Abstract
Objectives
The use of sonography in musculoskeletal research and clinical applications is increasing; however, measurement techniques for diagnosing carpal tunnel syndrome with sonography continue to be inconsistent. Novel methods of measurement using internal comparisons to identify swelling of the median nerve require investigation and comparison to currently used techniques.
Methods
The flattening ratio of the median nerve, bowing of the flexor retinaculum, and cross-sectional area of the median nerve were collected in the forearm, at the radio-carpal joint, and at the level of the pisiform in both symptomatic patients and asymptomatic control participants. Electrodiagnostic testing was completed in symptomatic patients as a diagnostic standard.
Results
Median nerve measurements were collected from 166 wrists of symptomatic and asymptomatic participants. The flattening ratio did not show any correlation to electrodiagnostic testing and was identical between both symptomatic and asymptomatic participants. Moderate to strong correlations were noted between electrodiagnostic testing results and sonographic measurements of the cross-sectional area at the pisiform, retinacular bowing, and both the ratio and change of the cross-sectional area between the forearm and pisiform. The area under the curve was large for all receiver operating characteristic curves for each measurement (0.759–0.899), and sensitivity was high (80.4%–82.4%).
Conclusions
Measurement of swelling through a ratio or absolute change had similar diagnostic accuracy as individual measurement of the cross-sectional area within the carpal tunnel. These measures may be useful for improving accuracy in more diverse clinical populations. Further refinement of protocols to identify the largest cross-sectional area within the carpal tunnel region and statistical methods to analyze clustered, multilevel outcome data are recommended to improve diagnostics.
Keywords: diagnostics, electrodiagnostics, median mononeuropathy, musculoskeletal
The use of sonography for investigation and diagnosis of musculoskeletal conditions has been rapidly increasing over the past few decades. Advances in the quality and portability of sonography have well positioned this technology as the tool of choice for research and clinical application in orthopedics, neurology, and other musculoskeletal practice settings.1 Full integration of sonography into clinical and research applications requires convincing diagnostic standards.
Evidence supporting this use of sonography as a diagnostic tool for median nerve conditions, specifically carpal tunnel syndrome, is inconsistent. Despite studies with high correlation and diagnostic accuracy, one review indicated that there remains a lack of convincing evidence to support the use of sonography in diagnosis of carpal tunnel syndrome.2 However, the conflicting evidence in previous studies may be a result of variable methods and techniques.3 Positive correlation of sonographic measurements to diagnostic reference standards, such as electrodiagnostic testing, shows the promise of sonography as a screening tool for carpal tunnel syndrome.4
The flattening ratio of the median nerve, anterior bowing of the flexor retinaculum, and measurement of the cross-sectional area of the median nerve are the three most common diagnostic measures that have been investigated. Of these, measurement of the cross-sectional area of the median nerve within the carpal tunnel at the level of the pisiform has been the most consistent in previous research literature. Studies that have attempted to take indirect measures of the cross-sectional area using the ellipsoid formula have shown lower diagnostic accuracy.5,6 Because of the irregular shape the median nerve frequently takes, most literature indicates that measurement of the cross-sectional area is best assessed through a direct trace.7,8 Similarly, a few studies have included the hypoechoic epineurium in the cross-sectional area measurement9–11; however, there is consensus in the literature that more precise measurement of the cross-sectional area is obtained along the inner hypoechoic border.
Diagnostic accuracy continues to vary across research studies using the cross-sectional area at the pisiform; therefore, measurement of the swelling of the median nerve has been suggested as a refined method.12 Anthropometry may cause natural variation in the size of the median nerve among individuals of both sexes and various body compositions. Therefore, comparison of the cross-sectional area of the median nerve in the carpal tunnel region to an unaffected site (ie, forearm) may provide more accurate information regarding changes within a specific individual.
Two methods for measuring the swelling of the median nerve have been proposed, but neither has been confirmed with extensive research. Swelling calculated as a ratio between the cross-sectional area at the distal radius and pisiform levels was noted to have very low sensitivity (6%), indicating that use of a wrist measurement may not be a good internal comparison.13 However, measurement of the cross-sectional area swelling as a ratio between the forearm and wrist14 has been shown to reduce the rate of false-negative results from 37% to 2% over a single measurement of the cross-sectional area at the pisiform.15 An alternative measurement of swelling calculated as the absolute change in the cross-sectional area between the forearm and carpal tunnel region has also shown high diagnostic accuracy at 96% to 100% sensitivity.16
The objective of this study was to investigate the utility of sonography for diagnosis of carpal tunnel syndrome compared to the current clinical reference standard of electrodiagnostic testing. This study will standardize data collection and measurement techniques based on previous literature while investigating the accuracy of both previously studied measures and new methods. Previous measurements to be evaluated include the flattening ratio, bowing of the flexor retinaculum, and cross-sectional area in the carpal tunnel region. Swelling of the median nerve will be evaluated through both the absolute change in the cross-sectional area and the ratio of the cross-sectional area in the carpal tunnel compared to the forearm.
Materials and Methods
From June through December 2010, all patients who entered the neurodiagnostic clinic at The Ohio State University for a nerve conduction study and electromyography (EMG) were screened for entry into the study. The study was explained to the patients, and they all provided written consent to participate and permission for the researchers to review test results. This study was approved by the Biomedical Institutional Review Board at The Ohio State University.
Study Participants
Only those patients with suspected idiopathic carpal tunnel syndrome were offered the option to participate in the study, which required a referral that indicated a diagnosis of carpal tunnel syndrome or primary symptoms of carpal tunnel syndrome. Patients were included in the study if they had numbness or tingling in the median nerve distribution of at least one hand that had lasted at least 3 weeks. Patients were excluded from the study if they had a history of trauma to the wrist or hand that included broken bones, if there was a history of surgery to the wrist or any permanently placed shunts or objects in the hand or wrist, if there was a known history of other systemic neurologic disorders or uncontrolled thyroid disorders, or if the patient was pregnant or within 3 months postpartum. The same exclusion criteria were used to recruit a convenience sample of asymptomatic nonclinical control participants. The study was limited to the working adult population 18 to 65 years of age. Participants with anatomic anomalies observed during electrodiagnostic testing or sonographic data collection were excluded from further analysis (ie, bifurcated median nerve and Martin-Gruber anastomosis). Participants with diabetes were not excluded from the study provided diabetic neuropathy was ruled out with electrodiagnostic testing. Similarly, participants with a persistent median artery were not excluded provided no other anatomic anomalies or obstruction of the artery was observed that may have been contributory to symptoms.
Anthropometric and demographic data included age, height, mass, sex, hand dominance, and wrist width and depth. Each participant’s body mass index (BMI) and wrist ratio (wrist depth divided by wrist width) were calculated. Clinical assessment included a subjective report of symptoms including the duration of symptoms, Symptom Severity Scale and Functional Severity Scale,17 and clinical provocative tests (Phalen, Tinel, and Durkin).
Wrists were evaluated separately and divided into symptomatic and asymptomatic wrists after completion of provocative tests and subjective symptom reports. Both wrists of the patients were included in the study as long as a bilateral investigation was prescribed by the referring physician and each wrist met all inclusion criteria. The wrists of the controls who had symptoms or positive provocative test results were excluded.
Electrodiagnostic Testing
Electrodiagnostic studies were completed on all symptomatic patients with a Synergy tower (Care Fusion, Inc, Middleton, WI). The asymptomatic controls did not receive electrodiagnostic testing. Nerve conduction studies and EMG testing were completed by a neurologist based on American Association of Electrodiagnostic Technologists and American Association of Neuromuscular and Electrodiagnostic Medicine guidelines. The skin temperature at the fingers was higher than 34°C for all participants before initiating nerve conduction protocols. Orthodromic sensory responses were obtained by placing stimulating electrodes at the proximal crease of digit 2 and in the palm, 8 cm from the recording site on the ventral forearm at the wrist crease. Distal sensory nerve action potentials were averaged. The sensory nerve action potential amplitude, velocity, and latency were measured for all participants. Compound muscle action potentials were obtained by placing the recording electrodes over the abductor pollicis brevis on the thenar eminence. The simulating electrodes were placed 7 cm proximally over the median nerve at the wrist and at the antecubital fossa. The distal motor latency, distal and proximal compound muscle action potential amplitudes, and conduction velocity were recorded. Additional nerve conductions on the ulnar nerve were always performed, and the comparisons to nerve conduction results in the contralateral arm and leg were completed as indicted to determine any underlying polyneuropathy. Needle EMG was completed in the abductor pollicis brevis, and additional muscles were studied to rule out proximal median nerve, brachial plexus, or radicular abnormalities.
Nerve conduction study results were considered diagnostic of carpal tunnel syndrome when the sensory conduction velocity was less than 50 m/s across the carpal tunnel. Additional abnormalities including a sensory nerve action potential amplitude of less than 10 μV, distal motor latency of greater than 4.2 milliseconds, a compound muscle action potential amplitude of less than 4.0 mV, and changes recorded by EMG were used to determine the severity of carpal tunnel syndrome. Electromyographic results were recorded as normal, acute, or chronic. The absence of any electrical diagnostic criterion resulted in classification of the wrist as normal. Reduction in the sensory conduction velocity with normal motor responses and EMG results was categorized as mild carpal tunnel syndrome. Sensory nerve abnormalities combined with prolonged distal motor latency but normal EMG results were considered to represent moderate carpal tunnel syndrome. The absence of sensory responses coupled with motor nerve changes and abnormal EMG results was categorized as severe carpal tunnel syndrome.
Sonography
Sonography was completed with a LOGIQ i ultrasound system (GE Healthcare, Milwaukee, WI) and a 12-MHz linear array transducer. The sonographic settings and image acquisition were based on a previously published protocol.1 The participants sat facing the examiner with the forearm supinated and resting on a flat surface. The hand, wrist, and fingers were in a neutral and relaxed position throughout the evaluation. Longitudinal and cross-sectional images of the median nerve were collected as follows: (1) in the distal third of the forearm 6 cm proximal to the distal wrist crease, (2) proximal to the entrance of the carpal tunnel at the radiocarpal joint, and (3) within the carpal tunnel at the level of the pisiform. One additional cross-sectional image was taken at the distal carpal tunnel to observe the flexor retinaculum between the trapezium and hook of the hamate.
Sonograms were collected in wrists of both the symptomatic patients and asymptomatic controls. During collection of the images, the researcher annotated the image and placed a mark on the image to identify the median nerve to ensure that appropriate structures were analyzed. For the symptomatic patients, sonographic data were collected on the same day and within 1 hour of electrodiagnostic testing. Because of recruitment methods and required subjective/clinical testing for inclusion criteria, researchers obtaining the sonograms were not blinded to the participant’s symptom status. However, the sonographers were blinded to the results of electrodiagnostic testing in the symptomatic patients. Quality assurance checks were completed for gray scale images on the ultrasound equipment at least monthly throughout data collection to ensure reliability in image collection and processing.
Image Processing
To ensure reliability of measurements, all image processing and measurements were completed by the first author. The reliability of measurements was established by the first authors in previous publications.1,18 On a random basis, measurements were periodically verified by the second author to ensure that protocols were being followed for image processing. The researcher completing measurements and the researcher completing reliability checks were both blinded to all other data, including the recruitment group and method, subjective reports of the participants, and results of electrodiagnostic testing. Without reducing the image resolution, images were magnified to improve the precision of measurements along the inner echogenic border of the median nerve. Each measurement was repeated 5 times; the highest and lowest measurements were excluded; and the remaining 3 measurements were averaged.19
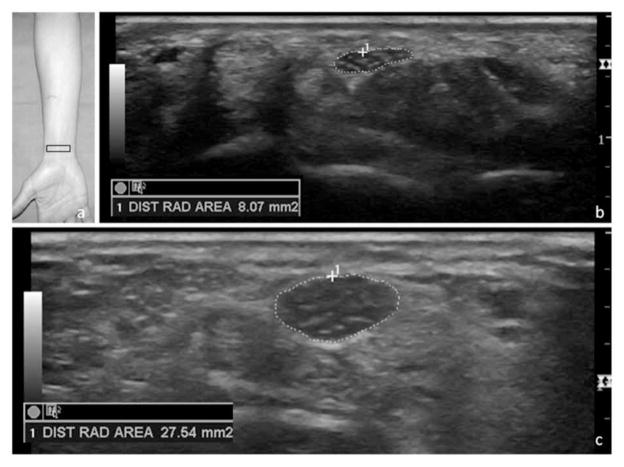
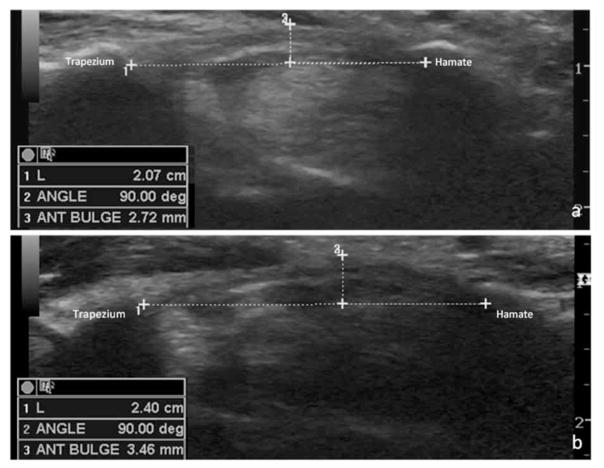
Measurements of the anteroposterior height (millimeters) and mediolateral width (millimeters) were taken at each of the 3 locations from the inside edge of the echogenic borders of the median nerve. The cross-sectional area (square millimeters) was obtained by a direct trace along the inner rim of the echogenic border of the nerve in each location (Figure 1). The height of the retinacular bulge was measured as the perpendicular distance from a line connecting the insertion points on the trapezium and hook of the hamate to the anterior-most point of the flexor retinaculum (Figure 2).
Figure 1.
Location of the transducer to obtain a cross-sectional image of the median nerve at the radial-carpal joint (a) with a sample image of a normal median nerve (b) and measurement of an enlarged median nerve in a symptomatic patient (c). DIST RAD indicates distal radius.
Figure 2.
Measurement of the retinacular bulge in the distal outlet of the carpal tunnel between the trapezium and hook of the hamate in an asymptomatic control participant (a) and a symptomatic patient (b). ANT indicates anterior; and L, length.
The flattening ratio was calculated by dividing the width by the height of the nerve in each of the locations. The cross-sectional area in the forearm was used as an internal reference for each participant. Cross-sectional area change scores were calculated as the absolute difference of the cross-sectional area at the distal radius and pisiform from the cross-sectional area in the forearm. The cross-sectional area ratio was calculated for each participant as the cross-sectional area at the distal radius and pisiform each divided by the cross-sectional area in the forearm.
Statistical Analysis
All demographic, anthropometric, and clinical data were compared between the symptomatic patients and asymptomatic controls to identify differences between the two groups on patient level variables. Frequencies, descriptive statistics, and distribution statistics for variables within each group were completed. χ2 testing was completed on all categorical data, and independent sample t tests were completed on continuous data to identify any differences between the two groups (P < .05).
Additional analyses were completed with data collected from symptomatic subjects. Kendall tau-b correlations were calculated for each variable versus the diagnostic group assignment in the symptomatic patients. Pearson correlations were completed between sonographic measurements and the primary electrodiagnostic testing measurements of the sensory conduction velocity and distal motor latency within the symptomatic group. Correlation results were used to identify specific sonographic measurements to be further analyzed with receiver operating characteristic curve analysis to calculate the sensitivity and specificity of sonographic measurements.
Results
Ninety-five individuals consented to participate in the study. After screening and application of all exclusion criteria, 47 symptomatic patients (83 wrists) and 44 asymptomatic controls (83 wrists) were included in the study. Wrists were excluded before the collection of data if the participant reported a history of surgery (n = 3) or wrist fracture (n = 3). In symptomatic patients, data were only included for wrists with subjective indications of median nerve conditions in the hands and at least 1 positive provocative test result. Wrists were excluded from analysis for any control participants who reported symptoms or had positive provocative test results (n = 4). After sonographic evaluation, wrists were excluded from analysis if bifurcation of the median nerve (n = 7) or Martin-Gruber anastomosis (n = 1) was visualized. A persistent median artery was documented in 6 wrists (3.2% of the total sample), but was not deemed a primary exclusion factor. Table 1 reports frequencies of exclusion criteria by group.
Table 1.
Number of Wrists (Percentage of Occurrence) Excluded by Group and Across All Participants Recruited Into the Study
| Reason for Exclusion | Symptomatic Patients (n = 98 wrists) | Asymptomatic Controls (n = 92 wrists) | Total |
|---|---|---|---|
| Bifurcated nerve | 4 (4.1) | 3 (3.3) | 7 (3.7) |
| Previous surgery | 3 (3.1) | 0 (0.0) | 3 (1.6) |
| History of wrist fracture | 1 (1.0) | 2 (2.2) | 3 (1.6) |
| Martin-Gruber anastomosis | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Symptoms not matching group assignment | 6 (6.1) | 4 (4.3) | 10 (5.3) |
| Total excluded | 15 (15.3) | 9 (9.8) | 24 (8.4) |
Significant differences were noted between the two groups in age, mass, BMI, and wrist ratio (P< .05; Table 2). All sonographic measurements were significantly larger in the symptomatic group versus the asymptomatic group with the exception of the flattening ratio, which was nearly identical between the two groups (Table 3). Although data for all sonographic measurements were noted to be normally distributed, the variability of data was much wider for the symptomatic group versus the control group on all measurements except the cross-sectional area in the forearm. Because the distribution of data for the cross-sectional area in the forearm was similar between groups and the means differed only by 0.50 mm2, the clinical relevance of this significant difference is questionable.
Table 2.
Descriptive Characteristics of Symptomatic Patients and Asymptomatic Control Participants
| Variable | Symptomatic Patients (n = 47) | Asymptomatic Controls (n = 44) | Test Statistic | df | P |
|---|---|---|---|---|---|
| Age, y | 45.6 (10.6) | 40.0 (12.1) | t = 2.355 | 89 | .021 |
| Female/male | 37/10 | 30/14 | χ2 = 1.301 | 1 | .254 |
| Right/left hand dominance | 44/3 | 37/7 | χ2 = 2.108 | 1 | .146 |
| Height, cm | 166.0 (8.3) | 168.8 (8.5) | t = −1.578 | 89 | .118 |
| Mass, kg | 88.0 (21.2) | 78.0 (19.7) | t = 2.339 | 89 | .022 |
| Body mass index, kg/m2 | 32.0 (7.4) | 27.4 (7.0) | t = 2.980 | 89 | .004 |
| Total wrists | 83 | 83 | NA | NA | NA |
| Dominant wrists | 44 | 42 | χ2 = 0.602 | 1 | .438 |
| Wrist ratio | 0.732 (0.041) | 0.710 (0.041) | t = 3.535 | 164 | .001 |
Values in parentheses are SD. NA indicates not applicable.
Table 3.
Comparison of Means (SDs) of Sonographic Measurements of the Median Nerve Between Symptomatic and Asymptomatic Wrists
| Measurement | Symptomatic Wrists (n = 83) | Asymptomatic Wrists (n = 83) | t | df | P |
|---|---|---|---|---|---|
| CSA in forearm, mm2 | 6.16 (1.28) | 5.64 (1.04) | 2.841 | 157.5 | .005 |
| CSA at distal radius, mm2 | 10.42 (3.82) | 7.95 (1.72) | 5.363 | 114.0 | <.001 |
| CSA at pisiform, mm2 | 11.36 (4.33) | 8.31 (1.89) | 5.883 | 112.2 | <.001 |
| Retinacular bulge, mm | 3.21 (0.55) | 2.83 (0.46) | 4.873 | 159.8 | <.001 |
| Flattening ratio at pisiform | 2.97 (0.67) | 2.97 (0.67) | −0.003 | 164 | .998 |
| CSA change: radius-forearm, mm | 4.26 (3.45) | 2.31 (1.73) | 4.615 | 120.9 | <.001 |
| CSA change: pisiform-forearm, mm | 5.20 (4.11) | 2.67 (1.84) | 5.127 | 113.5 | <.001 |
| CSA ratio: radius/forearm | 1.71 (0.55) | 1.44 (0.35) | 3.714 | 138.0 | <.001 |
| CSA ratio: pisiform/forearm | 1.88 (0.67) | 1.50 (0.36) | 4.440 | 125.3 | <.001 |
CSA indicates cross-sectional area..
Before analysis of electrodiagnostic testing and sonography in the symptomatic group, all wrist level data points were compared between the dominant and nondominant hands across all patients to ensure that no similarities or correlation occurred between the hands of individual patients that could influence the data.16 No patient effect was noted in the data when right and left wrists were compared and wrist data were deemed independent for analysis.
Nerve conduction study results were used to categorize symptomatic wrists into normal (n = 32), mild (n = 25), moderate (n = 23), and severe (n = 3). Moderate to strong significant correlations were noted between sonographic measurements and nerve conduction study results and the resulting diagnostic categorization of symptomatic wrists (Table 4). The strongest correlations were observed for the cross-sectional area at the pisiform (r = 0.678–0.746), absolute change between the cross-sectional area in the forearm and cross-sectional area at the pisiform (r = 0.648–0.706), and ratio of the cross-sectional area in the forearm to the cross-sectional area at the pisiform (r = 0.578–0.623) and to the cross-sectional area at the radius (r = 0.515–0.595). A moderate significant correlation was noted between the retinacular bulge measurement and nerve conduction study results. The BMI was noted to be moderately correlated to the sensory conduction velocity and the diagnostic classification, but no significant correlation was noted between the BMI and distal motor latency. In contrast, the wrist ratio was mildly correlated with distal motor latency and the diagnostic classification, but no significant correlation was noted to the sensory conduction velocity. Age and the flattening ratio showed no significant correlation to nerve conduction study results, and a mild correlation was noted in the cross-sectional area of the forearm to the sensory conduction velocity.
Table 4.
Correlation of Measurements to Nerve Conduction Study Data and Categorical Severity of Carpal Tunnel Syndrome Diagnosis in Symptomatic Patients (n = 47 patients, 83 wrists)
| Variable | DML, r | SCV, r | Severity, tau-b |
|---|---|---|---|
| Age | 0.262 | −0.0285 | 0.208 |
| Body mass index | 0.226 | −0.409a | 0.344b |
| Wrist ratio | 0.259b | −0.127 | 0.183b |
| CSA in forearm | 0.211 | −0.249b | 0.161 |
| CSA at distal radius | 0.515a | −0.595a | 0.517a |
| CSA at pisiform | 0.678a | −0.746a | 0.595a |
| Retinacular bulge | 0.467a | −0.466a | 0.385a |
| Flattening ratio at pisiform | 0.082 | 0.025 | 0.021 |
| CSA change: radius-forearm | 0.493a | −0.567a | 0.509a |
| CSA change: pisiform-forearm | 0.648a | −0.706a | 0.582a |
| CSA ratio: radius/forearm | 0.434a | −0.517a | 0.430a |
| CSA ratio: pisiform/forearm | 0.578a | −0.623a | 0.522a |
CSA indicates cross-sectional area; DML, distal motor latency; and SCV, sensory conduction velocity.
Significant at P < .001.
Significant at P < .05.
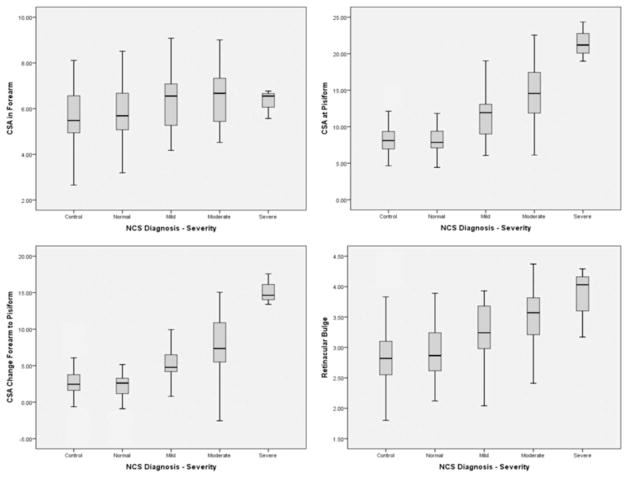
On the basis of correlation results, the cross-sectional area at the pisiform and the absolute change in the cross- sectional area from the forearm to the pisiform and retinacular bulge were further analyzed. A box plot of the cross-sectional area in the forearm shows the lack of any clear difference among the diagnostic groups, providing support for use of this measurement for internal comparison. Additional box plots display the increasing trend in the sizes of the diagnostic sonographic measurements by nerve conduction study severity category (Figure 3). The distribution of data in the moderate diagnostic group was much wider than in the other groups. Although a general upward trend was noted for the measurement of the retinacular bulge, the distribution of measurements was wider across all groups for this variable than displayed in the other plots. Asymptomatic control measurements included in the box plots show the similarity of measurements between the nerve conduction study normal classification and asymptomatic controls. The trends noted within the box plots are intended for descriptive discussion only and were not statistically analyzed.
Figure 3.
Box plots displaying data for various sonographic measurements by diagnostic group. CSA indicates cross-sectional area; and NCS, nerve conduction study.
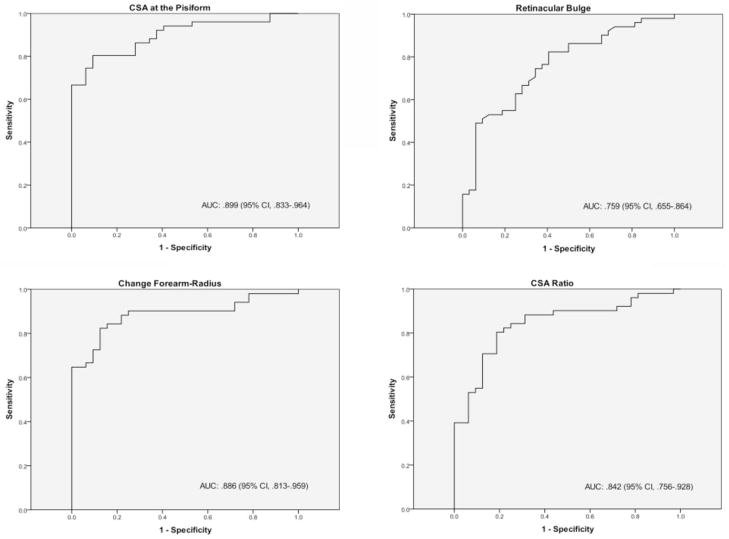
Receiver operating characteristic curve analysis was completed on the basis of positive or negative electrodiagnostic test results in the symptomatic patients. Receiver operating characteristic curves were generated for the 4 sonographic measurements that previous literature and correlational analysis suggested as significant. Receiver operating characteristic curves (area under the curve, 95% confidence interval) for the cross-sectional area at the pisiform (0.899, 0.833–0.964), retinacular bulge (0.759, 0.655–0.864), cross-sectional area change (0.886, 0.813–0.959), and cross-sectional area ratio (0.842, 0.756–0.928) are presented in Figure 4. Diagnostic thresholds were determined on the basis of receiver operating characteristic curves at 10.3 mm2, 2.94 mm, 4.16 mm2, and a ratio of 1.70 for each measurement, respectively (Table 5). The sensitivity of each measurement was either 80.4% or 82.4% with variable specificity (59.4%–90.6%).
Figure 4.
Receiver operating characteristic curve fitting for various sonographic measurements versus electrodiagnostic test results. AUC indicates area under the curve; CI, confidence interval; and CSA, cross-sectional area.
Table 5.
Sensitivity and Specificity of Various Sonographic Measurements
| Measurement | AUC | 95% CI | Threshold | Sensitivity, % | Specificity, % |
|---|---|---|---|---|---|
| CSA at pisiform | 0.899 | 0.833–0.964 | 10.3 mm2 | 80.4 | 90.6 |
| Retinacular bulge | 0.759 | 0.655–0.864 | 2.94 mm | 82.4 | 59.4 |
| CSA change: pisiform-forearm | 0.886 | 0.813–0.959 | 4.16 mm2 | 82.4 | 87.5 |
| CSA ratio: pisiform/forearm | 0.842 | 0.756–0.928 | 1.70 | 80.4 | 81.2 |
AUC indicates area under the curve; and CI, confidence interval.
Discussion
The results of this study confirm findings of previous research indicating the cross-sectional area of the median nerve at the pisiform as strongly correlated with electrodiagnostic testing, the reference standard of diagnosis for carpal tunnel syndrome. These data also confirm that, although carpal tunnel syndrome is commonly thought of as a compression neuropathy, the flattening ratio is not a useful measurement in diagnosis.10,20 More importantly, this study provides support for further investigation of the change in the cross-sectional area of the median nerve from the forearm within each individual. Furthermore, more detailed investigation is required to better differentiate various diagnostic severities and refine these new techniques to validate the utility of sonography in screening for median nerve conditions.
Although not as strongly correlated with electrodiagnostic testing as a single cross-sectional area measurement at the pisiform, cross-sectional area swelling between the forearm and carpal tunnel region within each individual shows promising utility in carpal tunnel syndrome screening. There was a relationship between the BMI and diagnostic measures in our symptomatic patients. However, it is not clear whether enlarged nerves are a pathologic result of an increased BMI or whether the nerve is naturally larger because of the increased overall anthropometric composition of the individual. The calculation of the absolute value change in the cross-sectional area between the forearm and pisiform has the potential to control for this unknown relationship and improve diagnostic accuracy.16 Other studies suggest that calculation of a ratio of these cross-sectional area measurements provides improved accuracy.14,15 The data in this study indicate stronger correlations and increased distribution of data for an absolute change score than with a ratio score, but both were comparable to the singular measurement of the cross-sectional area at the pisiform, indicating that further research is needed. Epidemiologic studies may provide information to better understand the impact of anthropometry, validating these comparative-type measurements.
Although the single cross-sectional area measurement at the pisiform stands up throughout the research literature, the moderate to strong correlation of the cross-sectional area measurement immediately proximal to the carpal tunnel at the radial-carpal joint may provide relevant diagnostic information. It is possible that space within the carpal tunnel may be restricted in some individuals because of edema within the carpal tunnel,21 space occupied by the flexor tendons,22 or excursion of the lumbricals into the carpal canal.23,24 Therefore, swelling at multiple levels within the entire carpal tunnel region is likely, and more substantial enlargement of the nerve may occur immediately proximal to or distal to the tunnel itself.25 By obtaining images and measurements at only one specific anatomic landmark within the carpal tunnel, the largest cross-sectional area may not be obtained on every individual.10 Improved diagnostic accuracy for clinical protocols may occur with measurement of the largest cross- sectional area in the entire carpal tunnel region.16,21
Swelling of the nerve and increased edema due to inflammation of the nerve or tendons could also be responsible for increased retinacular bowing in individuals with carpal tunnel syndrome. Previous research has been inconclusive regarding the utility of retinacular bowing, possibly because of difficulty in obtaining clear images of the retinaculum in the distal carpal tunnel.3 Diagnostic thresholds for retinacular bowing have ranged from 2.11 mm20 to nearly 3.7 mm10 in previous research. Differences were noted in sonographic measurements of the retinacular bulge in the distal tunnel between patients and controls in this study, and an increasing trend was noted across various severities within the symptomatic group; however, the area under the curve for this measurement was much smaller than that of the cross-sectional area. Although developing a threshold for diagnosis based on retinacular bowing may not be clinically realistic, the differences between patients and controls may assist in differential diagnosis of tenosynovitis or other conditions.
Calculated diagnostic thresholds are consistent with previous research at approximately 10 mm2 for the cross-sectional area at the pisiform and a threshold of greater than 3 mm for the height of anterior retinacular bowing. The sensitivity and specificity of these diagnostic threshold values were high. Because electrodiagnostic testing was used as the comparison, variable accuracy of electrodiagnostic testing may have influenced the accuracy of the sonographic measurements.24,26–28 However, the moderate to strong correlations of the sonographic measurements to the electrodiagnostic testing measurements supports continued investigation. Receiver operating characteristic curve analysis completed on the basis of multiple diagnostic categories rather than on positive or negative electrodiagnostic test findings may more accurately reflect the trend that is suggested by strong correlations. Furthermore, the results of this study may be limited because of the assumption of independence of data from wrists in the same individual. Further analysis of these data may best be completed by techniques that investigate clustered data instead of assuming independence.29,30
Despite some limitations, these statistics support previous work, indicating that the selected sonographic measurements can differentiate normal from severe carpal tunnel syndrome25 but are not as good at differentiating mild or moderate cases. This finding may promote the use of sonography as a screening tool to identify normal or severe cases, eliminating the need for electrodiagnostic testing.27 Additionally, because sonography has the ability to measure acute physiologic changes, there remains the possible utility of sonography to aid in evaluation of symptomatic patients who have normal electrodiagnostic test results.7 Previous research with this cross-section of participants resulted in sensitivity of 30.5% and specificity of 96.7%, and the results of this study do not show any difference in sonographic measurements between asymptomatic controls and patients with normal electrodiagnostic test results. Exploration of morphologic characteristics in various regions throughout the entire carpal tunnel region between patients with symptoms but negative electrodiagnostic test results and asymptomatic controls may provide a better understanding of acute changes related to the development of abnormalities. Identification and monitoring of acute physiologic changes may lead to improved interventions to prevent chronic disorders or diseases.
Further evaluation of the use of sonography for improved screening in these cases may lie in the development of additional gray scale, Doppler, dynamic, or qualitative evaluation techniques. In this study, numerous variations in morphologic characteristics were observed in the longitudinal view of the median nerve at the carpal tunnel level. The most convincing observation was of anteroposterior swelling of the nerve (Figure 5). A notch sign or waistline effect was noted in the longitudinal view of the nerve in other patients. Previous use of a qualitative scale for observing these changes in the longitudinal view has resulted in reported sensitivity of 50% and specificity of 95.8% to 100%.20,31 Combination of this qualitative measure with the quantitative measure of the cross-sectional area increased sensitivity to 89.1%.31
Figure 5.
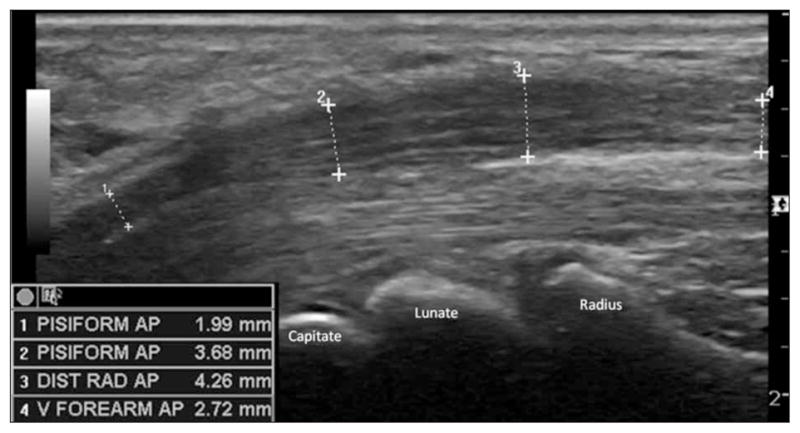
Longitudinal image of the median nerve in a symptomatic patient showing the anteroposterior (AP) swelling of the nerve in the carpal tunnel region (2 and 3) compared to proximal (4) and distal (1) measurements. DIST RAD indicates distal radius; and V, ventral.
The primary limitation of this study was the lack of a control group with comparative data. Although asymptomatic controls were recruited and included to provide a baseline comparison to the symptomatic patients, electrodiagnostic testing was not performed on the controls, which may have caused artificial significance of diagnostic results. Additionally, the patient population was older and had a larger BMI. However, because age and BMI have both been suggested as contributory factors to the development of carpal tunnel syndrome,32 these group differences may not have had a considerable impact on comparative outcomes. Future studies may better control these factors by matching controls to patients. Although the standardized data collection protocol should have reduced bias in this study, blinding of the sonographer collecting the data to participant status (ie, patient versus control) may strengthen future studies. Finally, whereas wrists with a diagnosis in the severe category were notably different in sonographic measurements from those in the other groups, the relatively low number of patients with a severe diagnosis, compared to other diagnostic outcomes, limits the interpretation of the results. Future studies with even distribution across all diagnostic categories or recruitment for comparison of specific categories are needed to gain a deeper understanding of the diagnostic utility of these sonographic measurements.
In conclusion, this study contributes confirmatory data for the use of gray scale sonographic measurements in the diagnosis of carpal tunnel syndrome through quality-controlled methods. Cross-sectional area measurements continue to show the most promise for clinical screening. Refined evaluation of the largest cross-sectional area within the entire region and of cross-sectional area swelling may improve the utility of sonography. Exploration of these and other new screening methods using gray scale, Doppler, and dynamic sonographic techniques can continue to expand the utility of sonography as a screening tool for carpal tunnel syndrome.
Acknowledgments
We thank GE Healthcare (Milwaukee, WI) for continued support of our research and for providing equipment and transducers to conduct this study. Funding for biostatistics and ethics support was provided by the National Institute of Health’s Clinical and Translational Sciences Award to The Ohio State University.
Abbreviations
- BMI
body mass index
- EMG
electromyography
References
- 1.Roll SC, Evans K. Feasibility of using a hand-carried sonographic unit for investigating median nerve pathology. J Diagn Med Sonography. 2009;25:241–249. [Google Scholar]
- 2.Seror P. Sonography and electrodiagnosis in carpal tunnel syndrome diagnosis: an analysis of the literature. Eur J Radiol. 2008;67:146–152. doi: 10.1016/j.ejrad.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Roll SC, Case-Smith J, Evans KD. Diagnostic accuracy of ultrasonography vs. electromyography in carpal tunnel syndrome: a systematic review of literature. Ultrasound Med Biol. 2011;37:1539–1553. doi: 10.1016/j.ultrasmedbio.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Beekman R, Visser LH. Sonography in the diagnosis of carpal tunnel syndrome: a critical review of the literature. Muscle Nerve. 2003;27:26–33. doi: 10.1002/mus.10227. [DOI] [PubMed] [Google Scholar]
- 5.Bayrak IK, Bayrak AO, Tilki HE, Nural MS, Sunter T. Ultrasonography in carpal tunnel syndrome: comparison with electrophysiological stage and motor unit number estimate. Muscle Nerve. 2007;35:344–348. doi: 10.1002/mus.20698. [DOI] [PubMed] [Google Scholar]
- 6.Swen WA, Jacobs JW, Bussemaker FE, de Waard JW, Bijlsma JW. Carpal tunnel sonography by the rheumatologist versus nerve conduction study by the neurologist. J Rheumatol. 2001;28:62–69. [PubMed] [Google Scholar]
- 7.Koyuncuoglu HR, Kutluhan S, Yesildag A, Oyar O, Guler K, Ozden A. The value of ultrasonographic measurement in carpal tunnel syndrome in patients with negative electrodiagnostic tests. Eur J Radiol. 2005;56:365–369. doi: 10.1016/j.ejrad.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Wong SM, Griffith JF, Hui AC, Tang A, Wong KS. Discriminatory sonographic criteria for the diagnosis of carpal tunnel syndrome. Arthritis Rheum. 2002;46:1914–1921. doi: 10.1002/art.10385. [DOI] [PubMed] [Google Scholar]
- 9.Moran L, Perez M, Esteban A, Bellon J, Arranz B, del Cerro M. Sonographic measurement of cross-sectional area of the median nerve in the diagnosis of carpal tunnel syndrome: correlation with nerve conduction studies. J Clin Ultrasound. 2009;37:125–131. doi: 10.1002/jcu.20551. [DOI] [PubMed] [Google Scholar]
- 10.Keleş I, Karagülle Kendi AT, Aydin G, Zög̑ SG, Orkun S. Diagnostic precision of ultrasonography in patients with carpal tunnel syndrome. Am J Phys Med Rehabil. 2005;84:443–450. doi: 10.1097/01.phm.0000163715.11645.96. [DOI] [PubMed] [Google Scholar]
- 11.Kaymak B, Ozçakar L, Cetin A, Candan Cetin M, Akinci A, Hasçelik Z. A comparison of the benefits of sonography and electrophysiologic measurements as predictors of symptom severity and functional status in patients with carpal tunnel syndrome. Arch Phys Med Rehabil. 2008;89:743–748. doi: 10.1016/j.apmr.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Visser LH, Smidt MH, Lee ML. Diagnostic value of wrist median nerve cross-sectional area versus wrist-to-forearm ratio in carpal tunnel syndrome. Clin Neurophysiol. 2008;119:2898–2899. doi: 10.1016/j.clinph.2008.08.022. author reply 2899. [DOI] [PubMed] [Google Scholar]
- 13.Saracgil SN, Karatas M, Yerli H, Isiklar I, Karadeli E. Diagnostic significance of ultrasonography in carpal tunnel syndrome and comparison with electrodiagnostic tests. Turk J Phys Med Rehabil. 2009;55:13–18. [Google Scholar]
- 14.Hobson-Webb LD, Massey JM, Juel VC, Sanders DB. The ultrasonographic wrist-to-forearm median nerve area ratio in carpal tunnel syndrome. Clin Neurophysiol. 2008;119:1353–1357. doi: 10.1016/j.clinph.2008.01.101. [DOI] [PubMed] [Google Scholar]
- 15.Hobson-Webb LD, Padua L. Median nerve ultrasonography in carpal tunnel syndrome: findings from two laboratories. Muscle Nerve. 2009;40:94–97. doi: 10.1002/mus.21286. [DOI] [PubMed] [Google Scholar]
- 16.Klauser AS, Halpern EJ, De Zordo T, et al. Carpal tunnel syndrome assessment with US: value of additional cross-sectional area measurements of the median nerve in patients versus healthy volunteers. Radiology. 2009;250:171–177. doi: 10.1148/radiol.2501080397. [DOI] [PubMed] [Google Scholar]
- 17.Levine DW, Simmons BP, Koris MJ, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75:1585–1592. doi: 10.2106/00004623-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Evans KD, Roll SC, Li X, Sammet S. A holistic evaluation of risk factors for work-related musculoskeletal distress among asymptomatic sonographers performing neurosonology: a pilot study. J Diagn Med Sonography. 2010;26:64–78. [Google Scholar]
- 19.Kwon BC, Jung KI, Baek GH. Comparison of sonography and electrodiagnostic testing in the diagnosis of carpal tunnel syndrome. J Hand Surg Am. 2008;33:65–71. doi: 10.1016/j.jhsa.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Wang LY, Leong CP, Huang YC, Hung JW, Cheung SM, Pong YP. Best diagnostic criterion in high-resolution ultrasonography for carpal tunnel syndrome. Chang Gung Med J. 2008;31:469–476. [PubMed] [Google Scholar]
- 21.Mallouhi A, Pülzl P, Trieb T, Piza H, Bodner G. Predictors of carpal tunnel syndrome: accuracy of gray-scale and color Doppler sonography. AJR Am J Roentgenol. 2006;186:1240–1245. doi: 10.2214/AJR.04.1715. [DOI] [PubMed] [Google Scholar]
- 22.Holtzhausen LM, Constant D, de Jager W. The prevalence of flexor digitorum superficialis and profundus muscle bellies beyond the proximal limit of the carpal tunnel: a cadaveric study. J Hand Surg Am. 1998;23:32–37. doi: 10.1016/s0363-5023(98)80085-3. [DOI] [PubMed] [Google Scholar]
- 23.Siegel DB, Kuzma G, Eakins D. Anatomic investigation of the role of the lumbrical muscles in carpal tunnel syndrome. J Hand Surg Am. 1995;20:860–863. doi: 10.1016/S0363-5023(05)80444-7. [DOI] [PubMed] [Google Scholar]
- 24.Cobb TK, An KN, Cooney WP. Effect of lumbrical muscle incursion within the carpal tunnel on carpal tunnel pressure: a cadaveric study. J Hand Surg Am. 1995;20:186–192. doi: 10.1016/S0363-5023(05)80005-X. [DOI] [PubMed] [Google Scholar]
- 25.Wong SM, Griffith JF, Hui AC, Lo SK, Fu M, Wong KS. Carpal tunnel syndrome: diagnostic usefulness of sonography. Radiology. 2004;232:93–99. doi: 10.1148/radiol.2321030071. [DOI] [PubMed] [Google Scholar]
- 26.Ziswiler H, Reichenbach S, Vogelin E, Bachmann LM, Villiger PM, Juni P. Diagnostic value of sonography in patients with suspected carpal tunnel syndrome: a prospective study. Arthritis Rheum. 2005;52:304–311. doi: 10.1002/art.20723. [DOI] [PubMed] [Google Scholar]
- 27.Naranjo A, Ojeda S, Mendoza D, Francisco F, Quevedo JC, Erausquin C. What is the diagnostic value of ultrasonography compared to physical evaluation in patients with idiopathic carpal tunnel syndrome? Clin Exp Rheumatol. 2007;25:853–859. [PubMed] [Google Scholar]
- 28.Yesildag A, Kutluhan S, Sengul N, et al. The role of ultrasonographic measurements of the median nerve in the diagnosis of carpal tunnel syndrome. Clin Radiol. 2004;59:910–915. doi: 10.1016/j.crad.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Obuchowski NA. Nonparmetric analysis of clustered ROC curve data. Biometrics. 1997;53:567–578. [PubMed] [Google Scholar]
- 30.Li G, Zhou K. A unified approach to nonparametric comparison of receiver operating characteristic curves for longitudinal and clustered data. J Am Stat Assoc. 2008;103:705–713. doi: 10.1198/016214508000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kele H, Verheggen R, Bittermann H, Reimers CD. The potential value of ultrasonography in the evaluation of carpal tunnel syndrome. Neurology. 2003;61:389–391. doi: 10.1212/01.wnl.0000073101.04845.22. [DOI] [PubMed] [Google Scholar]
- 32.Hussain T. Musculoskeletal symptoms among truck assembly workers. Occup Med (Lond) 2004;54:506–512. doi: 10.1093/occmed/kqh087. [DOI] [PubMed] [Google Scholar]