Abstract
Purpose: This article reviews the recent advances in understanding of the fundamental properties of circadian rhythms and discusses the clinical features, diagnosis, and treatment of circadian rhythm sleep disorders (CRSDs).
Recent Findings: Recent evidence strongly points to the ubiquitous influence of circadian timing in nearly all physiologic functions. Thus, in addition to the prominent sleep and wake disturbances, circadian rhythm disorders are associated with cognitive impairment, mood disturbances, and increased risk of cardiometabolic disorders. The recent availability of biomarkers of circadian timing in clinical practice has improved our ability to identify and treat these CRSDs.
Summary: Circadian rhythms are endogenous rhythms with a periodicity of approximately 24 hours. These rhythms are synchronized to the physical environment by social and work schedules by various photic and nonphotic stimuli. CRSDs result from a misalignment between the timing of the circadian rhythm and the external environment (eg, jet lag and shift work) or a dysfunction of the circadian clock or its afferent and efferent pathways (eg, delayed sleep-phase, advanced sleep-phase, non–24-hour, and irregular sleep-wake rhythm disorders). The most common symptoms of these disorders are difficulties with sleep onset and/or sleep maintenance and excessive sleepiness that are associated with impaired social and occupational functioning. Effective treatment for most of the CRSDs requires a multimodal approach to accelerate circadian realignment with timed exposure to light, avoidance of bright light at inappropriate times, and adherence to scheduled sleep and wake times. In addition, pharmacologic agents are recommended for some of the CRSDs. For delayed sleep-phase, non–24-hour, and shift work disorders, timed low-dose melatonin can help advance or entrain circadian rhythms; and for shift work disorder, wake-enhancing agents such as caffeine, modafinil, and armodafinil are options for the management of excessive sleepiness.
OVERVIEW OF THE HUMAN CIRCADIAN SYSTEM
Circadian rhythms are physiologic and behavioral cycles with a recurring periodicity of approximately 24 hours, generated by the endogenous biological pacemaker, the suprachiasmatic nucleus (SCN), located in the anterior hypothalamus.1 These rhythms control a variety of biological processes, such as sleep-wake cycle, body temperature, feeding, hormone secretion, glucose homeostasis, and cell-cycle regulation. The timing of these physiologic rhythms may become altered, leading to changes in the phase relationship of rhythms to each other, which can cause internal desynchronization. This loss of coordination of rhythms may have negative consequences on rest-activity cycles and other physiologic and behavioral functions.
Circadian Entrainment
Circadian rhythms are synchronized with the earth’s rotation by daily adjustments in the timing of the SCN, following the exposure to stimuli that signal the time of day. These stimuli are known as zeitgebers (German for “time-givers”), of which light is the most important and potent stimulus. The magnitude and direction of the change in phase depends on when within the circadian system the light pulse is presented. A plot of phase changes according to the time of light stimulus presentation provides a phase response curve. Exposure to light results in a phase response curve with delays in the early subjective night (ie, evening) and advances in the late subjective night (ie, early morning). In addition to light, feeding schedules, activity, and the hormone melatonin can also affect the circadian timing.1
The timing of melatonin secretion by the pineal gland is regulated by the SCN, with the onset of secretion approximately 2 hours before natural sleep time and being highest during the middle of the night.1 Melatonin onset measured in a dim light environment (DLMO) is a stable marker of circadian phase and is used in research as well as clinical practice to determine the timing of the endogenous circadian rhythm.
Neuroanatomy and Neurochemistry
The central circadian timing system has three distinct components: (1) a circadian pacemaker, the SCN, (2) input pathways for light and other stimuli that synchronize the pacemaker to the environment, and (3) output rhythms that are regulated by the pacemaker. The SCN is the central pacemaker that links the 24-hour changes in the external environment with the 24-hour changes in the internal environment (Figure 7-1).2 The SCN is composed from the “core” and “shell” subnuclei, which have distinct neurochemical properties.1 While γ-aminobutyric acid is the dominant neurotransmitter in the SCN, present in nearly all SCN neurons, SCN neuropeptides are highly localized within either the core or shell nuclei. The SCN core contains high density of vasoactive intestinal polypeptide, gastrin-releasing peptide, and bombesin-containing neurons. Somatostatin and neurophysin are dominant neurochemicals within the SCN shell.
Figure 7-1.
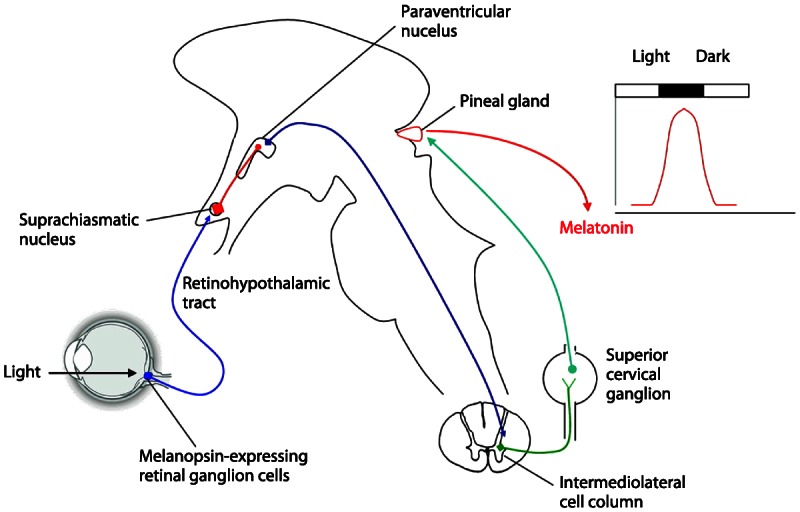
Schematic illustration of the pathway responsible for entrainment of melatonin secretion by light. The circadian regulation of melatonin secretion is dependent on an indirect pathway that originates in photosensitive ganglion cells in the retina and reaches the suprachiasmatic nucleus, the circadian pacemaker, via the retinohypothalamic tract. The suprachiasmatic nucleus controls the sympathetic output to the pineal gland, which is responsible for melatonin secretion via an inhibitory projection to the paraventricular nucleus of the hypothalamus. This pathway is responsible for the peak of melatonin secretion during darkness. Reprinted with permission from Benarroch EE, Neurology.2 © 2008, American Academy of Neurology. www.neurology.org/content/71/8/594.extract.
The SCN receives photic information from the retina via direct (retinohypothalamic) and indirect (retinogeniculate) pathways.3 The melanopsin-containing ganglion cells of the retina are the primary photoreceptors for the circadian system. The SCN also receives nonphotic information from the raphe nuclei. Several less-characterized afferents converge in the SCN from basal forebrain, pons, medulla, and posterior hypothalamus. The major efferents from the SCN project to the subparaventricular zone and the paraventricular nucleus of the hypothalamus, dorsomedial hypothalamus, thalamus, preoptic and retrochiasmatic areas, stria terminalis, lateral septum, and intergeniculate nucleus. The SCN also communicates via diffusion of humoral signals to the rest of the brain. These diffusible SCN outputs likely include transforming growth factor α, cardiotrophinlike cytokine, and prokineticin 2. A major development in chronobiology research has been the discovery of circadian clocks in non-SCN brain regions and almost all peripheral tissues.4 While the signals mediating communication between the SCN and peripheral oscillators remain under extensive investigation, it is clear that the central clock (ie, SCN) and peripheral clocks may have distinct circadian synchronizers. The SCN, however, is most likely dominant in maintaining circadian rhythmicity of peripheral clocks.
Genetic Regulation
Circadian rhythms are determined genetically by a core set of clock genes, including three Per genes (the period homolog 1 gene, Per1; the period homolog 2 gene, Per2; the period homolog 3 gene, Per3); the circadian locomotor output cycles kaput gene, Clock; the cycle gene, Bmal1; and two plant cryptochrome gene homologs (the cryptochrome 1 gene, Cry1, and the cryptochrome 2 gene, Cry2).5 These genes and their products interact to form transcription-translation feedback loops that provide the molecular basis of circadian rhythmicity. During the day, Clock interacts with BMal1 to activate transcription of the Per and Cry genes, resulting in high levels of these transcripts. PER and CRY proteins translocate to the nucleus and inhibit CLOCK–B-MAL1-mediated transcription. During the night, the PER-CRY repressor complex is degraded, and the cycle starts again (Figure 7-2). Circadian clock genes control a significant proportion of the genome. It is estimated that approximately 10% of all expressed genes are under regulation of the clock genes. Furthermore, peripheral tissues contain independent clocks. It is likely that peripheral clocks are synchronized by an input directly from the SCN or SCN-mediated messages. Several excellent reviews are available for more detailed overview of the molecular regulation of the circadian system,3,6,7
Figure 7-2.
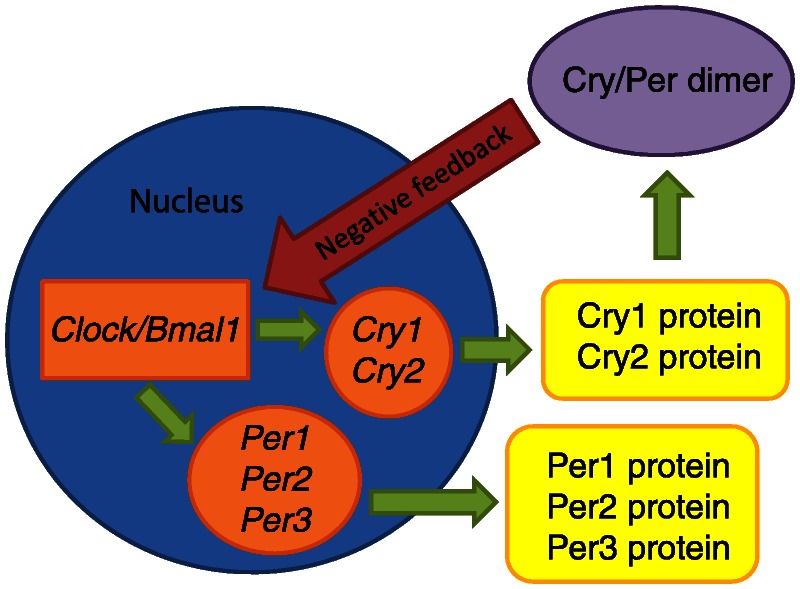
Simplified representation of the transcription cycle.
CIRCADIAN RHYTHM SLEEP DISORDERS
Circadian rhythm sleep disorders (CRSDs) are chronic patterns (for at least 1 month) of sleep-wake rhythm disturbances due to alterations of the circadian timing system or to a misalignment between the timing of the endogenous circadian rhythm and the sleep-wake times required by school or work schedules. As a result, patients present with impairments in sleep and wake functioning. The diagnosis of all CRSDs is based on a careful history and sleep diary with actigraphy. Polysomnography (PSG) is not routinely indicated to establish the diagnosis. However, PSG is indicated to assess for other comorbid sleep disorders. In addition to comorbid sleep disorders, psychiatric disorders—particularly depression and anxiety—are common in patients with nearly all types of CRSDs and should be considered in the differential diagnosis.
Delayed Sleep-Phase Disorder
Delayed sleep-phase disorder (DSPD) is characterized by a chronic or recurrent inability to fall asleep and wake up at socially acceptable times, resulting in symptoms of difficulty falling asleep and excessive daytime sleepiness, particularly in the morning. By definition, in relation to socially acceptable times, there is a more than 2-hour delay in the major sleep period. Patients have difficulty waking up in the morning and are often late for work or school. When patients are allowed to sleep at their biologically preferred time and wake up spontaneously after their major sleep period, sleep and daytime function normalize.
Epidemiology. The prevalence of DSPD is 0.2% to 10.0% depending on severity and the population groups surveyed. Milder cases are more prevalent, as are cases among adolescents and young adults. There appears to be no sex predilection, but among adolescents the phase delay occurs at an older age in males than in females, with males reaching their peak at age 21 and females at age 17. A familial predisposition for DSPD also occurs, and DSPD appears to be common (33%) in patients with hepatic cirrhosis.8
Pathophysiology. Multiple biological and behavioral factors contribute to the development of DSPD. The postulated mechanisms for DSPD include (1) decreased response to the phase-advancing effect of light in the morning,9 (2) increased sensitivity to the phase-delay response of evening light, and (3) a longer than normal time to complete one circadian cycle (ie, long circadian period). Familial cases and the demonstration of polymorphisms of circadian clock genes in DSPD indicate a genetic basis for this condition.10 Environmental, behavioral, and psychological factors also play a role in the development of DSPD. For example, individuals with a delayed circadian phase are more likely to work in the evening and be exposed to evening light, which can further delay the timing of circadian rhythms, or they wake up late, thus perpetuating the vicious cycle of late sleep and late wake times.11
Clinical presentation and diagnosis. Patients with DSPD usually fall asleep between 1:00 AM and 6:00 AM and wake up in the late morning to early afternoon, as outlined in the International Classification of Sleep Disorders, Second Edition: Diagnostic and Coding Manual. Conditioned insomnia and chronic sleep deprivation may develop as a complication of DSPD. In addition, patients with DSPD tend to have decreased academic and work performance, especially during morning hours, in addition to habitual tardiness and morning absences. Diagnosis is made by careful history and well-kept sleep diaries with or without actigraphy for a minimum of 7 days (preferably 14 days) (Case 7-1). Standardized chronotype questionnaires are useful tools to assess the chronotype of “eveningness” and “morningness.” In addition, a delay in the timing of objective circadian rhythms, such as the DLMO or urinary 6-sulfatoxymelatonin, is desirable to confirm the delayed circadian phase. Depression, anxiety, and personality disorders are more common among patients with DSPD.
Case 7-1
A 21-year-old man presented with nightly complaints of difficulty falling asleep that started 4 to 5 years earlier. He had no problem staying asleep, but it took several hours to fall asleep, and he had difficulty staying alert during the workday. He was concerned because his work performance was suffering and he had been seen dozing at his desk. Actigraphy recording of his sleep and wake cycle is shown in Figure 7-3.
Figure 7-3.
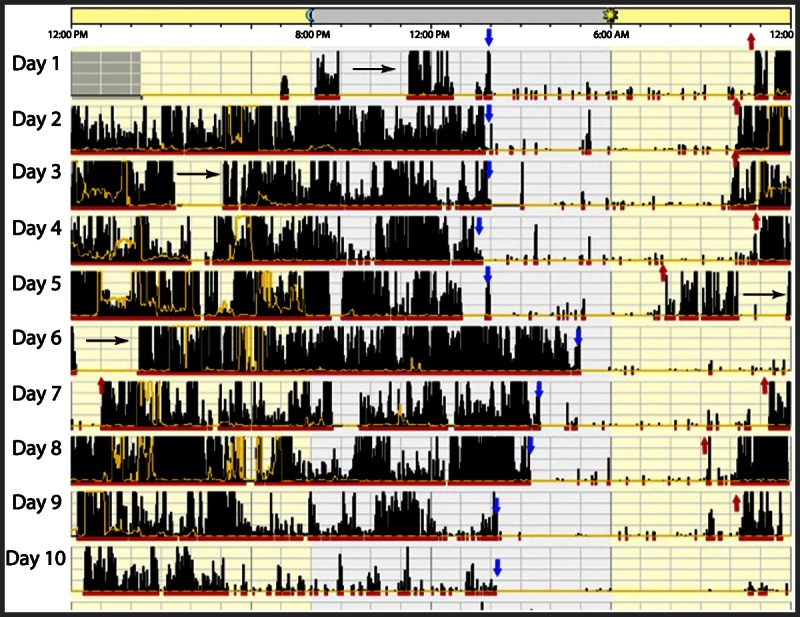
Representative actogram of patient with delayed sleep-phase disorder. The blue arrows indicate sleep onset and the red arrows indicate the end of the major sleep period. The black horizontal arrows indicate naps. In high-amplitude actigraphy, dense bars are representative of wakefulness, and low, sparse bars are representative of sleep. Note that sleep onset is 2:00 AM to 4:00 AM and the end of the major sleep period varies from 8:00 AM all the way to 1:00 PM (on day 7). On day 5, when total sleep duration was from 2:00 AM to 7:00 AM, the patient takes two naps, resulting in less sleep homeostatic drive, and therefore sleep onset the next night is not until 5:00 AM.
Comment: Typical of patients with delayed sleep-phase disorder, this man had significant sleep-onset insomnia andwas then sleepywhile atwork because of chronic sleep deprivation andbecause hewas forced to beawake during a part of his circadian sleep time.
Treatment. The American Academy of Sleep Medicine (AASM) practice parameters recommend appropriately timed morning light exposure and evening exogenous melatonin either alone or in combination as effective treatments for DSPD (Figure 7-4). The combination of light therapy and melatonin has been shown to have complementary benefits.12 Bright light (full spectrum or blue enriched) in the morning for 2 hours shortly after the minimum of the core body temperature rhythm (typically occurring 2 to 3 hours before natural wake-up time) has been shown to successfully advance circadian rhythms in patients with DSPD.13 Melatonin 0.5 mg to 5 mg given 5.0 to 6.5 hours before DLMO (13 to 14 hours after natural wake-up time)14 advances sleep and rise times, while administration closer to DLMO is less effective. Effectiveness lasts up to 1 year with daily melatonin intake, but relapses can occur in up to 90% of people after they discontinue their melatonin. Time to relapse ranges from 1 day to 6 months with the more severe DSPD cases relapsing faster.15 Furthermore, melatonin has been shown to improve depression in patients with DSPD.16 Although it has been reported that vitamin B12 may be effective as an adjunctive treatment to bright light, it was not recommended by the 2007 AASM practice parameters as a treatment for DSPD.17 There is also limited evidence suggesting a therapeutic benefit of combining light therapy with cognitive-behavioral therapy in adolescents with DSPD.18
Figure 7-4.
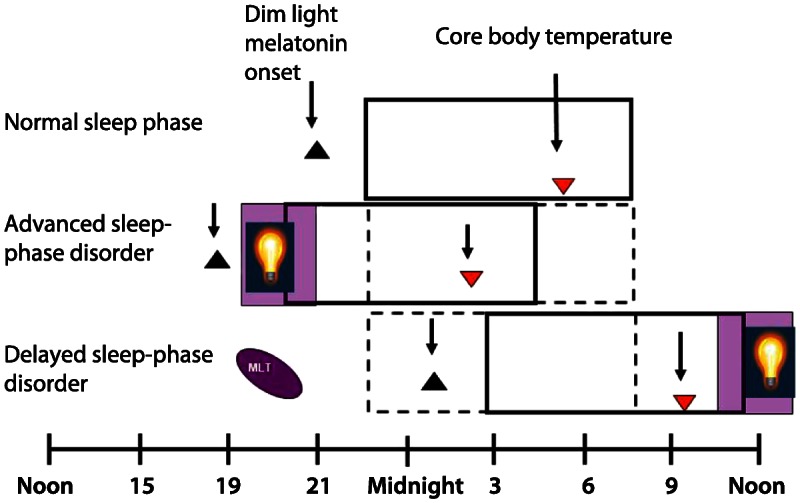
Summary of treatment approaches for delayed sleep-phase disorder and advanced sleep-phase disorder. Bright light administered before the nadir of body core temperature is a potent stimulus for delaying circadian phase. The most commonly used treatment for advanced sleep-phase disorder is early-evening light therapy, usually between 7:00 PM and 9:00 PM. This approach has been shown to improve sleep duration and sleep maintenance, as well as daytime performance. The combination of light therapy and melatonin has been shown to have complementary benefits. Bright light in the morning for 1 to 2 hours shortly after the minimum of the core body temperature rhythm advances circadian rhythms and 0.5 mg to 5 mg of melatonin taken 5 to 6.5 hours before dim light melatonin onset (13 to 14 hours after natural wake-up time) results in advanced sleep and wake times in patients with delayed sleep-phase disorder. MLT = melatonin.
Advanced Sleep-Phase Disorder
Advanced sleep-phase disorder (ASPD) is characterized by an advance in the phase of the major sleep episode in relation to the desired or required sleep and wake-up times. Patients have chronic or recurrent difficulty staying awake until the desired or socially acceptable bedtime, together with an earlier than desired wake-up time. When patients are allowed to choose their preferred schedule, sleep quality and duration are normal for age.
Epidemiology. ASPD is less common than DSPD. The estimated prevalence is 1% in the general population, which is likely an underestimate since many individuals successfully adapt their social and work schedules to the advanced sleep phase.19 Both sexes are equally affected by the disorder. The onset of ASPD is typically during middle age. Several studies suggest that age may be a risk factor for ASPD, likely on the basis of a phase advance of the circadian pacemaker with aging.17
Pathophysiology. A shortened circadian period has been postulated to be involved in the pathogenesis of ASPD. Genetic factors likely play an important role in the development of ASPD. Several families with ASPD inherited in an autosomal dominant mode have been described. Two gene mutations have been identified in some of these families, affecting the circadian clock gene hPer2 and the casein kinase 1 delta gene.20 These mutations result in a shortened circadian period. Additional mechanisms include an attenuated ability to phase delay because of a dominant phase advance region of the phase response curve to light and increased retinal sensitivity to light in the morning,21 resulting in a stronger advancing signal for the circadian clock.
Clinical presentation and diagnosis. Patients with ASPD typically present with symptoms of daytime sleepiness, most prominent in the late afternoon or early evening hours; sleep maintenance difficulty; and early morning awakening. Individuals with ASPD are usually sleepy and struggle to stay awake between 6:00 PM and 9:00 PM and wake up earlier than desired, between 2:00 AM and 5:00 AM.
The diagnosis of ASPD is based on a detailed sleep history accompanied by a sleep diary and, if feasible, actigraphy over a period of least 7 days (preferably 14 days) to demonstrate advanced sleep and wake times. Major depressive disorders should be carefully differentiated from ASPD. Standardized chronotype questionnaires are useful tools to assess the chronotype of eveningness and morningness. Patients with ASPD will score as “morning” types. In addition, an advance in the timing of objective circadian rhythms, such as the DLMO or urinary 6-sulfatoxymelatonin, is desirable to confirm the advanced circadian phase.
Treatment. The AASM practice parameter recommends sleep-wake scheduling and timed light exposure as the primary treatments for ASPD. Practical therapeutic approaches for ASPD include timed light exposure in the evening and avoiding light in early morning hours (Figure 7-4). Melatonin or hypnotics may be beneficial for sleep maintenance insomnia. Bright light administered before the nadir of body core temperature is a potent stimulus for delaying circadian phase. The most commonly used treatment for ASPD is early-evening light therapy, usually between 7:00 PM and 9:00 PM. This approach has been shown to improve sleep duration and sleep maintenance, as well as daytime performance. However, results of evening light therapy have not been uniformly positive.22 Compliance with timed light exposure can be challenging, and bright light may irritate the eyes, particularly in older adults. A chronotherapeutic approach of advancing bedtime by 3 hours every 2 days has been reported effective in ASPD; however, the scheduling constraint of this approach limits its use in clinical practice. Based on the phase response curve to melatonin, administration of melatonin in early morning will advance the circadian phase, although clinical evidence of the efficacy or safety of melatonin for the treatment of ASPD is lacking. While hypnotic agents are prescribed for the management of sleep maintenance symptoms associated with ASPD, their efficacy and safety have not been systematically studied.
Irregular Sleep-Wake Rhythm Disorder
Irregular sleep-wake rhythm disorder (ISWRD) is characterized by a temporally disorganized sleep and wake pattern, such that multiple sleep and wake periods occur throughout the 24-hour cycle. This disorder is more prevalent in older adults with dementia and in patients with developmental disorders.
Epidemiology. ISWRD is common among institutionalized older adults, particularly those with Alzheimer disease and those with late afternoon-evening agitation or sundowning. Age alone is not a risk factor for ISWRD, but age-related neurologic and psychiatric disorders are.23 ISWRD has also been described in patients with head trauma, children with developmental delay, and patients with schizophrenia, particularly those with positive symptoms,24 independent of daytime functioning.25
Pathophysiology. Multiple physiologic, behavioral, and environmental factors contribute to the development of ISWRD. The most likely mechanisms include central degeneration of SCN neurons and decreased exposure or input of external synchronizing agents, such as light and activity that result in a weakened central circadian oscillation and temporal disorganization of circadian rhythms.26 The problem is perpetuated by a variety of factors inherent in the lifestyles of older adults in nursing homes and other similar living facilities. Institutionalized elderly patients get significantly less exposure to light in both amount and intensity. This is a result of lower levels of light indoors and the fact that most eye diseases, such as cataracts, reduce light input to the SCN even further. Compounding the diminished light exposure is the reduction in other external synchronizers such as structured social and physical activities. Decreased mobility, adverse effect on sleep and alertness by medications, and increased napping also play a role in the development of ISWRD.26 Genetic factors have been implicated in the development of ISWRD in Alzheimer disease.27
Clinical presentation and diagnosis. In patients with ISWRD, sleep bouts occur in three or more short intervals of approximately 1 to 4 hours each, spread over 24 hours. The longest bout generally occurs between 2:00 AM and 6:00 AM. The overall amount of sleep per 24-hour period, however, is relatively normal for the patient’s age.23 Because of fragmented sleep and multiple daytime naps, patients usually present with symptoms of sleep maintenance insomnia and excessive daytime sleepiness. In addition to the typical symptomatology, diagnosis requires a history of a minimum of three irregular sleep-wake cycles in a 24-hour cycle recorded for 14 days by sleep diary and/or actigraphy.
Treatment. Creating a cognitively enriched environment with structured social and physical activity during the day is an important therapeutic modality, especially if combined with a healthy bedtime routine and a nocturnal environment conducive to sleep. Measures include minimizing noise and light during the scheduled sleep period and addressing issues such as nocturia and enuresis to reduce sleep disturbances at night. Light, however, remains the most effective therapeutic intervention. Exposure to 3000 lux to 5000 lux bright light for 2 hours every morning for 4 weeks has been shown to improve daytime alertness, decrease napping, consolidate nighttime sleep, and reduce nocturnal agitation.23 Melatonin alone has not been shown to be consistently effective in treating ISWRD in older adults or in patients with Alzheimer disease. However, effectiveness may be improved when melatonin at bedtime is combined with light during the day.21 Small open-label trials using doses of 2 mg to 20 mg of melatonin have shown some benefit in children with developmental disorders.28 Controlled-release formulation appeared more effective than immediate release in this subpopulation. The AASM practice parameters recommend using a combination of environmental and behavioral modifications and bright light therapy for ISWRD.
Non–24-Hour Sleep-Wake Disorder
Non–24-hour sleep-wake disorder (N24SWD) (nonentrained rhythm disorder formerly known as free-running rhythm disorder) is characterized by a chronic or recurrent pattern of sleep and wake cycles that are not synchronized to the 24-hour environment. Typically a consistent daily drift (usually to later and later times) of sleep-onset and wake-up times occurs.
Epidemiology. Sleep disturbances in people who are blind are common, and approximately 50% may have N24HSWD. Much less commonly, N24HSWD can occur in sighted people. Onset of symptoms typically occurs during the second or third decade of life.29 In blind and sighted individuals, there is a male predilection with a ratio of 2.6/1.30
Pathophysiology. The etiology of N24HSWD in blind people is clearly a marked decrease or absence of light perception. However, not all patients who are blind exhibit this lack of entrainment, because in some, light information from the retinal ganglion cells can still reach the SCN, or other synchronizing agents (such as structured social and physical activity) can sufficiently entrain circadian rhythms.30 Although the exact mechanism in sighted individuals remains to be elucidated, evidence suggests that a long circadian period that is beyond the normal range of entrainment is likely a risk factor.30 Other postulated mechanisms that can lead to an abnormal interaction between sleep homeostasis and endogenous circadian rhythms include (1) decreased photosensitivity,30 (2) alteration and reduction of social cues because of psychiatric illness–induced social withdrawal,21 (3) mutation in the creatinine kinase 1 ɛ (CK1ɛ) gene,21 and (4) desynchrony between the melatonin and sleep rhythms.30
Clinical presentation and diagnosis. The presenting symptoms depend on when the person is required to sleep in relation to his or her nonentrained endogenous circadian rhythm of sleep-wake propensity. Patients typically present with symptoms of insomnia, excessive daytime sleepiness, or both for several weeks. These symptomatic episodes alternate with days to weeks in which the patient is asymptomatic. The prevailing complaint is the interference of the sleep-wake schedule with work, school, and other social obligations.29 Patients with this disorder can have, at various times, excessive daytime sleepiness and sleep-onset insomnia or early morning awakenings. Napping is quite common, and careful analysis of sleep-wake rhythms may reveal two distinct sleep-wake cycle periods separated by phase jumps (ie, when sleep onset is delayed for more than 4 hours).21
Diagnosis is made by a careful history and documenting that the sleep complaints are present for at least 2 months. A minimum of 14 days of sleep diary and/or actigraphy can be very helpful. Actigraphy of a patient with N24HSWD is shown in Figure 7-5. Continuous core body temperature measurements, if feasible, or serial measurements of the timing of the melatonin rhythm from serum, saliva, or urine can be confirmatory as they exhibit the same non–24-hour rhythm as the disorder itself.21 Frequent comorbid psychiatric conditions, primarily mood disorders, need to be addressed as well. Care has to be taken to differentiate N24HSWD from DSPD. Most sighted patients with N24HSWD also have an evening chronotype. A misdiagnosis can be problematic, as chronotherapy for DSPD may induce a non–24-hour rhythm. In the largest cohort of sighted N24HSWD patients, one-fourth had received a previous misdiagnosis of DSPD.21
Figure 7-5.
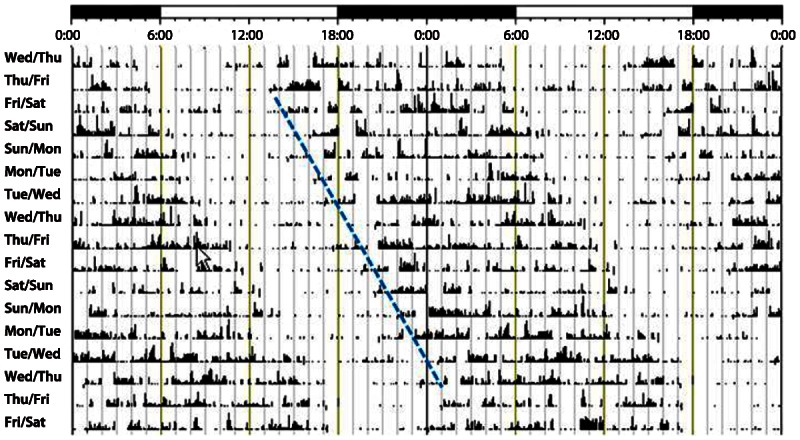
Actigraphy record of a sighted patient with non–24-hour sleep-wake disorder. Note the daily delay drift of the onset and offset of the sleep-wake rhythm with a circadian period that is longer than 24 hours.
Treatment. In blind patients with N24SWD, melatonin is the therapeutic mainstay together with strong structured behavioral and social cues, such as timing of meals, planned activities, and regular physical exercise.29 This same approach is recommended for sighted persons, with the additional option of bright light exposure in the morning shortly after awakening. Although the dose of melatonin for the treatment of N24HSWD varies among studies, a practical recommendation is to start with a higher dose (3 mg to 10 mg) 1 hour before bedtime or a few hours before predicted DLMO for the first month. Entrainment usually occurs within 3 to 9 weeks but must be maintained by regular low-dose (0.5 mg) melatonin to prevent a relapse. If the initiation dose fails, an alternate method is a 0.5-mg dose over a period of several months. Most blind patients whose circadian period is close to 24 hours can maintain entrainment with very low nightly doses of 20 μg to 300 μg. Vitamin B12 trials have been unsuccessful in sighted patients, but evidence from case reports suggests that a combination of timed melatonin doses of 0.5 mg to 5.0 mg taken nightly at 9:00 PM, exposure to bright light, and a regular sleep-wake schedule is successful in entraining these patients.17
Jet-Lag Disorder
Jet-lag disorder results from travel across several time zones and subsequent misalignment of the internal circadian clock and the destination’s local time. Symptoms of jet lag usually emerge within 1 to 2 days after travel. Main manifestations of jet lag are generalized malaise, sleep disturbances, impaired daytime alertness, poor appetite, diminished cognitive performance, depressed mood, irritability, and anxiety.
Pathophysiology. Internal desynchronization of physiologic rhythms resulting from time-zone changes is responsible for most of the symptoms of jet-lag disorder. The severity and type of jet-lag symptoms depend on several variables, including the number of time zones crossed and the direction of travel.31 Eastward travel may be more difficult to adapt to than westward travel, because the former requires advancing circadian rhythms and the latter a phase delay. Humans generally have an endogenous circadian period that is slightly longer than 24 hours, so that a delay shift is more easily achieved. Older adults may have more difficulty with circadian realignment than younger people.32 Typically, symptoms of jet lag subside within a few days but may persist for a few weeks in some travelers. The speed of this resynchronization is somewhat faster with westbound travel (1.0 hour per day) compared with eastbound travel (1.5 hours per day). Not all travelers crossing multiple time zones develop jet-lag disorder, but most will experience some level of sleep and wake disturbance.
Clinical presentation and diagnosis. Patients with jet-lag disorder typically present with symptoms of recurrent insomnia and daytime somnolence as a result of rapid travel across two or more time zones. The sleep disturbance leads to clinically significant impairment in daytime functioning. The most common sleep disturbances associated with jet lag are sleep fragmentation, early morning awakenings, and sleep-initiation insomnia. People traveling eastward develop difficulty falling asleep and awakening the next day. Westbound travelers experience excessive somnolence in the early evening, and early morning awakening. In addition to impairments of sleep and wake function, travelers affected by jet lag report gastrointestinal disturbances, menstrual irregularities, and the exacerbation of affective disorders. Cognitive impairment emerging from jet lag may have serious consequences, such as impaired decision-making for business travelers or impaired performance in athletes.33 Effects of jet lag not only affect travelers but can also have rather significant consequences for airline pilots and need to be considered when planning work schedules, stopover durations, and rest periods between flights.
Treatment. The main objective in treating jet lag is to improve sleep quality and daytime alertness by realigning the endogenous circadian rhythm with the required or desired sleep and wake times of the destination’s time zone. However, when the time in the destination is expected to be brief (2 days or less), circadian adaptation may be counterproductive and treatment should be aimed at improving or alleviating jet-lag symptoms.34
Nonpharmacologic treatment approaches are important in the management of jet-lag disorder. Strategic exposure and avoidance of exposure to light have been utilized as an effective treatment approach. Inappropriate timing of light exposure may result in further desynchronization of the circadian system at the destination. The optimal timing or avoidance of light exposure depends on the direction of travel and the number of time zones crossed.9 For example, after an eastbound flight from Chicago to Paris, passengers should avoid bright light in early morning and expose themselves to bright light in late morning and afternoon (to advance circadian rhythms). If flying westbound, efforts should be made to stay awake during the daylight hours, maximize light exposure in the afternoon and early evening, and not sleep until nighttime at the destination. Shifting the circadian clock by using timed exposure to light several days before travel may be useful in minimizing jet-lag symptoms but has practical limitations for a frequent business traveler.
Currently, no US Food and Drug Administration (FDA)–approved pharmacologic agents are available for the treatment of jet-lag disorder. Based on its ability to phase shift circadian rhythms and its potential soporific effect, melatonin has been studied. Administration of melatonin at doses of 0.5 mg to 10 mg in the early evening hours several days before eastbound travel followed by administration at bedtime at the destination effectively reduces symptoms of jet lag.22 Significant improvements with melatonin were demonstrated by self-reported sleep and mood measures as well as objective circadian measures (ie, melatonin and cortisol rhythms). Immediate-release melatonin appears to be more effective than extended-release formulations.34 A Cochrane Review of 10 randomized placebo-controlled trials of melatonin and air travel concluded that melatonin at doses of 2 mg to 5 mg taken before bedtime over 2 to 4 days is effective in reducing jet-lag symptoms.35 A combination approach incorporating melatonin with timed physical activity and light exposure usually results in additional improvements of symptoms.
Ramelteon, an MT1/MT2 melatonin receptor agonist with greater affinity for melatonin receptors and longer half-life compared to melatonin, may be effective in treating symptoms of jet lag. In a recent placebo-controlled study, a significant decrease in sleep latency was achieved with administration of ramelteon (1 mg), administered at bedtime for 4 nights at the new destination.36 In this study, beneficial effects of ramelteon were strongly influenced by light exposure since only participants maintained in constant dim light had significant differences in latency to persistent sleep. Further studies are needed to prove the efficacy of ramelteon for jet lag.
Several other pharmacologic agents, including caffeine and hypnotic medications, have been explored as options to alleviate jet-lag symptoms. Short-acting hypnotic medications can be used to treat insomnia associated with jet lag.9 Several studies demonstrated beneficial effects of caffeine on fatigue and the reduced alertness associated with jet lag. In a randomized placebo-controlled study of modafinil, improved alertness and other jet-lag symptoms were achieved after administration of 150 mg of modafinil.37 Based on the AASM practice parameters, timed melatonin administration is recommended as treatment for jet-lag disorder. Additional treatment options include maintaining home-based sleep hours for brief travel, short-term hypnotic use for insomnia, and caffeine to alleviate daytime sleepiness.
Shift Work Disorder
Shift work disorder (SWD) is characterized by a history of chronic (at least 1 month) excessive sleepiness during the required wake (work) time and/or insomnia symptoms during the associated required or desired sleep period that occurs in relation to unconventional work schedules.
Epidemiology. Almost 20% of the workforce in the developed world is engaged in shift work. The prevalence of SWD is approximately 1% in the general population and up to 10% among night and rotating shift workers. In the general population, men are slightly at higher risk than women for SWD.38 In certain populations, such as nurses, the prevalence of SWD can reach about 40%.38
Pathophysiology. The primary etiology of SWD is the opposition of required sleep and wake times to their endogenous circadian rhythm of sleep and wake propensity. This often results in shortened sleep duration by 1 to 4 hours. In addition, trying to stay awake during the night, when the circadian alertness signal is low, leads to excessive sleepiness during the work hours.21 The overnight shift is usually associated with the most severe symptoms, but patients may report symptoms of SWD with any shift that requires one to be awake at an adverse circadian time. Tolerance to the effects of shift work may vary with age, chronotype, comorbid sleep disorders, social situation, and distance of commute between home and work.21
Clinical presentation and diagnosis. The diagnosis is made by careful history of symptoms and work schedule. A minimum of 2 weeks of sleep logs with or without actigraphy can help not only by showing total sleep duration but also by demonstrating circadian-sleep misalignment.21 On nonworking days, individuals with SWD tend to revert back to more traditional daytime activities and night sleep schedules, contributing further to the circadian misalignment. Night shift workers and rotating shift workers get less sleep than day workers or evening shift workers. Night shift workers generally have no difficulty falling asleep but complain primarily of difficulty maintaining sleep during the late morning or afternoon. Excessive sleepiness is most marked during the last half of the work hours and while commuting to home at the end of the shift. Other symptoms of SWD include chronic fatigue, malaise, mood disorder, and nonspecific complaints, such as dyspepsia and decreased libido.29 Risk of alcohol and substance abuse is increased, as is the risk of weight gain, hypertension, and cardiovascular disease, and some studies suggest an association with breast and endometrial cancer.39 In addition to the medical comorbidities, SWD is accompanied by significant social and economic burdens in the form of accidents, lost days of work, poorer performance, and increased health care use.39
Treatment. The primary aim of treatment is to improve alertness during the required wake time and sleep quality during the scheduled sleep time. All patients with SWD should be counseled regarding conservative nonpharmacologic measures. These include optimizing the sleep environment (eg, darkened room, comfortable temperature, noise reduction), adherence to good sleep habits (eg, maintain a regular sleep and wake schedule, avoid excessive caffeine), patient and family education, and scheduled naps when possible.
Appropriately timed light therapy has been shown to accelerate circadian adaptation to night shift work. For night shift workers, bright light exposure ranging from 1000 lux to 10,000 lux either in 3- or 6-hour blocks or in 20-minute or 1-hour blocks (ending 2 hours before the end of the shift) has been shown to accelerate circadian adaptation to night work and improve both alertness and performance (Case 7-2).40 Complementary to light exposure during work, it is important to avoid bright light exposure during the morning commute by using appropriate eyewear.17
Case 7-2
A 26-year-old female security guard reported chronic fatigue and difficulty keeping up with her duties. She had started her job 4 months ago and worked 8- to 9-hour night shifts for 4 to 5 consecutive days (from 11:00 PM or midnight toz 7:00 AM). On days off she usually went to bed when her husband did at midnight and slept until late morning. She reported difficulty staying asleep during the day when off duty, and sleepiness and decreased concentration during her shift at night. She described malaise, anxiety, and decreased libido. When working, she consumed large amounts of caffeine and sugar to help her stay awake. Her sleep-wake diary before and after treatment is shown in Figure 7-6.
Figure 7-6.
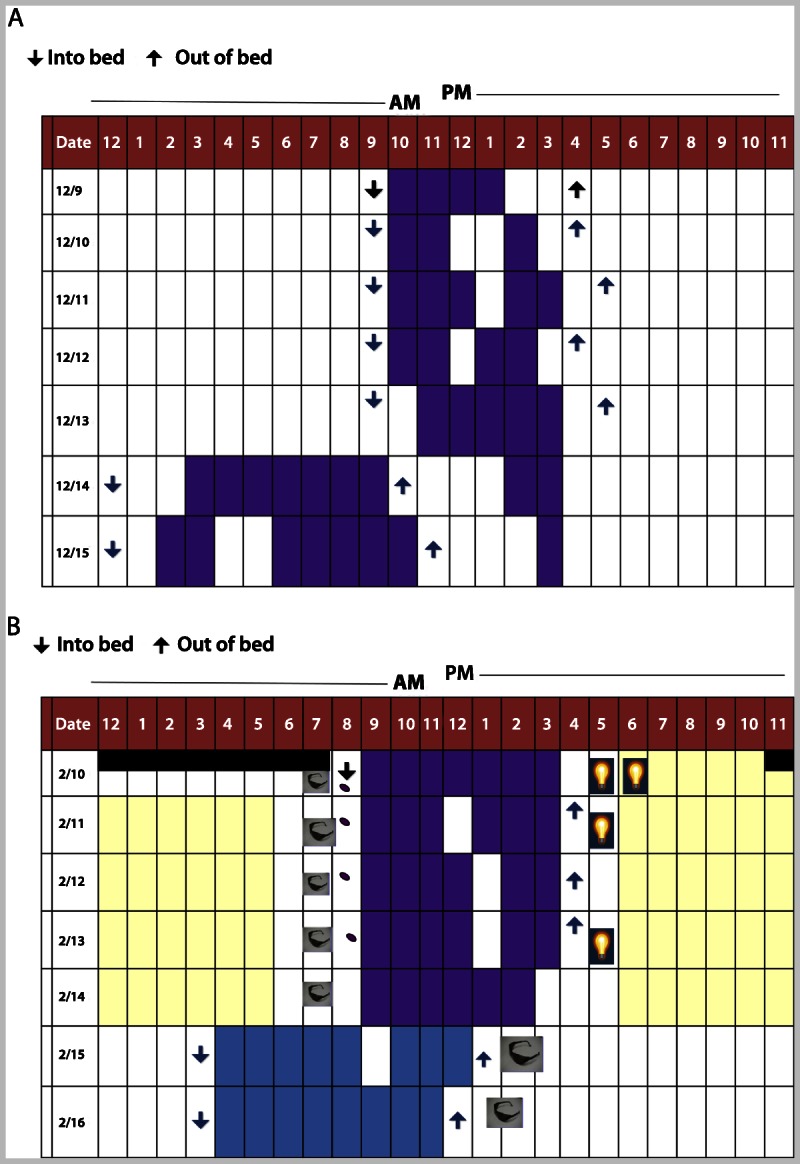
Sleep-wake diary of a patient with shift work disorder. A, Patient’s sleep diary for 1 week before treatment. B, Patient’s sleep diary for 1 week after 1 month of treatment with a combination of intermittent naps when possible before the shift, bright light exposure (yellow) during the shift, and avoidance of light on the morning commute (sunglasses) together with maintaining a dark and quiet bedroom. In addition, 3 mg of melatonin (purple pill) was started to improve sleep as needed. Bright light exposure for 30 minutes to 1 hour (light bulb) was also initiated shortly after awakening during work days. During days off, she was instructed to maintain a compromised sleep and wake schedule so that she would have time with her husband and friends, but not completely revert to a daytime schedule.
Comment: As shown in Figure 7-6, after appropriate treatment the patient is sleeping in the “compromised circadian position” with sleep and wake times that allow time for some social activities during her days off, yet the change in sleep-wake times between days off and days on duty is not dramatically different. This sleep-wake schedule allows for more sleep consolidation and longer sleep duration throughout the week.
Data on the use of melatonin, at various doses, have produced conflicting results. However, melatonin when taken at bedtime does appear to modestly improve daytime sleep, but without any significant impact on nighttime alertness or performance. All other pharmacologic modalities fail to address the circadian misalignment but can be used for improving alertness during work hours or sleep during the scheduled sleep time. Therefore, pharmacologic agents should be used in combination with light and behavioral modalities. Hypnotics are not specifically indicated for SWD but may be prescribed for the treatment of insomnia that is often seen in these patients. Caffeine combined with timed naps improves performance and alertness during the night shift.41 The wake-promoting agents modafinil (200 mg) and armodafinil (150 mg) have been shown to improve performance and alertness when taken at the beginning of the night shift.17 Both modafinil and armodafinil are approved by the FDA for the treatment of excessive sleepiness associated with SWD. The AASM practice parameters recommend planned napping before and/or during the work shift, timed light exposure, and stimulants such as caffeine or modafinil during the night shift to improve alertness.17
KEY POINTS
Circadian rhythms are physiologic and behavioral cycles with a recurring periodicity of approximately 24 hours, generated by the endogenous biological pacemaker, the suprachiasmatic nucleus, located in the anterior hypothalamus.
Circadian rhythms are synchronized with the earth’s rotation by daily adjustments in the timing of the suprachiasmatic nucleus, following the exposure to stimuli that signal the time of day. These stimuli are known as “zeitgebers” (German for “time-giver”), of which light is the most important and potent stimulus. The magnitude and direction of the change in phase depends on when within the circadian system the light pulse is presented.
Delayed sleep-phase disorder is characterized by chronic or recurrent inability to fall asleep and wake up at socially acceptable times, resulting in symptoms of difficulty falling asleep and excessive daytime sleepiness, particularly in the morning.
Diagnosis of delayed sleep-phase disorder is made by careful history and well-kept sleep diaries with or without actigraphy for a minimum of 7 days (preferably 14 days).
Bright light (full spectrum or blue enriched) in the morning for 2 hours shortly after the minimum of the core body temperature rhythm (typically occurring 2 to 3 hours before natural wake-up time) has been shown to successfully advance circadian rhythms in patients with delayed sleep-phase disorder.
Patients with advanced sleep-phase disorder typically present with symptoms of daytime sleepiness (most prominent in the late afternoon or early evening hours) sleep maintenance difficulty, and early morning awakening.
Practical therapeutic approaches for advanced sleep-phase disorder include timed light exposure in the evening and avoiding light in early morning hours. Melatonin or hypnotics may be beneficial for sleep-maintenance insomnia.
Creating a cognitively enriched environment with structured social and physical activity during the day is an important therapeutic modality for patients with irregular sleep-wake rhythm disorder, especially if combined with a healthy bedtime routine and a nocturnal environment conducive to sleep.
Non–24-hour sleep-wake disorder is characterized by a chronic or recurrent pattern of sleep and wake cycles that are not synchronized to the 24-hour environment. Typically a consistent daily drift (usually to later and later times) of sleep-onset and wake-up times occurs.
In blind patients with non–24-hour sleep-wake disorder, melatonin is the therapeutic mainstay together with strong structured behavioral and social cues such as timing of meals, planned activities, and regular physical exercise. This same approach is recommended for sighted persons, with the additional option of bright light exposure in the morning shortly after awakening.
Internal desynchronization of physiologic rhythms resulting from time-zone changes is responsible for most of the symptoms of jet-lag disorder. The severity of jet lag depends on several variables, including the number of time zones crossed and the direction of travel.
Nonpharmacologic treatment approaches are important in the management of jet-lag disorder. Strategic exposure and avoidance of exposure to light have been utilized as an effective treatment approach.
Based on the American Academy of Sleep Medicine practice parameters, timed melatonin administration is recommended as treatment for jet-lag disorder.
Shift work disorder is accompanied by significant social and economic burdens in the form of accidents, lost days of work, poorer performance, and increased health care use.
Night shift workers and rotating shift workers get less sleep than day workers or evening shift workers.
For night shift workers, bright light exposure ranging from 1000 lux to 10,000 lux either in 3- to 6-hour blocks or in 20-minute to 1-hour blocks (ending 2 hours before the end of the shift) has been shown to accelerate circadian adaptation to night work and improve both alertness and performance.
Footnotes
Relationship Disclosure: Dr Zee has received personal compensation for activities with Jazz Pharmaceuticals; Merck & Co, Inc; Perdue Pharma; Philips Respironics; Sanofi-Aventis; Takeda Pharmaceutical Company Limited; UCB; and Zeo, Inc. Dr Zee receives research support from Philips Respironics. Dr Attarian receives personal compensation for activities with American Physicians Institute. Dr Videnovic reports no disclosure.
Unlabeled Use of Products/Investigational Use Disclosure: Dr Zee discusses the unlabeled use of melatonin for the treatment of circadian disorders. Dr Attarian discusses the unlabeled use of melatonin and light boxes to advance or delay circadian rhythms. Dr Videnovic discusses the unlabeled use of melatonin, ramelteon, and supplemental light exposure to advance circadian rhythms and treat jet-lag disorder.
REFERENCES
- 1. Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev 2010; 90 (3): 1063– 1102 [DOI] [PubMed] [Google Scholar]
- 2. Benarroch EE. Suprachiasmatic nucleus and melatonin: reciprocal interactions and clinical correlations. Neurology 2008; 71 (8): 594– 598 [DOI] [PubMed] [Google Scholar]
- 3. Dardente H, Cermakian N. Molecular circadian rhythms in central and peripheral clocks in mammals. Chronobiol Int 2007; 24 (2): 195– 213 [DOI] [PubMed] [Google Scholar]
- 4. Cermakian N, Boivin DB. The regulation of central and peripheral circadian clocks in humans. Obes Rev 2009; 10 (10 suppl 2): 25– 36 [DOI] [PubMed] [Google Scholar]
- 5. Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 2008; 9 (10): 764– 775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet 2006; 15: R271– R277 [DOI] [PubMed] [Google Scholar]
- 7. Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet 2004; 5: 407– 441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montagnese S, Middleton B, Mani AR, et al. Sleep and circadian abnormalities in patients with cirrhosis: features of delayed sleep phase syndrome? Metab Brain Dis 2009; 24 (3): 427– 439 [DOI] [PubMed] [Google Scholar]
- 9. Kolla BP, Auger RR. Jet lag and shift work sleep disorders: how to help reset the internal clock. Cleve Clin J Med 2011; 78 (10): 675– 684 [DOI] [PubMed] [Google Scholar]
- 10. Archer SN, Carpen JD, Gibson M, et al. Polymorphism in the PER3 promoter associates with diurnal preference and delayed sleep phase disorder. Sleep 2010; 33 (5): 695– 701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crowley SJ, Carskadon MA. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiol Int 2010; 27 (7): 1469– 1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paul MA, Gray GW, Lieberman HR, et al. Phase advance with separate and combined melatonin and light treatment. Psychopharmacology (Berl) 2011; 214 (2): 515– 523 [DOI] [PubMed] [Google Scholar]
- 13. Smith MR, Revell VL, Eastman CI. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med 2009; 10 (3): 287– 294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crowley SJ, Acebo C, Fallone G, Carskadon MA. Estimating dim light melatonin onset (DLMO) phase in adolescents using summer or school-year sleep/wake schedules. Sleep 2006; 29 (12): 1632– 1641 [DOI] [PubMed] [Google Scholar]
- 15. van Geijlswijk IM, Korzilius HP, Smits MG. The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis. Sleep 2010; 33 (12): 1605– 1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rahman SA, Kayumov L, Shapiro CM. Antidepressant action of melatonin in the treatment of Delayed Sleep Phase Syndrome. Sleep Med 2010; 11 (2): 131– 136 [DOI] [PubMed] [Google Scholar]
- 17. Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin 2010; 5 (4): 701– 715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gradisar M, Dohnt H, Gardner G, et al. A randomized controlled trial of cognitive-behavior therapy plus bright light therapy for adolescent delayed sleep phase disorder. Sleep 2011; 34 (12): 1671– 1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ando K, Kripke DF, Ancoli-Israel S. Delayed and advanced sleep phase symptoms. Isr J Psychiatry Relat Sci 2002; 39 (1): 11– 18 [PubMed] [Google Scholar]
- 20. Xu Y, Padiath QS, Shapiro RE, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature 2005; 434 (7033): 640– 644 [DOI] [PubMed] [Google Scholar]
- 21. Reid KJ, Zee PC. Circadian rhythm sleep disorders. Handb Clin Neurol 2011; 99: 963– 977 [DOI] [PubMed] [Google Scholar]
- 22. Morgenthaler TI, Lee-Chiong T, Alessi C, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep 2007; 30 (11): 1445– 1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zee PC, Vitiello MV. Circadian rhythm sleep disorder: irregular sleep wake rhythm type. Sleep Med Clin 2009; 4 (2): 213– 218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Afonso P, Brissos S, Figueira ML, Paiva T. Schizophrenia patients with predominantly positive symptoms have more disturbed sleep-wake cycles measured by actigraphy. Psychiatry Res 2011; 189 (1): 62– 66 [DOI] [PubMed] [Google Scholar]
- 25. Wulff K, Dijk DJ, Middleton B, et al. Sleep and circadian rhythm disruption in schizophrenia. Br J Psychiatry 2012; 200 (4): 308– 316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou QP, Jung L, Richards KC. The management of sleep and circadian disturbance in patients with dementia. Curr Neurol Neurosci Rep 2012; 12 (2): 193– 204 [DOI] [PubMed] [Google Scholar]
- 27. Hida A, Kusanagi H, Satoh K, et al. Expression profiles of PERIOD1, 2, and 3 in peripheral blood mononuclear cells from older subjects. Life Sci 2009; 84 (1–2): 33– 37 [DOI] [PubMed] [Google Scholar]
- 28. Braam W, Smits MG, Didden R, et al. Exogenous melatonin for sleep problems in individuals with intellectual disability: a meta-analysis. Dev Med Child Neurol 2009; 51 (5): 340– 349 [DOI] [PubMed] [Google Scholar]
- 29. Kanathur N, Harrington J, Lee-Chiong T., Jr Circadian rhythm sleep disorders. Clin Chest Med 2010; 31 (2): 319– 325 [DOI] [PubMed] [Google Scholar]
- 30. Uchiyama M, Lockley S. Non-24-hour sleep-wake syndrome in sighted and blind patients. Sleep Med Clin 2009; 4: 195– 211 [DOI] [PubMed] [Google Scholar]
- 31. Bjorvatn B, Pallesen S. A practical approach to circadian rhythm sleep disorders. Sleep Med Rev 2009; 13 (1): 47– 60 [DOI] [PubMed] [Google Scholar]
- 32. Moline ML, Pollak CP, Monk TH, et al. Age-related differences in recovery from simulated jet lag. Sleep 1992; 15 (1): 28– 40 [DOI] [PubMed] [Google Scholar]
- 33. Srinivasan V, Singh J, Pandi-Perumal SR, et al. Jet lag, circadian rhythm sleep disturbances, and depression: the role of melatonin and its analogs. Adv Ther 2010; 27 (11): 796– 813 [DOI] [PubMed] [Google Scholar]
- 34. Suhner A, Schlagenhauf P, Johnson R, et al. Comparative study to determine the optimal melatonin dosage form for the alleviation of jet lag. Chronobiol Int 1998; 15 (6): 655– 666 [DOI] [PubMed] [Google Scholar]
- 35. Herxheimer A, Petrie KJ. Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst Rev 2002; (2): CD001520. [DOI] [PubMed] [Google Scholar]
- 36. Zee PC, Wang-Weigand S, Wright KP, Jr, et al. Effects of ramelteon on insomnia symptoms induced by rapid, eastward travel. Sleep Med 2010; 11 (6): 525– 533 [DOI] [PubMed] [Google Scholar]
- 37. Rosenberg RP, Bogan RK, Tiller JM, et al. A phase 3, double-blind, randomized, placebo-controlled study of armodafinil for excessive sleepiness associated with jet lag disorder. Mayo Clin Proc 2010; 85 (7): 630– 638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Flo E, Pallesen S, Mageroy N, et al. Shift work disorder in nurses—assessment, prevalence and related health problems. PLoS One 2012; 7 (4): e33981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Culpepper L. The social and economic burden of shift-work disorder. J Fam Pract 2010; 59 (1 suppl): S3– S11 [PubMed] [Google Scholar]
- 40. Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev 2002; 6 (5): 407– 420 [PubMed] [Google Scholar]
- 41. Ker K, Edwards PJ, Felix LM, et al. Caffeine for the prevention of injuries and errors in shift workers. Cochrane Database Syst Rev 2010; (5): CD008508. [DOI] [PMC free article] [PubMed] [Google Scholar]