Abstract
Background & Aims
The diagnosis of drug-induced liver injury relies upon exclusion of other causes, including viral hepatitis A, B, and C. Hepatitis E virus (HEV) infection has been proposed as another cause of suspected drug-induced liver disease. We assessed the frequency of HEV infection among patients with drug-induced liver injury in the United States.
Methods
The drug-induced liver injury network (DILIN) is a prospective study of patients with suspected drug-induced liver injury; clinical information and biological samples are collected to investigate pathogenesis and disease progression. We analyzed serum samples, collected from patients enrolled in DILIN, for immunoglobulin (Ig)G and IgM against HEV; selected samples were tested for HEV RNA.
Results
Among 318 patients with suspected drug-induced liver injury, 50 (16%) tested positive for anti-HEV IgG and 9 (3%) for anti-HEV IgM. The samples that contained anti-HEV IgM (collected 2 to 24 weeks after onset of symptoms) included 4 that tested positive for HEV RNA, genotype 3. Samples from the 6-month follow-up visit were available from 4 patients; they were negative for anti-HEV IgM, but levels of anti-HEV IgG increased with time. Patients that had anti-HEV IgM were mostly from older men (89%; mean age, 67 years) and 2 were HIV positive. Clinical reassessment of the 9 patients with anti-HEV IgM indicated that acute hepatitis E was the most likely diagnosis for 7 and might be the primary diagnosis for 2.
Conclusion
HEV infection contributes to a small but important proportion of cases of acute liver injury that are suspected of being drug induced. Serologic testing for HEV infection should be performed—particularly if clinical features are compatible with acute viral hepatitis.
Keywords: Viral hepatitis, jaundice, isoniazid, liver biopsy, causality assessment, liver disease, drug toxicity, treatment, cirrhosis
Introduction
Drug-induced liver injury is the leading cause of acute liver failure and the primary reason for regulatory action leading to failed drug approval, market withdrawal, usage restrictions and warnings to practicing physicians in the United States.1 The diagnosis of drug-induced liver injury is often difficult because of the lack of specific biomarkers and the diversity of its clinical presentation.2 The diagnosis is primarily one of exclusion and is made only after elimination of common causes of liver disease, such as alcoholic hepatitis, metabolic and genetic liver diseases, bile duct obstruction, and hepatitis A, B, and C virus infection (HAV, HBV, and HCV).
Hepatitis E virus (HEV) infection is another cause of acute liver injury but is rarely considered in the differential diagnosis of drug-induced liver injury, largely because hepatitis E is thought to be rare in the Western World and unlikely to occur unless there is a history of recent travel to an endemic area such as Asia, Africa or Central or South America.3 Several recent findings have served to alter this opinion. First, indigenous cases of acute hepatitis E have been reported in the United States as well as Europe, Japan, and New Zealand caused by HEV genotype 3 strains which are endemic to domestic and wild animals, particularly swine.4-12 In addition, recent population-based surveys in the United States have shown that at least 20% of adults are reactive for IgG anti-HEV, and thus have serological evidence of previous HEV infection.13,14 Finally, a publication from the United Kingdom suggested that up to 12% of cases of acute liver injury initially attributed to medications were actually due to unsuspected acute HEV infection.15
The aims of the current study were to assess whether acute hepatitis E accounts for some cases of suspected drug-induced liver injury in the United States and whether testing for HEV infection is warranted in the routine evaluation of patients with acute liver disease of unknown cause.
Material and Methods
Patient identification and causality analysis
The Drug Induced Liver Injury Network (DILIN) consists of multiple (previously 5, and currently 8) U.S. clinical sites and a data coordinating center that have enrolled patients with suspected drug-induced liver injury into a prospective study since 2004. The rationale, design and conduct of the DILIN, as well as a summary of the first 300 enrolled cases have been described.16,17 All enrolled cases were subjected to formal causality assessment independently by three investigators, and a final causality score was obtained by consensus.18 At the same time, a Roussel Uclaf Causality Assessment (RUCAM) score19 was determined and cases were graded for severity using a five-point scale developed by the DILIN.16
Serologic and Virologic Testing
Serum samples were obtained at the time of enrollment, which might be as long as 6 months after the onset of liver injury, and were stored at -80 degrees Celsius in a central repository. For the current study, serum samples from the first 318 patients enrolled were tested for IgM and IgG anti-HEV using enzyme immunoassays of established sensitivity and specificity.20,21 Samples with IgM anti-HEV and those with strongly positive reactions for IgG anti-HEV were further tested for HEV RNA using nested reverse transcription polymerase chain reaction (RT-PCR)22 and the PCR products were separated by electrophoresis on ethidium bromide-stained agarose gels, extracted from the gel and directly sequenced to provide the consensus sequence. A BLAST search of GenBank nucleotide sequences was performed to determine HEV genotype. Details of the ELISA assays for anti-HEV and the PCR for HEV RNA are provided in Supplementary Material.
Histological Analysis
When available, liver biopsies (n=3) were reviewed by a hepatic pathologist (D.E.K.) who was unaware of the medications implicated and results of HEV testing. Histological features of inflammation, fibrosis, steatosis, cholestasis, vascular injury and other findings were systematically recorded, along with a description of the overall pattern of injury.
Repeat Causality Analysis
Cases positive for HEV IgM were subjected to repeat causality analysis by three independent reviewers after the results of HEV serological and RT-PCR testing were available. Cases were again judged for the likelihood that the implicated medication was responsible for the liver injury as “definite” (>95% likelihood), “highly likely” (75-94%), “probable” (50-74%), “possible” (25-49%) or “unlikely” (<25%).18 Cases were also judged using the same scale as to the likelihood that the liver injury was due to acute hepatitis E based upon the clinical, biochemical and histological findings.
Data analysis
Pairwise comparisons were performed between the cases with no serological evidence of HEV infection versus patients with evidence of active or recent HEV infection (defined by presence of HEV IgM) and those with distant and resolved HEV infection (defined by presence of IgG without IgM anti-HEV). The Wilcoxon test was used for continuous variables, Fisher’s exact test for binary outcomes, and the Pearson chi-squared test for other categorical variables.
IRB approval
All details of the DILIN Prospective study were reviewed and approved by the institutional review boards of each clinical site and the data coordinating center. Each enrolled subject signed an informed consent that allowed future testing on archived biosamples. In addition, the protocol for anti-HEV testing was specifically approved by the institutional review board of the National Institute of Allergy and Infectious Diseases of the intramural program of the National Institutes of Health.
Results
Serological Testing
Among 318 patients tested, 50 (16%) were reactive for IgG anti-HEV, 9 of whom (3%) were also reactive for IgM anti-HEV. The demographical and clinical features of patients with both IgG and IgM anti-HEV (Group 1, n=9), with IgG anti-HEV alone (Group 2, n=41), and with no markers of HEV infection (Group 3, n= 268) are shown in Table 1. Comparing the three groups, patients with anti-HEV reactivity were on average older (67 and 62 versus 47 years: both comparisons p = 0.001) and those with IgM anti-HEV were more often men (90% versus 44% and 41%: p = 0.003). Initial and peak serum bilirubin, alanine aminotransferase (ALT) and alkaline phosphatase levels were similar in the three groups of patients. Furthermore, the three groups did not different in distribution of pattern of serum enzyme elevations, severity scores or causality scores.
Table 1.
Demographical and Clinical Features of Three Cohorts Based upon HEV Serology
| Feature | Group 1 Both IgM & IgG anti-HEV Positive (n=9) | Group 2 Only IgG anti-HEV Positive (n=41) | Group 3 Both IgG and IgM anti-HEV negative (n=268) | p values (1 vs 3/2 vs 3) |
|---|---|---|---|---|
|
| ||||
| Male sex (n, %) | 8 (89%) | 18 (44%) | 104 (39%) | <.003/<.53 |
|
| ||||
| Age (years)* | 67.2 (12.9) | 61.8 (13.2) | 47.0 (16.7) | <.001/<.001 |
|
| ||||
| Time to onset (days)** | 140 (42-504) | 70.5 (25-334) | 39.0 (19-101) | 0.10/0.03 |
|
| ||||
| Peak bilirubin (mg/dL)* | 10.8 (8.1) | 10.1 (9.9) | 11.9 (11.3) | 0.90/0.33 |
|
| ||||
| Peak ALT (U/L)* | 1183 (1251) | 678 (564) | 916 (1031) | 0.55/0.24 |
|
| ||||
| Peak Alk P (U/L)* | 270 (163) | 368 (363) | 439 (481) | 0.26/0.36 |
|
| ||||
| Initial R value* | 10.7 (11.6) | 11.5 (16.7) | 11.5 (15.3) | 0.58/0.69 |
|
| ||||
| Pattern of injury (n, %) *** | ||||
| Hepatocellular | 5 (56%) | 22 (54%) | 146 (55%) | 0.43/0.09 |
| Mixed | 3 (33%) | 13 (32%) | 51 (19%) | |
| Cholestatic | 1 (11%) | 6 (15%) | 71 (27%) | |
|
| ||||
| Severity Score (n, %) *** | ||||
| 1 (No jaundice) | 1 (11%) | 11 (28%) | 71 (27%) | |
| 2 (Jaundice, not Hospitalized) | 1 (11%) | 5 (13%) | 55 (21%) | 0.38/0.72 |
| 3 (Jaundice, Hospitalized) | 4 (44%) | 15 (38%) | 87 (33%) | |
| 4 (Liver failure) | 3 (33%) | 6 (15%) | 37 (14%) | |
| 5 (Death or transplant) | 3 (8%) | 12 (5%) | ||
|
| ||||
| Causality score (n, %)*** | ||||
| 1 (Definite) | 0 (0%) | 10 (25%) | 80 (31%) | |
| 2 (Highly likely) | 5 (56%) | 17 (43%) | 108 (41%) | 0.27/0.31 |
| 3 (Probable) | 2 (22%) | 4 (10%) | 40 (15%) | |
| 4 (Possible) | 2 (22%) | 5 (12%) | 25 (10%) | |
| 5 (Unlikely) | 0 (0%) | 4 (10%) | 9 (3%) | |
Mean and standard deviation,
Median and 25-75th range,
See text for full explanation of pattern of injury, and scoring systems for severity and causality
Abbreviations: ALT, alanine aminotransferase; Alk P, alkaline phosphatase; R value, ratio of ALT to Alk P in expressed as times the upper limit of the normal range [ratios <2 are considered “cholestatic”, 2-5 “mixed” and >5 “hepatocellular” injury]; HEV, hepatitis E virus.
Demographic and Clinical Features of IgM anti-HEV Positive Cases
Selective demographic and clinical features of the nine IgM anti-HEV positive cases are given in Table 2, and detailed case summaries of each patient are provided as Supplementary Data. The cases included eight men and one woman; eight were non-Hispanic whites and one was multiracial. The average age was 67 years (range 42 to 83 years). Initial serum bilirubin levels ranged from 0.4 to 15.1 mg/dL (mean = 7.0 mg/dL) and peak levels were only slightly higher (mean = 10.8 mg/dL). Initial ALT levels ranged from 196 to 3838 U/L (mean = 1073 U/L) and alkaline phosphatase from 113 to 632 U/L (mean = 225 U/L). Based upon calculation of the R score (ALT divided by alkaline phosphatase both expressed as multiple of the upper limit of the normal range19), the biochemical pattern of serum enzyme elevations was hepatocellular (R > 5) in 5, cholestatic in 1 (R < 2) and “mixed” in 3 (R 2-5). Three patients gave a history of fever and one of rash, but these symptoms were not prominent and no patient had documented eosinophilia. Antinuclear antibody was present in low titers in two patients (1:40 and 1:80) and smooth muscle antibody in seven (titers ranging from 1:8-1:320) but other autoimmune features (arthritis, rash, hyperglobulinemia) were not common. All patients were symptomatic, eight were jaundiced, and seven were hospitalized for the liver injury. The clinical course was considered severe in three patients who manifested signs of hepatic failure such as elevations in INR > 1.5, ascites or hepatic encephalopathy. Two of these patients had a clinical syndrome resembling “acute on chronic” hepatitis, both having evidence of pre-existing liver disease (alcoholic and nonalcoholic fatty liver disease).
Table 2.
Clinical Features of Nine Cases of Suspected Drug Induced Liver Disease that Tested Positive for IgM anti-HEV
| Case No | Drug | Age years | Sex | Time to Onset | Peak Bilirubin (mg/dL) | Peak ALT (U/L) | Peak Alk P (U/L) | HEV RNA | Causality* | HEV ** Likelihood |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Azithromycin | 74 | M | 13 days | 8.9 | 2428 | 210 | Negative | 2 → 4 | 2 |
| 2 | Nevirapine | 42 | M | 42 days | 0.9 | 560 | 190 | Positive | 4 → 4 | 3 |
| 3 | Isoniazid | 64 | M | 8.5 months | 13.2 | 429 | 127 | Positive | 2 → 4 | 3 |
| 4 | Ezetimibe | 64 | M | 1 year | 5.1 | 557 | 180 | Negative | 3 → 4 | 3 |
| 5 | Allopurinol | 80 | M | 7 weeks | 10.6 | 235 | 210 | Negative | 2 → 3 | 4 |
| 6 | Telithromycin | 83 | M | 16 days | 28.2 | 196 | 200 | Negative | 2 → 3 | 4 |
| 7 | Nevirapine | 61 | M | 1.4 years | 16.7 | 708 | 446 | Positive | 3 → 5 | 1 |
| 8 | Dietary supplement | 59 | F | 4.5 months | 4.5 | 3838 | 207 | Negative | 4 → 5 | 1 |
| 9 | Pravastatin | 79 | M | 12.7 years | 8.6 | 1564 | 632 | Positive | 3 → 5 | 1 |
DILIN Causality Assessment18: scored as either 1 (definite), 2 (highly likely), 3 (probable), 4 (possible) or 5 (unlikely). First number corresponds to the initial causality assessment done before availability of HEV serology, while second number (after →) corresponds to causality assessment upon re-review with the HEV serology known.
Likelihood that the acute liver injury was due to acute hepatitis E: scored identically to causality assessment.
Abbreviations: M, male; F, female; ALT, alanine aminotransferase; Alk P, alkaline phosphatase; HEV, hepatitis E virus.
Information on exposures to farm animals or raw pork was not specifically sought but none offered such information or gave a history of travel to an endemic area. One patient became ill while travelling to Prague, Czech Republic, but symptoms arose upon his arrival, making infection from exposure in the United States more likely. The patients were geographically diverse and presented at 4 of the 5 DILIN centers including Indiana (n=5), San Francisco (n=2), Connecticut (n=1) and North Carolina (n=1) between 2004 and 2006. The initially implicated medications included nevirapine (as a part of antiretroviral therapy) in two patients, and isoniazid, allopurinol, telithromycin, pravastatin, ezetimibe, azithromycin and an herbal product in one case each. The latency to first documented laboratory abnormalities ranged from 13 days to 12.7 years, being less than one month in two, 1 to 6 months in four and greater than 6 months in three cases.
HEV RNA results
Four patients with IgM anti-HEV were also reactive for HEV RNA, and all four harbored genotype 3. Sequencing analyses showed that the four were not closely related phylogenetically (data not shown) and therefore were not likely due to a single source or contamination, presenting in different geographic areas (Indiana, San Francisco and North Carolina). The four cases with viremia included both patients with HIV infection. Follow-up serum samples, drawn approximately 6 months after enrollment, were available from four IgM anti-HEV-positive subjects: all had an increase in IgG anti-HEV titer, but IgM anti-HEV had diminished in titer or had become negative, consistent with seroconversion after acute infection. All were also negative for HEV RNA (two were positive on the earlier specimen) (Table 3).
Table 3.
Initial and 6 month Follow-up Anti-HEV titers *
| Case No | Initial IgM anti-HEV | Final IgM anti-HEV | Initial IgG anti-HEV | Final IgG anti-HEV | Initial HEV RNA | Final HEV RNA |
|---|---|---|---|---|---|---|
| 3 | 16,384 | 100 | 4,096 | 16,384 | Pos | Neg |
| 5 | 4,096 | 100 | 8,192 | 131,072 | Neg | Neg |
| 8 | 4,096 | <100 | 4,096 | 8,192 | Neg | Neg |
| 9 | 65,536 | 500 | 8,192 | 65,536 | Pos | Neg |
Antibody titers were calculated from serial dilution data using linear regression and curve fitting with Microsoft Excel and GraphPad Prism software.
Causality Analysis/Re-analysis
The initial causality assessment for the nine cases concluded that 4 were highly likely, 3 probably and 2 possibly due to drug-induced liver injury. RUCAM scores of cases ranged from 5 to 10, values of 3 to 5 (n=1) indicating a “possible”, values of 6 to 8 (n=5) a ‘probable” and those above 8 (n=3) a “highly probable” likelihood.21 Thus, most cases were considered compatible with drug-induced liver injury on initial assessment in the absence of anti-HEV results.
On reassessment after the results of anti-HEV testing were available, the causality scores changed in eight cases (remaining “possible” in one) and no case was considered more than “probably” due to drug-induced liver injury (Table 2). Even with the information on HEV serology, two cases (implicated medications being allopurinol and telithromycin) were still considered probably due to drug-induced liver injury rather than hepatitis E. Both patients presented late during the course of illness and initial clinical features and laboratory results were not available. The remaining seven cases were considered more likely to be acute hepatitis E than drug-induced liver injury, although four were still considered “possibly” due to the medication. Three cases were considered “definite” acute hepatitis E.
Liver histology of IgM anti-HEV positive cases
Liver biopsy tissue was available from three patients. Case 2 developed serum enzyme elevations without jaundice during antiretroviral therapy that persisted for 4 months and had a liver histology (Figure 1) suggestive of chronic hepatitis with focal bridging necrosis and hepatocyte rosette formation, minimal steatosis, a lymphocytic portal infiltrate with scattered eosinophils and only rare plasma cells, and bridging fibrosis. The bile ducts showed mild injury with reactive changes, but without cholestasis. Case 4 developed mild acute liver injury with jaundice after taking ezetimibe for one year and underwent liver biopsy during recovery when serum bilirubin (1.4 mg/dL) and ALT levels (60 U/L) were close to normal. The biopsy (not shown) showed mild steatosis, ballooning and bridging fibrosis with focal copper accumulation consistent with nonalcoholic steatohepatitis which he was thought to have before onset of the acute injury (based upon chronic ALT elevations and obesity). Hepatocyte rosetting was also present which is atypical of nonalcoholic fatty liver disease and is consistent with regeneration following a more severe injury in the immediate past. Case 7 developed jaundice and a hepatitis-like syndrome 1.5 years after starting an antiretroviral regimen including nevirapine and on liver biopsy (Figure 2) had changes of lobular disarray, spotty hepatocyte necrosis without confluence, marked lobular but scant portal inflammation, mild intrahepatic cholestasis but no bile duct injury, steatosis or fibrosis. These changes were compatible with either viral hepatitis or acute hepatocellular drug injury.
Figure 1. Liver injury initially attributed to nevirapine (Case #2) and later considered probably due to chronic HEV infection.
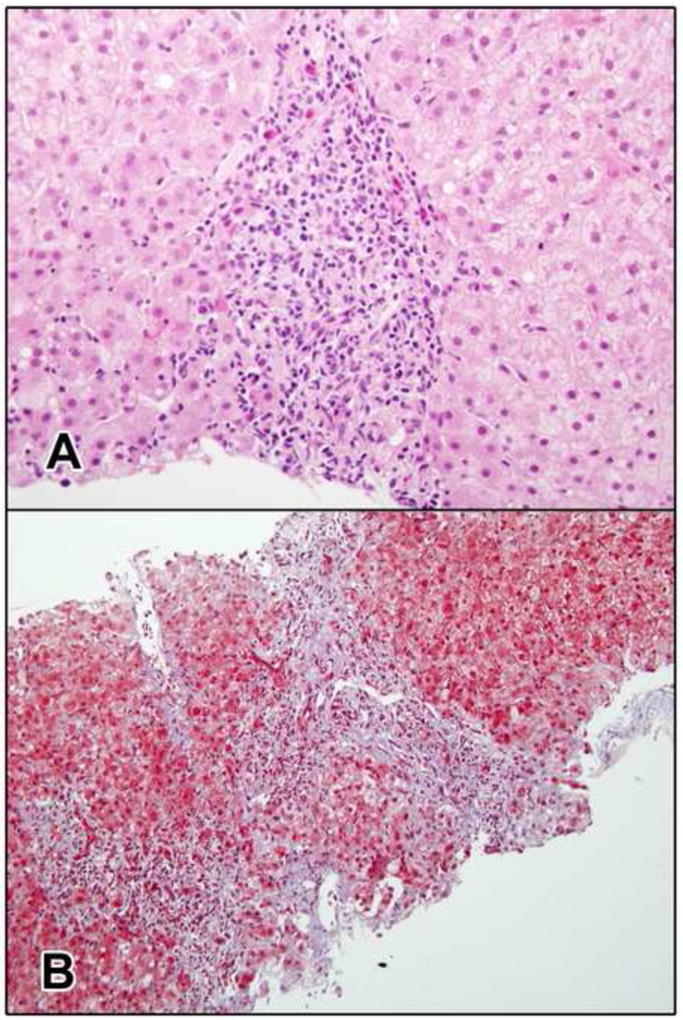
a. The portal area is expanded by a chronic inflammatory infiltrate with interface hepatitis indicative of chronic hepatitis. Several eosinophils are present (H&E, 400x). b. There is portal fibrotic expansion with early bridging fibrosis. Ductular reaction is present at the edges of the portal areas (Masson trichrome, 200x).
Figure 2. Acute liver injury attributed to nevirapine (Case #7) and later considered due to hepatitis E.
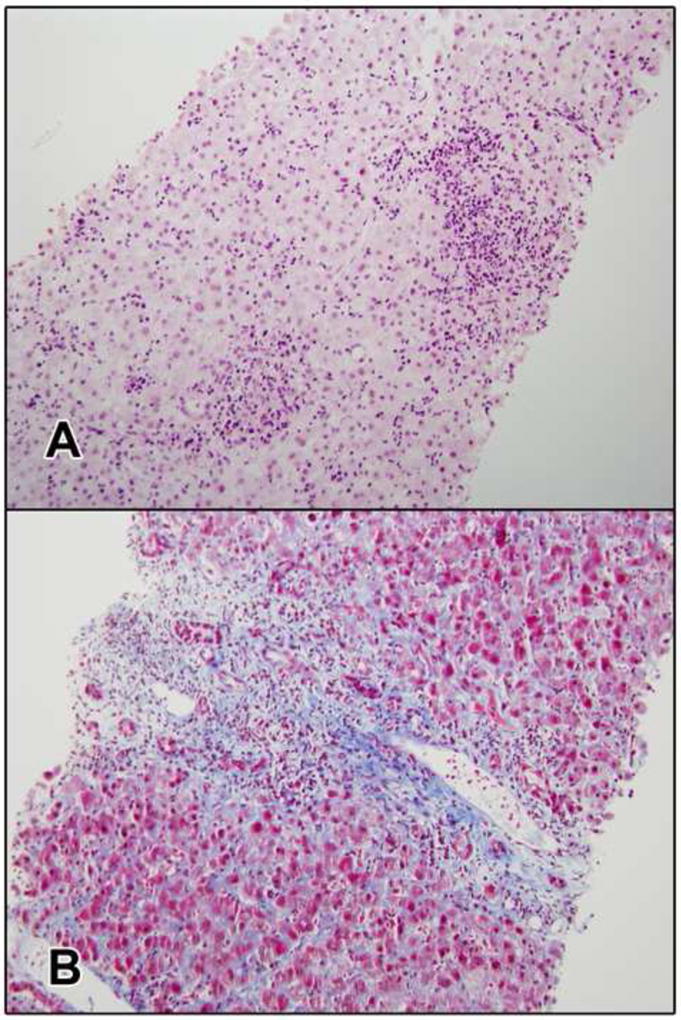
a. The biopsy shows an acute hepatitis pattern with mild canalicular cholestasis. There portal inflammation with many plasma cells and a few eosinophils and moderate interface hepatitis. The parenchyma shows and numerous foci of lobular inflammation with acidophil bodies (H&E, 200x). b. There is early periportal fibrosis and mild ductular reaction (Masson trichrome, 200x).
Discussion
The accurate diagnosis of drug-induced liver injury is critically important not only for patient care, but also for drug development, as even a single episode of severe liver damage associated with a drug during pre-marketing clinical testing may undermine its subsequent approval and marketing.25 For the patient, the occurrence of liver injury may preclude the use of a medication that is critically needed such as a first-line anticonvulsant, anti-retroviral or anti-tuberculosis medication. Nevertheless, the accurate diagnosis of drug-induced liver disease is challenging, not only because of the lack of specific markers, but also because the clinical features are non-specific and often similar to those that occur in acute viral hepatitis or other causes of liver injury. In essence, the diagnosis requires exclusion of a wide array of other causes of liver injury, including viral and autoimmune hepatitis, bile duct obstruction, sepsis, hepatic ischemia, and metabolic disorders.2
While viral serology for hepatitis A, B and C are part of the standard evaluation of suspected drug-induced liver disease, testing for evidence of HEV infection is rarely done, largely because this form of acute viral hepatitis is considered rare in developed countries.3,26 Indeed, until recently almost all reported cases of hepatitis E in the United States were described in travelers returning from endemic areas of infection, such as Asia, Northeast Africa, the Middle East, and Mexico. However, there have been increasing numbers of cases of indigenous or “autochthonous” HEV infection described in patients in developed countries who deny travel to endemic areas.4-12,15,27-30 These non-travel associated cases are typically caused by genotype 3 HEV which is commonly found in swine even in developed countries.4,10,11,21,24 Indeed, careful medical history of non-travel related cases has often identified exposure to domesticated and wild animals or recent consumption of undercooked pork or wild game.6,10-12 Finally, recent reports have demonstrated that chronically immunosuppressed patients, such as organ transplant recipients or HIV infected individuals, can develop chronic HEV infection with high levels of virus and may represent a reservoir for human infection in western countries.31,32 Importantly, chronic HEV infection can lead to progressive hepatic fibrosis, cirrhosis and end-stage liver disease, and recent pilot studies have suggested that chronic HEV infection can be eradicated by a relatively short course of peginterferon or ribavirin.33,34
In this study, evidence of HEV infection was sought in an unselected group of cases of drug-induced liver injury that had been accrued at five medical centers in the United States between the years 2004 and 2009. Nine of 318 cases (3%) had serological evidence of ongoing or recent acute hepatitis E. Interestingly, all except one patient was male and the average age was almost 2 decades older than the cohort of patients without anti-HEV reactivity. These findings are similar to reports on indigenous cases of hepatitis E from the United Kingdom, in which 78% of cases were in men and the average age was 65 years.30 The reasons for these associations are not known. One explanation is that genotype 3 HEV infection may be largely subclinical in young, healthy individuals and more likely to be symptomatic and icteric in older men or patients with significant co-morbidities. Indeed, among the nine patients described here, eight had major co-morbidities including HIV infection, chronic obstructive pulmonary disease, asthma, alcoholism, obesity, tuberculosis, diabetes, atherosclerotic cardio- or cerebro-vascular disease, and lymphoma. In the absence of specific testing, it is not surprising that hepatitis E and drug-induced injury might be confused clinically; the clinical features of hepatitis E like those of other forms of acute hepatitis can resemble hepatitis caused by medications.15,26,29,35
A careful reanalysis of the nine cases of suspected drug-induced liver injury that were positive for IgM anti-HEV, however, demonstrated that some cases were still considered to be more likely due to drug hepatotoxicity than acute hepatitis E. These findings suggest that the recent acute HEV infection detected by IgM anti-HEV testing did not account for the acute liver injury in all cases and that co-incidental, subclinical hepatitis E may have preceded the acute liver injury caused by the implicated medication.
An important finding of this study was that four cases had HEV RNA detectable in serum, all of which were genotype 3. These results indicate that the acute hepatitis E is due to local sources, possibly related to exposure to farm animals or eating pork. Two cases occurred in patients with HIV infection who were on chronic antiretroviral therapy, the liver injury initially being attributed to nevirapine. Indeed, one of the patients had chronic hepatitis on liver biopsy and was still HEV RNA positive almost 24 weeks after initial presentation with serum enzyme elevations. Such findings suggest that immunosuppressed individuals should avoid eating undercooked pork or game, and that such individuals with chronic unexplained liver disease should be tested for HEV infection.
In the remaining five patients the absence of viremia may have been due to the delay between the onset of symptoms and blood sampling for the study, as patients were typically referred by local physicians to the DILIN investigators days or even weeks after initial presentation. Thus, the nine blood samples were obtained an average of 37 days after onset of illness. In previous studies of acute hepatitis E, IgM anti-HEV was generally present in serum when symptoms arose and persisted for 4 to 6 months after resolution, whereas HEV RNA was detectable during the incubation period of the disease and early during the clinical illness; and in some cases was not longer detectable by the time of onset of jaundice or clinical symptoms.3,34,35 In studies of acute hepatitis in which patients were tested at or near the onset of illness, serum HEV-RNA was detected in only 50-66% of anti-HEV IgM positive cases.30,36 HEV RNA is also detectable in stool during the acute phase of infection, although the reliability of testing stool versus serum has not been adequately evaluated.
While acute hepatitis E due to genotype 1 tends to be symptomatic and icteric, the clinical spectrum of genotype 3 HEV infection has not been fully defined. The patients described here often had severe liver injury. Eight patients were jaundiced, the single anicteric case appearing to have chronic hepatitis E (in the setting of HIV infection). Three patients developed features of hepatic failure including two with an “acute-on-chronic” syndrome developing hepatic encephalopathy and/or ascites concurrent with onset of jaundice. Nevertheless, the high rate of anti-HEV positivity in the U.S. population14 suggests that many cases of acute HEV infection are subclinical. Indeed, among the 9 patients described here, 2 were considered still to have drug-induced liver injury and a recent subclinical HEV infection.
Another explanation for the current results is false positivity in the serological assays. This explanation, however, is unlikely because highly validated assays for IgM and IgG anti-HEV were used, four cases were reactive for HEV RNA in serum, and follow up testing in four subjects demonstrated a decline in IgM and rise in IgG anti-HEV titers. At present, there are no FDA approved assays for anti-HEV with proven specificity and sensitivity. Research testing results can be obtained from the Centers for Disease Control and Prevention (http://www.cdc.gov/hepatitis/) or by special arrangements with research laboratories. The results of the current study demonstrate the critical need for commercially available, sensitive and reliable assays for IgM anti-HEV and HEV RNA.
In summary, a small proportion of cases of suspected drug-induced liver injury in the United States may be related to concurrent and unsuspected acute hepatitis E. Testing for HEV infection should be considered when the pattern of injury resembles acute viral hepatitis and when features of the clinical presentation and latency (time to onset) are unusual. Such testing is particularly apropos when the medications are critically important (e.g., antiretrovirals, anti-tuberculosis agents). Testing for IgG anti-HEV alone is not helpful because 20% or more of otherwise healthy adults in Western countries are likely to test positive.13,14 Tests for IgM anti-HEV are most appropriate for screening for acute infection, with positive results confirmed by HEV RNA testing or follow up serology (disappearance of IgM anti-HEV and appearance or rise in titer of IgG anti-HEV). The presence of serological evidence of acute hepatitis E should also prompt a search for possible sources of infection, such as foreign travel, exposure to farm animals, consumption of undercooked pork or wild game, and close personal contact with chronically immunosuppressed persons. Finally, these findings underscore the need for sensitive and reliable, commercially available assays for HEV infection in the United States and reexamination of the possible benefit of an HEV vaccine.37,38
Supplementary Material
Acknowledgments
Source of Funding: The DILIN network is supported by the National Institute of Diabetes and Digestive and Kidney Diseases, NIH under the following cooperative agreements: 1U01DK065021, 1U01DK065193, 1U01DK065201, 1U01DK065193, 1U01DK065184, 1U01DK065211, 1U01DK065238, and 1U01DK065176. This study was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases and the National Cancer Institute, NIH
Abbreviations
- DILI
drug induced liver injury
- HAV
hepatitis A virus
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HEV
hepatitis E virus
- anti-HEV
antibody to hepatitis E virus
- DILIN
the Drug Induced Liver Injury Network
- NHANES
National Health and Nutrition Evaluation Survey
- RT-PCR
reverse transcriptase-polymerase chain reaction
- ELISA
enzyme-linked immunosorbent assay
- RUCAM
Rousel Uclaf Causality Assessment Method
- NIH
National Institutes of Health
Footnotes
Conflicts of interest: Dr. Chalasani has received consulting fees regarding drug-induced liver injury in the past 12 months from the following companies: Teva pharmaceuticals, KaroBio, J&J, Salix Pharmaceuticals, and Gilead Sciences and has received research support from Eli Lilly for research on drug-induced liver disease. Dr. Fontana is on the Speaker‘s Bureau of Genentech and Gilead Sciences and has received research support or consulting fees from Bristol-Myers Squibb, GlaxoSmithKline and Medtronic. Drs. Davern, Hayashi, Kleiner, Engle, Nguyen, Emerson, Purcell, Gu, Serrano and Hoofnagle have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Naga Chalasani, Email: nchalasa@iupui.edu.
Robert J. Fontana, Email: rfontana@med.umich.edu.
Paul H. Hayashi, Email: paul_hayashi@med.unc.edu.
Petr Protiva, Email: petr.protiva@yale.edu.
David E. Kleiner, Email: Kleinerd@mail.nih.gov.
Ronald E. Engle, Email: rengle@niaid.nih.gov.
Hanh Nguyen, Email: htnguyen@niaid.nih.gov.
Suzanne U. Emerson, Email: semerson@niaid.nih.gov.
Robert H. Purcell, Email: rpurcell@niaid.nih.gov.
Hans L. Tillmann, Email: hans.tillmann@duke.edu.
Jiezhun Gu, Email: jiezhun.gu@duke.edu.
Jose Serrano, Email: SerranoJ@extra.niddk.nih.gov.
Jay H. Hoofnagle, Email: HoofnagleJ@extra.niddk.nih.gov.
References
- 1.Watkins PB, Seeff LB. Drug-induced liver injury: summary of a single topic clinical research conference. Hepatology. 2006;43:618–31. doi: 10.1002/hep.21095. [DOI] [PubMed] [Google Scholar]
- 2.Fontana RJ, Seeff LB, Andrade RJ, Björnsson E, Day CP, Serrano J, Hoofnagle JH. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52:730–42. doi: 10.1002/hep.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145–54. doi: 10.1002/rmv.384. [DOI] [PubMed] [Google Scholar]
- 4.Schlauder GG, Dawson GJ, Erker JC, et al. The sequence and phylogenetic analysis of a novel hepatitis E virus isolated from a patient with acute hepatitis reported in the United States. J Gen Virol. 1998;79(Pt 3):447–56. doi: 10.1099/0022-1317-79-3-447. [DOI] [PubMed] [Google Scholar]
- 5.Levine DF, Bendall RP, Teo CG. Hepatitis E acquired in the UK. Gut. 2000;47:740. doi: 10.1136/gut.47.5.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ijaz S, Arnold E, Banks M, et al. Non-travel-associated hepatitis E in England and Wales: demographic, clinical, and molecular epidemiological characteristics. J Infect Dis. 2005;192:1166–72. doi: 10.1086/444396. [DOI] [PubMed] [Google Scholar]
- 7.Mansuy JM, Peron JM, Abravanel F, et al. Hepatitis E in the south west of France in individuals who have never visited an endemic area. J Med Virol. 2004;74:419–24. doi: 10.1002/jmv.20206. [DOI] [PubMed] [Google Scholar]
- 8.Dalton HR, Fellows HJ, Gane EJ, et al. Hepatitis E in New Zealand. J Gastroenterol Hepatol. 2007;22:1236–40. doi: 10.1111/j.1440-1746.2007.04894.x. [DOI] [PubMed] [Google Scholar]
- 9.Halbur PG, Kasorndorkbua C, Gilbert C, et al. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J Clin Microbiol. 2001;39:918–23. doi: 10.1128/JCM.39.3.918-923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yazaki Y, Mizuo H, Takahashi M, Nishizawa T, Sasaki N, Gotanda Y, Okamoto H. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol. 2003;84(Pt 9):2351–7. doi: 10.1099/vir.0.19242-0. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda H, Okada K, Takahashi K, Mishiro S. Severe hepatitis E virus infection after ingestion of uncooked liver from a wild boar. J Infect Dis. 2003;188:944. doi: 10.1086/378074. [DOI] [PubMed] [Google Scholar]
- 12.Wichmann O, Schimanski S, Koch J, et al. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J Infect Dis. 2008;198:1732–41. doi: 10.1086/593211. [DOI] [PubMed] [Google Scholar]
- 13.Meng XJ, Wiseman B, Elvinger F, et al. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol. 2002;40:117–22. doi: 10.1128/JCM.40.1.117-122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuniholm MH, Purcell RH, McQuillan GM, Engle RE, Wasley A, Nelson KE. Epidemiology of hepatitis E virus in the United States: results from the Third National Health and Nutrition Examination Survey, 1988-1994. J Infect Dis. 2009;200:48–56. doi: 10.1086/599319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalton HR, Fellows HJ, Stableforth W, et al. The role of hepatitis E virus testing in drug-induced liver injury. Aliment Pharmacol Ther. 2007;26:1429–35. doi: 10.1111/j.1365-2036.2007.03504.x. [DOI] [PubMed] [Google Scholar]
- 16.Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, Rochon J DILIN Study Group. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Drug Induced Liver Injury Network (DILIN) Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockey DC, Seeff LB, Rochon J, et al. US Drug-Induced Liver Injury Network. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51:2117–26. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of International Consensus Meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–30. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 20.Engle RE, Yu C, Emerson SU, Meng XJ, Purcell RH. Hepatitis E virus (HEV) capsid antigens derived from viruses of human and swine origin are equally efficient for detecting anti-HEV by enzyme immunoassay. J Clin Microbiol. 2002;40:4576–80. doi: 10.1128/JCM.40.12.4576-4580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu C, Engle RE, Bryan JP, Emerson SU, Purcell RH. Detection of immunoglobulin M antibodies to hepatitis E virus by class capture enzyme immunoassay. Clin Diagn Lab Immun. 2003;10:579–586. doi: 10.1128/CDLI.10.4.579-586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johne R, Plenge-Bönig A, Hess M, Ulrich RG, Reetz J, Schielke A. Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT PCR. J Gen Virol. 2010;91:750–758. doi: 10.1099/vir.0.016584-0. [DOI] [PubMed] [Google Scholar]
- 23.Meng XJ, Purcell RH, Halbur PG, et al. A novel virus in swine is closely related to the human hepatitis E virus. PNAS USA. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Ling R, Erker JC, H, et al. A divergent genotype of hepatitis E virus in Chinese patients with acute hepatitis. J Gen Virol. 1999;80:169–177. doi: 10.1099/0022-1317-80-1-169. [DOI] [PubMed] [Google Scholar]
- 25.Davern TJ, Chalasani N. Drug-induced liver injury in clinical trials: as rare as hens’ teeth. Am J Gastroenterol. 2009;104:1159–61. doi: 10.1038/ajg.2009.76. [DOI] [PubMed] [Google Scholar]
- 26.Krawczynski K. Hepatitis E. Hepatology. 1993;17:932–41. [PubMed] [Google Scholar]
- 27.Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48:494–503. doi: 10.1016/j.jhep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis. 2008;8:698–709. doi: 10.1016/S1473-3099(08)70255-X. [DOI] [PubMed] [Google Scholar]
- 29.Chau TN, Lai ST, Tse C, Ng TK, Leung VK, Lim W, Ng MH. Epidemiology and clinical features of sporadic hepatitis E as compared with hepatitis A. Am J Gastroenterol. 2006;101:292–6. doi: 10.1111/j.1572-0241.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 30.Dalton HR, Stableforth W, Thurairajah P, et al. Autochthonous hepatitis E in Southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:784–90. doi: 10.1097/MEG.0b013e3282f5195a. [DOI] [PubMed] [Google Scholar]
- 31.Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–7. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 32.Haagsma EB, Niesters HG, van den Berg AP, et al. Prevalence of hepatitis E virus infection in liver transplant recipients. Liver Transpl. 2009;15:1225–8. doi: 10.1002/lt.21819. [DOI] [PubMed] [Google Scholar]
- 33.Kamar N, Rostaing L, Abravanel F, et al. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin Infect Dis. 2010;50:e30–e33. doi: 10.1086/650488. [DOI] [PubMed] [Google Scholar]
- 34.Kamar N, Rostaing L, Abravanel F, et al. Ribavirin therapy inhibits viral replication on patients with chronic hepatitis E virus infection. Gastroenterology. 2010;139:1612–8. doi: 10.1053/j.gastro.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Chauhan A, Jameel S, Dilawari JB, Chawla YK, Kaur U, Ganguly NK. Hepatitis E virus transmission to a volunteer. Lancet. 1993;341:149–50. doi: 10.1016/0140-6736(93)90008-5. [DOI] [PubMed] [Google Scholar]
- 36.El-Syed Zaki M, El-Deen Zaghloul MH, El Sayed O. Acute sporadic hepatitis E in children: diagnostic relevance of specific immunoglobulin M and immunoglobulin G compared with nested reverse transcriptase PCR. FEMS Immunol Med Microbiol. 2006;48:16–20. doi: 10.1111/j.1574-695X.2006.00111.x. [DOI] [PubMed] [Google Scholar]
- 37.Shrestha MP, Scott RM, Joshi DM, et al. Safety and efficacy of a recombinant hepatitis E Vaccine. N Engl J Med. 2007;357:895–903. doi: 10.1056/NEJMoa061847. [DOI] [PubMed] [Google Scholar]
- 38.Zhu F-C, Zhang J, Zhang X-F, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomized, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895–902. doi: 10.1016/S0140-6736(10)61030-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


