosmol/kg), yielding K-methanesulfonate as the main constituent. For recording of combined slow and fast IPSCs, under conditions allowing for both GABAA and GABAB currents, internal Cl- was slightly raised and pipettes were filled with a solution containing (in mM): 120 KOH, 20 KCl, 0.3 EGTA, 10 HEPES, 0.3 Na3GTP, 2 MgATP and 5 disodium creatine-phoshate, adjusted to pH 7.3 with methanesulfonic acid (280–290 mosmol/kg), yielding K-methanesulfonate as the main constituent. Whole-cell patch-clamp recordings were carried out using a MultiClamp 700B amplifier (Molecular Devices, Union City, CA). Giga seals (>1 GΩ) were always obtained before break-in. For isolated GABAA or GABAB receptor responses, neurons were voltage-clamped at a Vhold of either -70 mV or -50 mV, and whole-cell capacitances and series resistances were noted. Resistances were compensated by 70-80% (lag 10 μs), and recordings were discontinued if series resistance changed by more than 20% or exceeded 20 MΩ (typical series resistances 10-14 MΩ). For mixed GABAA and GABAB responses, the membrane was held between ECl (-47 mV) and EK (-100 mV), and thus fast GABAA IPSCs are inward, but slow GABAB IPSCs are outward.
Data acquisition and analysis
All recordings were low-pass filtered (8-pole Bessel) at 3 kHz, digitized at 20 kHz, and acquired using a BNC-2110 D/A converter and a PCI-6014 board (National Instruments, Austin, TX) and custom-written LabVIEW 6.1–based software (EVAN v. 1.4, courtesy of Istvan Mody). As there was no apparent difference in cell size between pyramidal cells of WT and SSADH KO mice (21.1 ± 0.6 pF, n = 27, and 22.0 ± 0.5 pF, n = 29, respectively), the currents were presented without capacitance normalization in histograms. Unpaired two-tailed Student's t-tests were used to compare means with P < 0.05 as the significance level. Data are presented as means ± SEM, except for the averaged EC50 (means ± standard deviation), with n indicating the number of neurons. Concentration-response curves were generated by washing in different agonist concentrations, and a Hill function was used to fit current amplitudes normalized to the maximal current obtained with a saturating concentration of agonist: (y = 1/(1 + ([EC50]/[x])h), where EC50 is the concentration yielding the half-maximal response and h is the Hill coefficient. On these curves, each point represents the mean ± SEM across all experiments. Correlation of concentration-response curves was analyzed with linear regression in GraphPad Prism (Graph Pad Software, Inc., La Jolla, CA, USA).
Solutions and drugs
Baclofen, CGP55845, kynurenic acid were from Tocris (Avonmouth, UK), sodium 4-hydroxybutyrate (GHB) was from Lancaster Synthesis (Eastgate, England), while pyruvic acid was from MP Biomedicals (Irvine, CA). All other drugs and reagents were from Sigma (St. Louis, MO).
Results
Similar function of presynaptic GABABRs on inhibitory nerve terminals in WT and SSADH KO mice
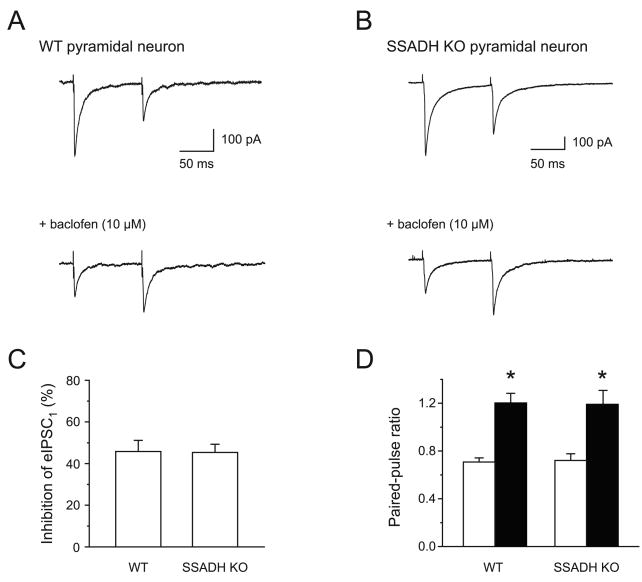
Initially, the present study focused on presynaptic GABABR mediated inhibition of GABAergic transmission onto layer 2/3 pyramidal neurons by minimal stimulation of putative single GABAergic axons onto neurons clamped at -70 mV (Jensen and Mody, 2001). Using stimulating intensities of 20-40% above threshold, GABAA receptor-mediated IPSCs (eIPSCs) were elicited in layer 2/3 pyramidal neurons in the presence of the glutamate receptor antagonist kynurenic acid (3 mM) (Fig. 1). Using paired-pulse stimulation with an inter-pulse interval of 100 ms, the ratio of the second eIPSC (eIPSC2) relative to the first (eIPSC1) was 0.71 ± 0.03 (n = 15) in WT and 0.72 ± 0.06 (n = 13, P > 0.05) in SSADH KO mice (Fig. 1). Furthermore, upon activating GABAB receptors with baclofen (10 μM), the amplitude of eIPSC1 was depressed identically in WT (0.54 ± 0.05, n = 8) and SSADH KO mice (0.55 ± 0.04, n = 8). Finally, the increase in the paired-pulse ratio associated with lowering of the release probability by baclofen was similar in WT and SSADH. Thus, paired-pulse ratios in baclofen were 1.20 ± 0.08 for WT (n = 8) versus 1.19 ± 0.12 for KO (n = 8) (P > 0.05). These results indicate that the presynaptic GABAB receptor function at GABAergic synapses is similar in WT and SSADH KO mice.
Fig. 1. Function of presynaptic GABABRs in neocortical layer 2/3 pyramidal cells in WT and SSADH KO mice.
(A) Whole-cell recordings of evoked IPSCs (eIPSCs) in neocortical layer 2/3 pyramidal cells using extracellular stimulation. In the WT (wild type) slice, the averaged eIPSC showed paired-pulse depression of 0.50 (amplitude of eIPSC2 relative to eIPSC1) at a 100 ms interval. The GABABR agonist baclofen (10 μM) depressed eIPSC1 by 59%, and converted the paired-pulse depression into facilitation. (B) In the SSADH KO slice, paired-pulse depression of eIPSCs was observed. Baclofen depressed eIPSC1 by 66%, again turning depression to facilitation. (C) Baclofen reduced the amplitude of eIPSC1 in pyramidal cells equally in WT and SSADH KO mice. eIPSC1 was depressed by 45.6 ± 7% in WT slices (n = 8), and by 45.9 ± 5% in SSADH KO slices (n = 6). (D) The histogram shows paired-pulse depression of eIPSC2, expressed as a ratio of eIPSC1 (100 ms inter-pulse interval) for WT (left, n = 15) and SSADH KO (right, n = 13). Baclofen (10 μM) (filled bars) equally converted the paired-pulse ratio to facilitation in WT (left, n = 8) and SSADH KO (right, n = 6) mice, indicating similar properties of presynaptic GABABRs.
Postsynaptic GABABR mediated inhibition is reduced in SSADH KO mice
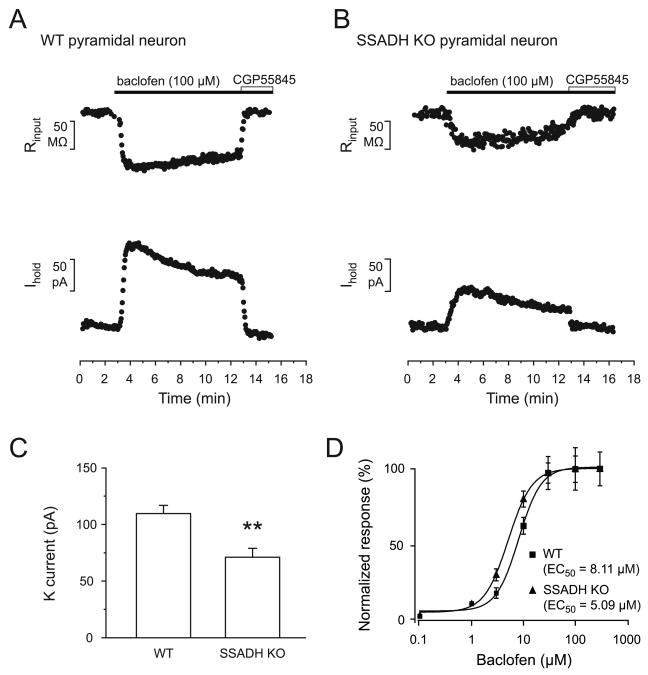
In order to investigate possible changes in the postsynaptic GABABR function in SSADH deficient mice, outward currents induced by baclofen during 10 min wash in experiments from the layer 2/3 pyramidal neurons were recorded. A supramaximal concentration of baclofen (100 μM) led to outward currents, which was associated with a concurrent decrease of the membrane resistance (Rin), indicating the increase of a potassium conductance, that could be blocked by the GABAB antagonist CGP55845 (8 μM) (Fig. 2A, B). In pyramidal neurons of WT mice, baclofen (100 μM) induced a current of 110.7 ± 6.5 pA and a decrease of Rin of 108.6 ± 13.1 MΩ (n = 9) (Fig. 2A, C). The current desensitized to 66.4 ± 5.4% during 10 minutes of continuous agonist application (Fig. 2A). In pyramidal neurons of SSADH KO mice, baclofen (100 μM) induced a potassium current of 71.1 ± 7.9 pA and a decrease of Rin of 71.3 ± 12.0 MΩ (n = 12) (Fig. 2B, C). In SSADH KO mice, the baclofen-evoked current was desensitizing to 68.2 ± 5.4% during 10 minutes of agonist application (Fig. 2B). As a result, SSADH KO mice exhibited lower GABABR mediated potassium currents in neocortical pyramidal neurons compared to WT mice, while the currents desensitized to similar extents upon acute baclofen exposure.
Fig. 2. Postsynaptic GABABR-mediated currents are reduced in layer 2/3 pyramidal cells in SSADH KO mice.
(A-B) The GABABR agonist baclofen (100 μM) induced outward currents in pyramidal neurons in WT (A) and SSADH KO mice (B), which were fully blocked by GABAB antagonist CGP55845. Note the concomitant reduction in input resistance (upper traces). The neurons were voltage-clamped at -50 mV. (C) Bar graphs representing the peak amplitude of the baclofen-induced outward currents in pyramidal cells of WT (n = 9) and SSADH KO (n = 12) mice (***: P < 0.001). (D) Concentration-response curves for baclofen are shown for pyramidal neurons of WT (squares; total n = 27) and SSADH KO (triangles; total n = 28). The mean EC50 of baclofen-evoked currents were 8.11 μM and 5.09 μM in pyramidal neurons of WT and SSADH KO mice respectively.
EC50 for baclofen at postsynaptic GABABRs in WT and SSADH KO mice
Previously, it was reported that chronic administration of GHB leads to cell-specific changes in the coupling efficiency between GABAB receptors and GIRK channels and, thus, potentially a strengthening in GABAB receptor mediated inhibition (Labouebe et al., 2007, Mutneja et al., 2005). To examine if the sensitivity to GABAB receptor agonist was affected in SSADH KO mice, a concentration-response relationship for baclofen (0.1 - 300 μM) was constructed using a population of responses from different pyramidal neurons of WT and SSADH KO mice (Fig. 2D). Averaged currents were normalized to maximal responses and fitted using the Hill equation. No major change in EC50 for baclofen between WT (8.11 ± 4.3 μM, h = 2.0, n = 27) and SSADH KO mice (5.09 ± 1.4 μM, h = 1.9, n = 28) was found. These data suggest that the coupling between GABAB receptors and GIRK channels is not affected in SSADH KO mice.
Accumulation of GABA or GHB in the slices is unlikely to explain reduced postsynaptic baclofen responses
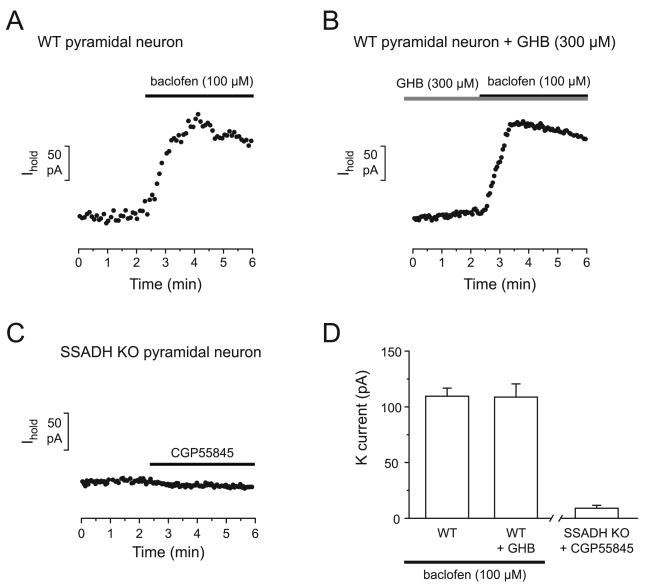
The partial agonist at GABABRs GHB, which is increased in the brains of SSADH KO mice, might bind to GABABRs in the slice during the recording and compete with the effect of baclofen (Mathivet et al., 1997). To examine if this could be mimicked in WT slices, experiments in the presence of GHB (300 μM) were performed. Ten min preincubation of WT slices with GHB (300 μM) did not decrease the response of the pyramidal neurons to baclofen (100 μM) in WT mice. Indeed, in the presence of GHB (300 μM) wash in of baclofen (100 μM) induced a potassium current of 109.0 ± 11.0 pA (n = 5) in WT mice, which was similar to the response without GHB (110.7 ± 6.5 pA, P > 0.05) (Fig. 3).
Fig. 3. Reduced baclofen currents in SSADH KO mice are not explained by accumulation of endogenous GHB or GABA in the slice.
(A-B) Representative recordings of currents induced by baclofen (100 μM) in the absence (A) or presence (B) of GHB (300 μM) in pyramidal neurons of WT mice. GHB (300 μM) did not affect the peak response to baclofen. (C) Recording of the effect of CGP55845 in pyramidal neurons of SSADH KO mice. Application of CGP55845 to a SSADH KO slice revealed little tonic activation of GABABRs by endogenous agonists. (D) Bar graphs represent the peak amplitude of the outward current evoked by baclofen (100 μM) in control (n = 9) and in the presence of GHB (300 μM) (n = 5) in WT slices. The rightmost bar shows the amplitude of tonic GABAB currents (n = 5) in the absence of exogenous agonists in SSADH KO mice.
Furthermore, in SSADH KO mice it is possible that the accumulation of both GABA and GHB in the slice could lead to tonically activated GABABRs, masking the baclofen-evoked potassium currents during slice recordings. To test if a tonic GABABR activation could be detected in SSADH KO, CGP55845 (8 μM) was applied to KO slices during recordings. CGP55845 application revealed a very small tonic potassium current of 9.0 ± 2.6 pA (n = 6). As a consequence, endogenous GABAB agonists are unlikely to interfere significantly with the baclofen induced GABAB response. This finding argues against the possibility that reduced postsynaptic baclofen responses are due to elevated endogenous GABAB agonists in SSADH KO mouse slices.
Responses to adenosine are reduced in SSADH KO mice
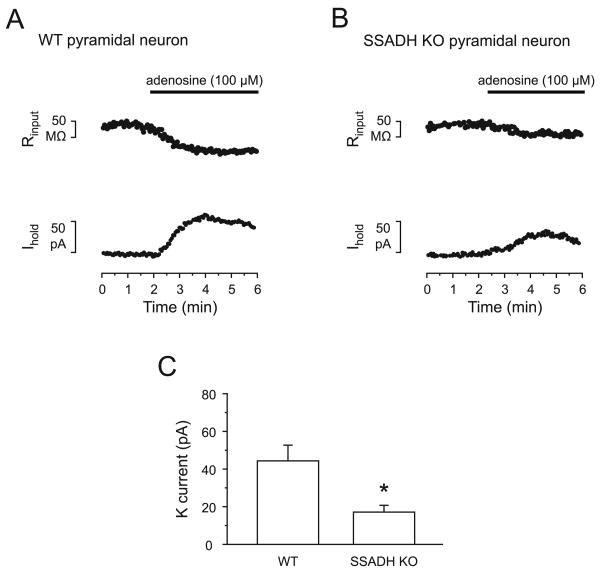
It was shown earlier that prolonged activation of Gαi/o coupled GPCRs can lead to heterologous desensitization of responses to similar acting neurotransmitters (Cornelisse et al., 2007, Blanchet and Luscher, 2002, Wetherington and Lambert., 2002), e.g. that baclofen exposure can decrease the response to other GPCRs, including adenosine receptors. To examine if the decreased GABABR response in SSADH deficiency includes alterations in downstream effector proteins, the responses to adenosine were tested. Wash in of adenosine (100 μM) led to outward currents in pyramidal neurons, also indicative of the activation of a potassium conductance. In WT mice, adenosine (100 μM) induced a current of 44.3 ± 8.3 pA and a decrease of Rin of 52.3 ± 5.7 MΩ (n = 5) (Fig. 4A, C). In SSADH KO mice, adenosine (100 μM) induced a significantly smaller current of 17.2 ± 3.6 pA and a decrease of Rin of 24.4 ± 5.6 MΩ (n = 6, P < 0.05) (Fig. 4B, C), i.e. only 39% of the WT adenosine current.
Fig. 4. Decreased responsiveness to adenosine in SSADH KO mice.
(A-B) Adenosine (100 μM) induced outward currents in pyramidal neurons in WT (A) and SSADH KO mice (B). Responses to adenosine (100 μM) were smaller in SSADH KO than in WT. Note the concomitant reduction in the input resistance (upper traces). The neurons were voltage-clamped at -50 mV. (C) Bar graphs representing the average peak amplitude of adenosine-induced outward currents in pyramidal cells of WT (n = 5) and SSADH KO (n = 6) mice (*: P < 0.05). In SSADH KO, the adenosine current was 39% of WT.
GABAB receptor responses mediated by synaptically released GABA are decreased in SSADH KO
Synaptically released GABA can generate chloride-channel dependent fast IPSCs mediated by GABAARs, and potassium-channel dependent slow IPSCs mediated by GABABRs (Nicoll, 1988, Mott et al., 1999). To determine if alterations in the GABABR mediated pathway in SSADH KO mice will attenuate synaptic potassium currents in neocortex, the magnitude of evoked slow IPSCs mediated by synaptically released GABA in WT and KO mice was compared. To ensure that the stimulating electrode activated inhibitory interneurons, fast IPSCs in the absence of GABAARs antagonists were monitored, and recordings were done under conditions allowing for combined GABAA and GABAB responses (Luscher et al., 1997). The membrane was held between ECl (-47 mV) and EK (-100 mV), and thus fast GABAA IPSCs were inward (Vhold -70 mV), while slow GABAB IPSCs (Vhold -50 mV) were outward. In order to induce a substantial synaptic release of GABA, extracellular stimulations consisting of 7 pulses at 100 Hz were employed (Mott et al., 1999). Again, average evoked fast IPSCs mediated by GABAARs responses were unaltered in SSADH KOs (not shown, but see Fig. 1). However, using a holding potential of -50 mV, a significant decrease to 54% of control in the synaptically evoked GABABR mediated responses was seen in SSADH KO compared to WT. In WT mice, the amplitude of the slow IPSCs averaged 70.1 ± 5.7 pA (n = 12) (Fig. 5A, C) while, in SSADH KO mice, the slow IPSCs were significantly smaller and averaged 38.2 ± 4.4 pA (n = 15) P < 0.001 (Fig. 5B, C).
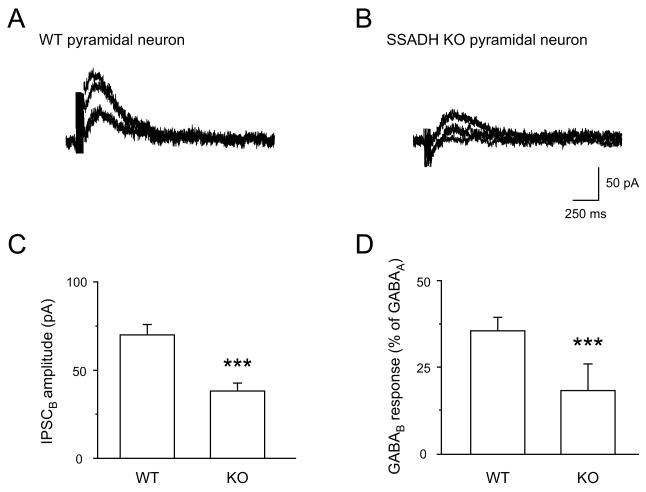
Fig. 5. Reduced synaptically evoked slow GABAB receptor IPSCs in SSADH KO mice.
(A-B) Slow GABABR-mediated IPSCs in pyramidal neurons of WT (A) and SSADH KO (B) mice. Currents were obtained in whole-cell configuration, by increasing the extracellular stimulus intensity above threshold evoking increasing GABABR IPSCs. Stimuli consisted of 7 pulses at 100 Hz, and the membrane was held at -50 mV (EK = -103 mV) to minimize a GABAAR component (ECl -47 mV) (C) Bar graphs representing the amplitude of the slow IPSCs in pyramidal cells of WT (n = 12) and SSADH KO (n = 15) mice (***: P < 0.001). (D) Bar graphs representing the amplitude of the GABAB IPSC normalized to the amplitude of the GABAA IPSC obtained from pyramidal cells of WT (n = 11) and SSADH KO (n = 20) mice (***: P < 0.001).
For further analysis, the amplitude of GABABR mediated responses were normalized to the peak amplitude of the GABAAR response obtained from the same cell. In WT, such normalized responses were 35.8 ± 4.0% (n = 11) (Fig. 5D) while, in SSADH KO mice, normalized GABABRs mediated responses averaged only 18.4 ± 2.7% (n = 20) (Fig. 5D) (P < 0.01), illustrating the significantly reduced GABABR mediated potassium currents during comparable synaptic releases of GABA.
Discussion
In the present study, presynaptic GABABR dependent inhibition of GABA release onto pyramidal cells was not affected in SSADH deficiency, indicating that the presynaptic receptors controlling calcium influx and the transmitter release machinery were not disturbed. On the other hand, the postsynaptic GABABR function was significantly altered, since we found a reduction in postsynaptic GABABR mediated currents in neurons of SSADH KO mice. GHB in relevant pathophysiological concentrations failed to mimic this effect in neurons in WT slices. Moreover, despite the increased levels of GABA there was no major basal GABAB receptor activation in neurons of SSADH KOs. Although chronic GHB exposure may lead to cell-specific changes in the coupling efficiency between GABABRs and GIRK channels (Labouebe et al., 2007), we found no major change in the EC50 for baclofen in SSADH KO mice. On the other hand, neurons of SSADH KO mice exhibited decreased responsiveness to the similarly acting neurotransmitter adenosine, suggesting a heterologous desensitization and pointing to alterations in proteins in the cascade downstream of GABABRs. Finally, slow GABAB IPSCs were decreased to 54% in KOs pointing to a physiological relevance of our pharmacological findings.
Overall, our results suggest that a differential plasticity of these types of pre- and postsynaptic GABABRs may operate in the rodent brain in vivo. The defective GABABRs function could play an important role in the seizure phenotype in SSADH knockout mice, and potentially also in the human disorder as well.
SSADH deficiency is associated with increased GABA and GHB
SSADH KO mice represent a genetic model of the severe case of human SSADH deficiency (Hogema et al., 2001). These mice manifest spike-and-wave discharge (SWD) and behaviors typical of absence seizures starting from age P10-14 evolving to myoclonic and generalized convulsive seizures from age P18, that progress into lethal status epilepticus (Cortez et al., 2004, Hogema et al., 2001). These changes have been electrophysiologically investigated in detail in vivo (Cortez et al., 2004). Comparable to human patients, SSADH deficient mice manifest accumulation of GABA (2-fold) and GHB (60-fold) in the brain (Jansen et al., 2008, Hogema et al., 2001). GHB is a weak agonist of GABAB receptors and present in low concentrations in the normal brain (2-4 μM), which is likely lower than what is necessary to activate GABABRs (Vayer et al., 1988). Of relevance for the SSADH KO model, in pharmacological models of absence seizures, where administration of 3.5 mmol/kg of GHB induced spike-and-wave activity, rat brain concentrations of GHB reached 240 μM (Snead, 1991), comparable to the concentration found in brain of SSADH deficiency (150-240 μM) (Hogema et al., 2001). This led to the hypothesis that raised GHB is involved in the brain pathophysiology in SSADH deficiency. However, it is equally likely that accumulation of GABA might also play an important role by over-activating GABA receptors. Indeed, both GHB and GABAB antagonists partially rescue the lethal phenotype of SSADH KO mice (Hogema et al., 2001).
Presynaptic GABABRs function on inhibitory terminals is similar in WT and SSADH KO mice
The presynaptic function of GABABRs on GABAergic nerve terminals was not affected in SSADH KO mice upon activating GABABRs with baclofen, since eIPSC1 was depressed similarly in WT and SSADH KO mice. Also, the increase in the paired-pulse ratio associated with lowering of the release probability by baclofen was similar in WT and SSADH KOs. The synapses giving rise to these IPSCs are probably part of a perisomatic inhibitory system, since they show depressing GABAA responses. The presented data suggests that there could be a differential susceptibility for downregulation of postsynaptic G-protein-coupled GABABR function (discussed below), compared with presynaptic GABABRs onto neocortical pyramidal neurons. Supporting this, cell-type specific mechanisms of desensitization of Gαi/o coupled pre- and postsynaptic receptors during prolonged agonist treatment have been reported. For example, postsynaptic GABABRs and 5-HT1ARs of pyramidal neurons of CA1 seem to be resistant to downregulation by high levels of agonists in transporter knockout mouse models (Jensen et al., 2003, Mannoury la Cour et al., 2001). On the other hand, in dorsal raphe neurons, prolonged increased agonist levels caused by either serotonin transporter knockout (Mannoury la Cour et al., 2001) or chronic treatment with serotonin uptake inhibitor fluoxetine (Cornelisse et al., 2007) led to reduced somatodendritic 5-HT1AR and GABABR responses. Similarly, in rat brain slices, postsynaptic, but not presynaptic, μ-opioid receptors can be acutely desensitized by the selective agonist DAMGO (Blanchet and Luscher, 2002). Finally, chronic treatment with agonists in cultured hippocampal neurons can desensitize postsynaptic, but not presynaptic, GABABRs (Wetherington and Lambert, 2002).
The molecular background for these differences are currently unknown, although a plausible explanation can be different mechanisms of G-protein regulation in GABAergic interneuron nerve terminals versus the somatodendritic area of pyramidal cells (Cruz et al., 2004, Labouebe et al., 2007), or different subcellular expression of GABABRs isoforms GABAB1a and GABAB1b. There is also growing evidence of cell or cell-compartment specific composition of GABABR heterodimers (Bischoff et al., 1999, Huang, 2006). Indeed, it was shown that GABAB1a containing heterodimers are predominantly expressed at the glutamatergic presynaptic terminals (Shaban et al., 2006, Vigot et al., 2006, Waldmeier, Kaupmann and Urwyler, 2008), while GABAB1b containing heterodimers are mainly found at postsynaptic sites of pyramidal neurons of the neocortex (Perez-Garci et al., 2006). Interestingly, GABAergic presynaptic terminals seem to be equipped with both heterodimer isoforms (Tiao et al., 2008); they are less sensitive to low concentrations of GHB in neocortex and thalamus (Gervasi et al., 2003, Jensen and Mody, 2001, Li et al., 2007) and might be more stable with respect to agonist induced desensitization.
Postsynaptic GABABRs mediated inhibition is reduced in SSADH KO mice
We found that postsynaptic GABABR mediated currents are significantly decreased in SSADH KO mice. This effect could potentially be explained by downregulation of the GABABR function in the SSADH KO neurons and/or by pathological extracellular conditions in slices from SSADH KO mice, such as high levels of GABA, GHB, or potassium. The latter is, however, unlikely since a similar change in membrane resistance was observed. On the other hand, we recently reported that GABA is elevated in slices of SSADH KO mice to a sufficient level for activation of extrasynaptic GABAARs mediating a tonic current (Drasbek et al., 2008). Interestingly, in this study we found that tonic GABAB mediated currents in KO mice were absent or very small. Similarly, GABA transporter 1 deficient (GAT1 KO) mice show similar results in CA1 pyramidal neurons (Jensen et al., 2003), where no tonic GABABRs mediated current could be revealed, despite a prominent GABAAR current. This difference in basal activation levels between somatodendritic extrasynaptic GABAARs and GABABRs could reflect a different cell-compartment specific localization, density or sensitivity of extrasynaptic GABAARs and GABABRs (Brown et al., 2002, Kulik et al., 2003, Schuler et al., 2001). Finally, accumulation of GHB (a partial agonist of GABAB receptors) in the brain slices of SSADH KO mice might have reduced the effect of baclofen, by competing for the binding sites (Mathivet et al., 1997). However, exposure of WT slices to GHB in pathophysiologically relevant concentrations failed to affect the magnitude of the baclofen induced potassium currents. Overall, decreased GABABR mediated inhibition that we found in SSADH KO mice most likely reflects downregulation of GABABR function, rather then pathological conditions in the slices of KO mice during recordings.
Heterologous desensitization of postsynaptic GABABRs in SSADH KO mice
Several factors could be involved in altered function of GABABRs, including GABABR2 subunit phosphorylation at several serines by Protein kinase A or 5′AMP-dependent protein kinase (AMPK) (Couve et al., 2002, Kuramoto et al., 2007). There is also evidence that constitutive turnover of GABABRs in neurons is modulated by receptor activation or inhibition (Fairfax et al., 2004, Grampp et al., 2008, Wilkins, Li and Smart, 2008). Furthermore, the coupling between GABABR and GIRK channels are affected by phosphorylation of GABABRs (Kuramoto et al., 2007) or changes in the expression or functioning of GIRK channel subunits (Cruz et al., 2004, Huang, Feng and Hilgemann, 1998, Labouebe et al., 2007, Logothetis et al., 2007).
GABABR signaling is also regulated by Regulators of G-protein signaling (RGS) proteins, which accelerate the rate of hydrolysis of GTP bound to the Gα subunit (Jaen and Doupnik, 2006, Mutneja et al., 2005). Chronic administration of GHB affects the coupling efficiency between GABABRs and GIRK channels in dopaminergic neurons of ventral tegmental area due to downregulation of RGS2 (Labouebe et al., 2007). To examine if receptor-effector coupling is affected during SSADH deficiency we compared the concentration-response relationships for baclofen in WT and SSADH KO mice. We found no major change in the EC50 for baclofen and the rapid desensitization in pyramidal neurons of SSADH KOs showed no difference as well. Our results suggest that there is no major change in RGS activity, GIRK composition or GABABR phosphorylation in neurons associated with SSADH deficiency.
Finally, heterologous desensitization could account for our results on adenosine responses. This phenomenon is well described for opiate receptors, sharing pathways with GABABRs (Terwilliger et al., 1991), when chronic administration of opiates to locus coeruleus neurons decreases responses to somatostatin and baclofen (Blanchet and Luscher, 2002). Similarly, the serotonin up-take inhibitor fluoxetine reduces responsiveness of dorsal raphe neurons to serotonin and baclofen (Cornelisse et al., 2007) and relevant to our study, incubation of hippocampal neurons with baclofen leads to decreased responses to adenosine (Wetherington and Lambert, 2002). Our data show that SSADH deficiency leads to decreases in both GABAB and adenosine receptor induced GIRK currents. Although a parallel downregulation of both receptor types in SSADH mice cannot be excluded, it is more likely that SSADH deficiency induces alterations in GABABR effector systems. Thus, we predict a decreased response of neocortex neurons to all neurotransmitters sharing similar pathways with GABABRs. Overall, our data support the possibility of downregulation of GABABRs or GIRKs, arguing against major changes in RGSs or GIRK subunit composition. However, future experiments are required to answer these questions in this mouse model of epilepsy.
Functional relevance
It is likely that the loss of slow postsynaptic inhibition could explain the progression into tonic-clonic seizures following the desensitization of postsynaptic, but not presynaptic, inhibitory GABABRs. Accordingly, endogenous GABA and GHB accumulated in the brain could induce loss of postsynaptic inhibition on pyramidal cells, and this is likely to increase neocortical excitability and seizure prevalence that may ultimately be lethal.
Acknowledgments
We are grateful to Lene Wind Steffensen for her generous help with PCR, and Lone Overgaard for technical assistance. This study was supported by grants from the Lundbeck Foundation (Denmark), Velux Foundation (Denmark), Gangsted Foundation (Denmark), the Danish Medical Research Council, NIH NS 40270-08, HD 58553-02/03 and a grant from the Pediatric Neurotransmitter Disease Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Bischoff S, Leonhard S, Reymann N, Schuler V, Shigemoto R, Kaupmann K, Bettler B. Spatial distribution of GABA(B)R1 receptor mRNA and binding sites in the rat brain. J Comp Neurol. 1999;412:1–16. [PubMed] [Google Scholar]
- Blanchet C, Luscher C. Desensitization of mu-opioid receptor-evoked potassium currents: initiation at the receptor, expression at the effector. Proc Natl Acad Sci U S A. 2002;99:4674–4679. doi: 10.1073/pnas.072075399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG. GABA(B) receptor: a site of therapeutic benefit. Curr Opin Pharmacol. 2006;6:37–43. doi: 10.1016/j.coph.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Brown JT, Gill CH, Farmer CE, Lanneau C, Randall AD, Pangalos MN, Collingridge GL, Davies CH. Mechanisms contributing to the exacerbated epileptiform activity in hippocampal slices of GABAB1 receptor subunit knockout mice. Epilepsy Res. 2003;57:121–136. doi: 10.1016/j.eplepsyres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha4beta3delta GABA(A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzi A, Wu Y, Frantseva MV, Perez Velazquez JL, Cortez MA, Liu CC, Shen LQ, Gibson KM, Snead OC., 3rd Succinic semialdehyde dehydrogenase deficiency: GABA(B) receptor-mediated function. Brain Res. 2006;1090:15–22. doi: 10.1016/j.brainres.2006.02.131. [DOI] [PubMed] [Google Scholar]
- Calver AR, Robbins MJ, Cosio C, Rice SQ, Babbs AJ, Hirst WD, Boyfield I, Wood MD, Russell RB, Price GW, Couve A, Moss SJ, Pangalos MN. The C-terminal domains of the GABA(b) receptor subunits mediate intracellular trafficking but are not required for receptor signaling. J Neurosci. 2001;21:1203–1210. doi: 10.1523/JNEUROSCI.21-04-01203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelisse LN, Van der Harst JE, Lodder JC, Baarendse PJ, Timmerman AJ, Mansvelder HD, Spruijt BM, Brussaard AB. Reduced 5-HT1A- and GABAB receptor function in dorsal raphe neurons upon chronic fluoxetine treatment of socially stressed rats. J Neurophysiol. 2007;98:196–204. doi: 10.1152/jn.00109.2007. [DOI] [PubMed] [Google Scholar]
- Cortez MA, Wu Y, Gibson KM, Snead OC., 3rd Absence seizures in succinic semialdehyde dehydrogenase deficient mice: a model of juvenile absence epilepsy. Pharmacol Biochem Behav. 2004;79:547–553. doi: 10.1016/j.pbb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Couve A, Thomas P, Calver AR, Hirst WD, Pangalos MN, Walsh FS, Smart TG, Moss SJ. Cyclic AMP-dependent protein kinase phosphorylation facilitates GABA(B) receptor-effector coupling. Nat Neurosci. 2002;5:415–424. doi: 10.1038/nn833. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Luscher C. Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Drasbek KR, Hoestgaard-Jensen K, Jensen K. Modulation of extrasynaptic THIP conductances by GABA(A)-receptor modulators in mouse neocortex. J Neurophysiol. 2007;97:2293–2300. doi: 10.1152/jn.00651.2006. [DOI] [PubMed] [Google Scholar]
- Drasbek KR, Vardya I, Delenclos M, Gibson KM, Jensen K. SSADH deficiency leads to elevated extracellular GABA levels and increased GABAergic neurotransmission in the mouse cerebral cortex. J Inherit Metab Dis. 2008;31:662–668. doi: 10.1007/s10545-008-0941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfax BP, Pitcher JA, Scott MG, Calver AR, Pangalos MN, Moss SJ, Couve A. Phosphorylation and chronic agonist treatment atypically modulate GABA(B) receptor cell surface stability. J Biol Chem. 2004;279:12565–12573. doi: 10.1074/jbc.M311389200. [DOI] [PubMed] [Google Scholar]
- Gervasi N, Monnier Z, Vincent P, Paupardin-Tritsch D, Hughes SW, Crunelli V, Leresche N. Pathway-specific action of gamma-hydroxybutyric acid in sensory thalamus and its relevance to absence seizures. J Neurosci. 2003;23:11469–11478. doi: 10.1523/JNEUROSCI.23-36-11469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson KM, Jakobs C, Pearl PL, Snead OC. Murine succinate semialdehyde dehydrogenase (SSADH) deficiency, a heritable disorder of GABA metabolism with epileptic phenotype. IUBMB Life. 2005;57:639–644. doi: 10.1080/15216540500264588. [DOI] [PubMed] [Google Scholar]
- Grampp T, Notz V, Broll I, Fischer N, Benke D. Constitutive, agonist-accelerated, recycling and lysosomal degradation of GABA(B) receptors in cortical neurons. Mol Cell Neurosci. 2008;39:628–637. doi: 10.1016/j.mcn.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Hogema BM, Gupta M, Senephansiri H, Burlingame TG, Taylor M, Jakobs C, Schutgens RB, Froestl W, Snead OC, Diaz-Arrastia R, Bottiglieri T, Grompe M, Gibson KM. Pharmacologic rescue of lethal seizures in mice deficient in succinate semialdehyde dehydrogenase. Nat Genet. 2001;29:212–6. doi: 10.1038/ng727. [DOI] [PubMed] [Google Scholar]
- Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Huang ZJ. GABA(B) receptor isoforms caught in action at the scene. Neuron. 2006;50:521–524. doi: 10.1016/j.neuron.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Jaen C, Doupnik CA. RGS3 and RGS4 differentially associate with G-protein-coupled receptor-Kir3 channel signaling complexes revealing two modes of RGS modulation. Precoupling and collision coupling. J Biol Chem. 2006;281:34549–34560. doi: 10.1074/jbc.M603177200. [DOI] [PubMed] [Google Scholar]
- Jansen EE, Struys E, Jakobs C, Hager E, Snead OC, Gibson KM. Neurotransmitter alterations in embryonic succinate semialdehyde dehydrogenase (SSADH) deficiency suggest a heightened excitatory state during development. BMC Dev Biol. 2008;8:112. doi: 10.1186/1471-213X-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K, Mody I. GHB depresses fast excitatory and inhibitory synaptic transmission via GABA(B) receptors in mouse neocortical neurons. Cereb Cortex. 2001;11:424–9. doi: 10.1093/cercor/11.5.424. [DOI] [PubMed] [Google Scholar]
- Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I. GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J Neurophysiol. 2003;90:2690–2701. doi: 10.1152/jn.00240.2003. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, Bettler B. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- Knerr I, Pearl PL, Bottiglieri T, Snead OC, Jakobs C, Gibson KM. Therapeutic concepts in succinate semialdehyde dehydrogenase (SSADH; ALDH5a1) deficiency (gamma-hydroxybutyric aciduria). Hypotheses evolved from 25 years of patient evaluation, studies in Aldh5a1-/- mice and characterization of gamma-hydroxybutyric acid pharmacology. J Inherit Metab Dis. 2007;30:279–294. doi: 10.1007/s10545-007-0574-2. [DOI] [PubMed] [Google Scholar]
- Kulik A, Vida I, Luján R, Haas CA, López-Bendito G, Shigemoto R, Frotscher M. Subcellular localization of metabotropic GABA(B) receptor subunits GABA(B1a/b) and GABA(B2) in the rat hippocampus. J Neurosci. 2003;23:11026–35. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto N, Wilkins ME, Fairfax BP, Revilla-Sanchez R, Terunuma M, Tamaki K, Iemata M, Warren N, Couve A, Calver A, Horvath Z, Freeman K, Carling D, Huang L, Gonzales C, Cooper E, Smart TG, Pangalos MN, Moss SJ. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53:233–247. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouebe G, Lomazzi M, Cruz HG, Creton C, Lujan R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, Luscher C. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;10:1559–1568. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- Li Q, Kuhn CM, Wilson WA, Lewis DV. Effects of gamma hydroxybutyric acid on inhibition and excitation in rat neocortex. Neuroscience. 2007;150:82–92. doi: 10.1016/j.neuroscience.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis DE, Jin T, Lupyan D, Rosenhouse-Dantsker A. Phosphoinositide-mediated gating of inwardly rectifying K(+) channels. Pflugers Arch. 2007;455:83–95. doi: 10.1007/s00424-007-0276-5. [DOI] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Mannoury la Cour C, Boni C, Hanoun N, Lesch KP, Hamon M, Lanfumey L. Functional consequences of 5-HT transporter gene disruption on 5-HT(1a) receptor-mediated regulation of dorsal raphe and hippocampal cell activity. J Neurosci. 2001;21:2178–2185. doi: 10.1523/JNEUROSCI.21-06-02178.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY. Ligand-induced signal transduction within heterodimeric GABA(B) receptor. Proc Natl Acad Sci U S A. 2001;98:14643–14648. doi: 10.1073/pnas.251554798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- Mathivet P, Bernasconi R, De Barry J, Marescaux C, Bittiger H. Binding characteristics of gamma-hydroxybutyric acid as a weak but selective GABA(B) receptor agonist. Eur J Pharmacol. 1997;321:67–75. doi: 10.1016/s0014-2999(96)00916-8. [DOI] [PubMed] [Google Scholar]
- Mott DD, Li Q, Okazaki MM, Turner DA, Lewis DV. GABAB-Receptor-mediated currents in interneurons of the dentate-hilus border. J Neurophysiol. 1999;82:1438–1450. doi: 10.1152/jn.1999.82.3.1438. [DOI] [PubMed] [Google Scholar]
- Mutneja M, Berton F, Suen KF, Luscher C, Slesinger PA. Endogenous RGS proteins enhance acute desensitization of GABA(B) receptor-activated GIRK currents in HEK-293T cells. Pflugers Arch. 2005;450:61–73. doi: 10.1007/s00424-004-1367-1. [DOI] [PubMed] [Google Scholar]
- Nicoll RA. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988;241:545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Nicoll RA. My close encounter with GABA(B) receptors. Biochem Pharmacol. 2004;68:1667–1674. doi: 10.1016/j.bcp.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Nylen K, Velazquez JL, Likhodii SS, Cortez MA, Shen L, Leshchenko Y, Adeli K, Gibson KM, Burnham WM, Snead OC., 3rd A ketogenic diet rescues the murine succinic semialdehyde dehydrogenase deficient phenotype. Exp Neurol. 2008;210:449–457. doi: 10.1016/j.expneurol.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garci E, Gassmann M, Bettler B, Larkum ME. The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron. 2006;50:603–616. doi: 10.1016/j.neuron.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Prosser HM, Gill CH, Hirst WD, Grau E, Robbins M, Calver A, Soffin EM, Farmer CE, Lanneau C, Gray J, Schenck E, Warmerdam BS, Clapham C, Reavill C, Rogers DC, Stean T, Upton N, Humphreys K, Randall A, Geppert M, Davies CH, Pangalos MN. Epileptogenesis and enhanced prepulse inhibition in GABA(B1)-deficient mice. Mol Cell Neurosci. 2001;17:1059–1070. doi: 10.1006/mcne.2001.0995. [DOI] [PubMed] [Google Scholar]
- Robbins MJ, Calver AR, Filippov AK, Hirst WD, Russell RB, Wood MD, Nasir S, Couve A, Brown DA, Moss SJ, Pangalos MN. GABA(B2) is essential for G-protein coupling of the GABA(B) receptor heterodimer. J Neurosci. 2001;21:8043–8052. doi: 10.1523/JNEUROSCI.21-20-08043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R, Bischoff S, Kaupmann K, van der Putten H, Bettler B. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)) Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der Putten H, Kaupmann K, Bettler B, Luthi A. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci. 2006;9:1028–1035. doi: 10.1038/nn1732. [DOI] [PubMed] [Google Scholar]
- Snead OC. The gamma-hydroxybutyrate model of absence seizures: correlation of regional brain levels of gamma-hydroxybutyric acid and gamma-butyrolactone with spike wave discharges. Neuropharmacology. 1991;30(2):161–7. doi: 10.1016/0028-3908(91)90199-l. [DOI] [PubMed] [Google Scholar]
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Tiao JY, Bradaia A, Biermann B, Kaupmann K, Metz M, Haller C, Rolink AG, Pless E, Barlow PN, Gassmann M, Bettler B. The sushi domains of secreted GABA(B1) isoforms selectively impair GABA(B) heteroreceptor function. J Biol Chem. 2008;283:31005–31011. doi: 10.1074/jbc.M804464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AJ, Whittle SR. Biochemical dissection of the gamma-aminobutyrate synapse. Biochem J. 1983;209:29–41. doi: 10.1042/bj2090029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayer P, Ehrhardt J, Gobaille S, Mandel P, Maitre M. Gamma hydroxybutyrate distribution and turnover rates in discrete brain regions of the rat. Neurochem Int. 1988;12:53–59. doi: 10.1016/0197-0186(88)90148-9. [DOI] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, Vacher CM, Muller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmeier PC, Kaupmann K, Urwyler S. Roles of GABAB receptor subtypes in presynaptic auto- and heteroreceptor function regulating GABA and glutamate release. J Neural Transm. 2008;115:1401–1411. doi: 10.1007/s00702-008-0095-7. [DOI] [PubMed] [Google Scholar]
- Wetherington JP, Lambert NA. GABA(B) receptor activation desensitizes postsynaptic GABA(B) and A(1) adenosine responses in rat hippocampal neurones. J Physiol. 2002;544:459–467. doi: 10.1113/jphysiol.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins ME, Li X, Smart TG. Tracking cell surface GABAB receptors using an alpha-bungarotoxin tag. J Biol Chem. 2008;283:34745–34752. doi: 10.1074/jbc.M803197200. [DOI] [PMC free article] [PubMed] [Google Scholar]