Abstract
Transport of large neutral amino acids (LNAA) across the blood brain barrier (BBB) is facilitated by the L-type amino acid transporter, LAT1. Peripheral accumulation of one LNAA (e.g., phenylalanine (phe) in PKU) is predicted to increase uptake of the offending amino acid to the detriment of others, resulting in disruption of brain amino acid homeostasis. We hypothesized that selected non-physiological amino acids (NPAAs) such as DL-norleucine (NL), 2-aminonorbornane (NB; 2-aminobicyclo-(2,1,1)-heptane-2-carboxylic acid), 2-aminoisobutyrate (AIB), and N-methyl-aminoisobutyrate (MAIB), acting as competitive inhibitors of various brain amino acid transporters, could reduce brain phe in Pahenu2 mice, a relevant murine model of PKU. Oral feeding of 5% NL, 5% AIB, 0.5% NB and 3% MAIB reduced brain phe by 56% (p<0.01), −1% (p=NS), 27% (p<0.05) and 14% (p<0.01), respectively, compared to untreated subjects. Significant effects on other LNAAs (tyrosine, methionine, branched chain amino acids) were also observed, however, with MAIB displaying the mildest effects. Of interest, MAIB represents an inhibitor of the system A (alanine) transporter that primarily traffics small amino acids and not LNAAs. Our studies represent the first in vivo use of these NPAAs in Pahenu2 mice, and provide proof-of-principle for their further preclinical development, with the long-term objective of identifying NPAA combinations and concentrations that selectively restrict brain phe transport while minimally impacting other LNAAs and downstream intermediates.
Introduction
Phenylketonuria (OMIM 261600) represents a heritable amino aciduria in which supraphysiological accumulation of phe is predicted to alter large neutral amino acid (LNAA) transport into brain. Depletion of specific brain LNAAs may have particularly deleterious effects, since these are precursors of critical downstream intermediates. These include S-adenosylmethionine (SAMe) derived from methionine, and the monoamine neurotransmitters dopamine and serotonin, derived from tyrosine and tryptophan, respectively. Among multiple mammalian amino acid transporters, at least four transport LNAAs (including phe (phe; F), tyrosine (tyr; Y), tryptophan (trp; W), leucine (leu; L), isoleucine (ile; I), valine (val; V), and methionine (met; M)) across brain capillaries (e.g., the blood brain barrier (BBB)) and intestinal mucosa (Choi and Pardridge 1986; Smith et al 1987). These transporters (LAT 1–4; all Na+-independent) comprise the system L nutrient transport system (Chrostowski et al 2009; Lin et al 2004; Christensen 1990; Table 1), originally characterized in Ehrlich ascites tumor cells as a system susceptible to 2-aminonorbornane (NB; Fig. 1) inhibition. Collectively, these LATs promote LNAA movement down a concentration gradient from blood, via endothelia, to the extracellular fluid (ECF) side and serve to insure homeostatic maintenance of brain LNAA levels. Under pathological situations (e.g. PKU), elevated levels of phe will overwhelm LAT homeostasis and increase self-uptake to the detriment of other LNAAs. A schematic diagram of the amino acid transport systems at the BBB, and their corresponding amino acid specificity, is depicted in Fig. 2.
Table 1.
Mammalian system L transporters using LNAAs as substrates
| Transporter | Expressiona | Amino Acids Transportedb | Reference |
|---|---|---|---|
| LAT-1 | Li (fetal), BM, Br, Pl, Te | L,I,V,F,Y,W,M,H | Kanai et al 1998 |
| LAT-2c | Je, Ile, Ki, Pl, Br, Te, SM | Y,F,W,T, N,I,C,S,L,V,Q (H,A,M,G)d | Segawa et al 1999 |
| LAT-3 | Pa, Li (fetal, adult), SM | I,L,V,F | Babu et al 2003 |
| LAT-4e | Pl, Ki, Leuc | F,L,I,M | Bodoy et al 2005 |
Legend:
(tissue abbreviations)=Li, liver; BM, bone marrow; Br, brain; Pl, placenta; Te, testes; Je, jejunum; Ile, ileum; Ki, kidney; SM, skeletal muscle; Pa, pancreas; Leuc, leucocytes;
(amino acid single letter codes)=H, his; T, thr; N, asn; C, cys; S, ser; Q, gln; A, ala; G, gly (see text for other amino acid abbreviations);
isoform of LAT-1;
lower affinity for amino acids in parentheses;
isoform of LAT-3
Fig. 1.
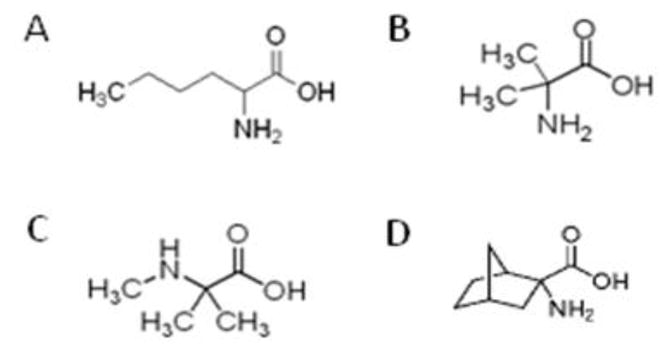
NPAAs evaluated in the current study; A, norleucine (NL); B, 2-aminoisobutyric acid (AIB); C, N-methyl-2-aminoisobutyric acid (MAIB); D, 2-aminonorbornane (NB). MAIB is a selective competitive inhibitor of the system A transport system (see Fig. 2). Structures were obtained from web resources (www.wikipedia.com; www.sigma-aldrich.com).
Fig. 2.
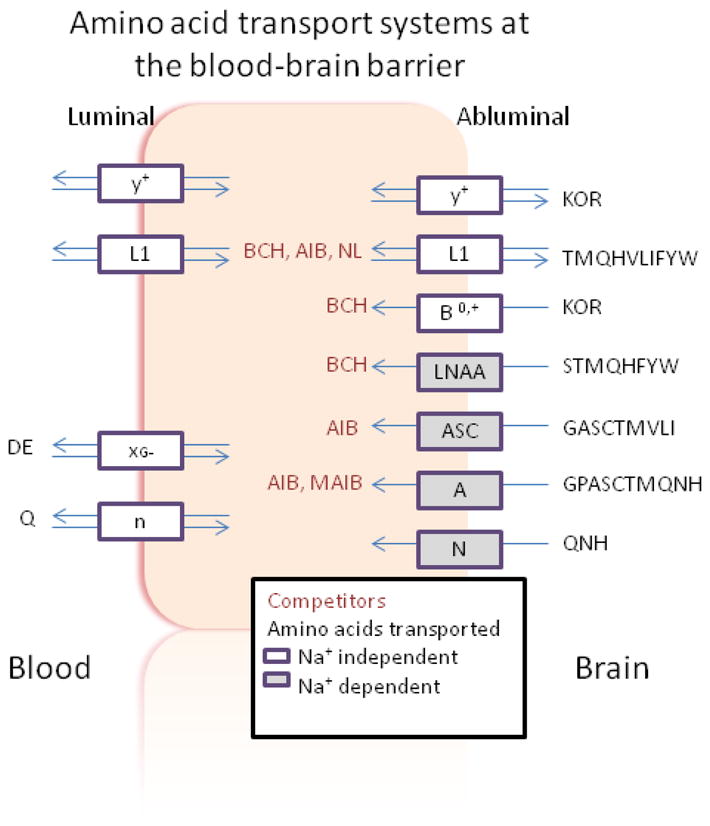
Schematic diagram of amino acid transport systems at the blood brain barrier. The sodium dependence of each system is indicated. The putative location for NPAA inhibition is depicted in the center of the diagram. Transport systems are enclosed in rectangles, and transported amino acids are presented in single letter format. Note that the LNAA transporter represents the general L (LAT) system, having overlap with the L1 system. Figure adapted from Broer and Brookes (2001).
Several NPAAs have been employed as inhibitors of different brain amino acid transport systems (Fig. 2; Tovar et al 1988; Tews and Harper 1986; Tews et al 1991; Tews et al 1990; McKean et al 1968). The prototypical LAT inhibitor, NB, is a bridged compound with high specificity for the LAT-1 system. Tews and Harper (1986) demonstrated that dietary supplementation of NL (Fig. 1) effectively lowered brain phe levels in the rat, while Ennis and coworkers (1994) presented evidence that AIB (Fig. 1) could compete with phe for transport on the LAT-1. Methyl-aminoisobutyrate (MAIB; Fig. 1) represents a specific inhibitor of the system A transporter with preference for small amino acids, although it can traffic glutamine, threonine, and methionine, the latter two generally considered LNAAs (Pisoni et al 1987). The preceding reports, and others documenting competition between LNAAs themselves for access to the brain (Christensen 1948; Knudsen et al 1995) have led to the concept of LNAA supplementation as an approach to lowering brain phe in PKU patients. Along these lines, Pietz and coworkers (1999) demonstrated that LNAA supplementation could decrease phe levels, and others (Matalon et al 2006; Michals-Matalon et al 2007) modified this diet with successful outcomes in PKU patients. Nonetheless, long term use of these LNAA-derived diets may be hampered by negative nitrogen balance (Dotremont et al 1995; van Spronsen and Enns 2010), which highlights the potential utility of applying NPAAs that are predicted to be non-metabolizable.
A systematic evaluation of NPAA intervention, targeting LNAA transport, has not been presented in any mammalian system. Zinnanti and coworkers (2009) provided proof-of-principle for this therapeutic approach employing dietary supplementation of NL in mice with intermediate maple syrup urine disease. NL feeding in these animals (in which leu, ile and val accumulate in response to the primary defect in branched-chain ketoacid dehydrogenase) extended lifespan while improving both brain biochemistry and neurobehavioral outcomes in affected animals. We have extended the work of Zinnanti and coworkers by implementing a preliminary characterization of the NPAAs described in Fig. 1 in a murine model of PKU, Pahenu2 mice. Outcome measures included body weights, dietary consumption and movement, and metabolic measures including LNAAs in brain and blood, monoamine neurotransmitters and methionine analogues in brain, and blood chemistries under selected dietary regimens. The current report summarizes the findings of our pilot studies.
Materials and Methods
Animal husbandry and subject number
For breeding, breeder pairs were established with male Pahenu2 and female heterozygous Pahenu2 subjects. Offspring were genotyped employing genomic DNA derived from tail clips obtained at weaning (20 days of life). Subsequently, PCR and restriction endonuclease digestion with BsmAl was performed, followed by visualization using 4% agarose gel electrophoresis (Zagreda et al 1999). Since our breeding scheme did not provide wild-type (WT) controls, and we sought to employ heterozygous Pahenu2 subjects as controls (thereby obviating the need for additional husbandry to generate WT subjects), we characterized monoamines and amino acids in WT subjects (of identical genetic background, C57Bl6) and compared to heterozygous Pahenu2 mice (Table 2). No significant differences were observed for eight of thirteen metabolites. Conversely, 5-HT, HVA, 5-HT turnover, total BCAA, and Met did differ significantly, although there was overlap of ranges (for all but HVA) and mean values were within 2 SD of each other. Based upon these comparative data, we felt justified in our pilot studies employing heterozygous Pahenu2 mice as internal controls.
Table 2.
Monoamines and amino acids in brain of heterozygous Pahenu2 (T) subjects and wild-type (WT) controls
| Metabolite | T mice | WT mice | t-test |
|---|---|---|---|
| DA | 8.10 ± 1.03 (n=12; 6.11–10.24) | 8.80 ± 1.24 (n=7; 6.62–10.21) | ns |
| 5-HT | 7.60 ± 4.34 (n=12; 2.92–15.36) | 3.09 ± 0.23 (n=7; 2.65–3.35) | <0.05 |
| DOPAC | 0.70 ± 0.06 (n=5; 0.63–0.81) | 0.66 ± 0.08 (n=7; 0.54–0.79) | ns |
| 3-MT | 0.56 ± 0.09 (n=12; 0.43–0.71) | 0.51 ± 0.25 (n=7; 0.25–0.85) | ns |
| HVA | 1.08 ± 0.10 (n=5; 0.97–1.23) | 1.71 ± 0.24 (n=7; 1.41–2.10) | <0.001 |
| 5-HIAA | 3.01 ± 1.78 (n=12; 0.91–5.40) | 1.99 ± 0.48 (n=7; 1.39–2.80) | ns |
| DA Turnover | 0.28 ± 0.09 (n=12; 0.20–0.55) | 0.27 ± 0.06 (n=7; 0.24–0.41) | ns |
| HT Turnover | 0.39 ± 0.09 (n=12; 0.31–0.56) | 0.65 ± 0.15 (n=7; 0.46–0.84) | <0.001 |
| DA Release | 0.06 ± 0.01 (n=5; 0.05–0.07) | 0.06 ± 0.02 (n=7; 0.03–0.09) | ns |
| Phe | 66 ± 16 (n=12; 31–87) | 59 ± 33 (n=8; 32–124) | ns |
| Tyr | 64 ± 17 (n=5; 45–88) | 52 ± 42 (n=8; 13–120) | ns |
| BCAA | 155 ± 30 (n=5; 108–189) | 104 ± 21 (n=7; 77–144) | <0.01 |
| Met | 71 ± 14 (n=12; 57–95) | 38 ± 16 (n=8; 14–63) | <0.001 |
Legend: Values shown are mean ± 1 SD, with number of animals and range in parentheses. BCAA represents total BCAA (the sum of valine, isoleucine and leucine) with HT Turnover representing turnover of serotonin; DA turnover=(DOPAC+HVA)/DA; HT-Turnover=5-HIAA/5-HT; DA Release=3-MT/DA. Statistical analyses employed an unpaired t test.
A sample size of five facilitated simple parametric statistical analysis (t test, ANOVA). This sample size was based upon power calculations utilized in another study in which hepatocyte transplantation was the study parameter. In that work, an n=5 sample size (hepatocyte-transplanted imsud mice vs. PBS-transplanted imsud mice; Skvorak et al 2009) enabled detection of a 20% reduction in alloisoleucine level with a significance of p<0.05. Accordingly, this number of subjects was adopted in the current study. The interventional protocol was approved by the institutional IACUC (protocol L0214).
Diet preparation and intervention
Dietary NPAA administration was chosen to most closely mimic the clinical setting. NPAAs included 5% 2-aminoisobutyric acid (AIB), 5% DL-norleucine (NL), 0.5% norbornane (NB; 2-aminobicyclo(2,2,1)heptane-2-carboxylic acid), and 3% N-methyl-aminoisobutyric acid (MAIB) (all obtained from Sigma Aldrich), and control chow (2018 Teklad Global Rodent Chow, 18 % protein, or extruded sterilizable chow 2018X). We characterized 5% (w/w) NL and AIB based upon the previous report of Zinnanti et al (2009) employed in the imsud murine model. The expense of NB (~$350/g) prohibited its use at 5%; accordingly, we chose 0.5% w/w (a serendipitous choice). Two experiments were performed, with the first evaluating NL, NB and AIB in regular 18% Teklad mouse chow (18R), and the second utilizing MAIB in sterilizable, extruded 18% protein chow. Following our initial trial with NL, AIB and NB intervention, our mouse colony suffered a Helicobacter infection, likely associated with our unsterilized 18% diet. Accordingly, for MAIB intervention we were constrained to employ an extrudable, sterilizable diet of identical protein content. We chose to initially examine a regular protein chow (as opposed to a low-protein chow) in order to gauge the potential for eventual dietary relaxation of protein intake in the clinical setting.
Chow was made in batches of 300 g. 18R was made with 300 mL of filtered water and mixed with drug and dehydrated 24 hours at 46°C. 18X chow was made with 500 ml of autoclaved water and dehydrated 20–24 hours at 46°C. Drugs were not autoclaved, and assumed stable at 46°C. Dietary intervention was begun at 3.5 weeks of age (post weaning and genotyping) and maintained for 3 weeks. Animals were housed under a 12 hour light/dark cycle with ad libitum access to food and water. Mouse weights were recorded three times weekly, and food consumption calculated weekly. Brain halves and sera were collected into 1.5 mL tubes at sacrifice. Brain halves were flash-frozen with liquid nitrogen prior to storage at −80°C.
Analyses of LNAA including SAMe and SAH
We used stable-isotope dilution liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) to determine SAM, SAH, methionine (met), tryptophan (trp), phenylalanine (phe), tyrosine (tyr), isoleucine (ile), leucine (leu) and valine (val) in serum and brain tissue from Pahenu2 mice. Stable isotope internal standards (2H3-SAMe, 2H3-methionine, 2H3-tryptophan, 2H3-phenylalanine, 2H7-tyrosine, 13C6-isoleucine, 2H3-leucine and 2H8-valine) were obtained from either CDN Isotopes or Cambridge Isotope Laboratories. Isotopically labeled SAH was not commercially available and 2H4-SAH was prepared by enzymatic synthesis utilizing an Axis Homocysteine EIA kit (Axis-Shield Diagnostics Ltd, UK). Composition of the reaction mixture were as follows: 900 μl phosphate buffer (reagent A), 50 μl adenosine, dithiothreitol and citric acid (reagent B), 50 μl recombinant S-adenosyl-L-homocysteine hydrolase (reagent C) and 100 μl 1 mmol/L 2H4-D,L-homocysteine. The mixture was incubated at 37 °C for 30 minutes. Upon completion of the reaction, 2H4-SAH was purified by HPLC with ultraviolet absorbance at 260 nm by injecting 25μl fractions and the eluate fractions containing 2H4-SAH were pooled. 2H4-SAH was separated with a Spheroclone ODS2 analytical column (250 × 4.6 mm, 5 μm; Phenomenex) with H2O-methanol (90:10, by volume), containing 500 μL/L formic acid as mobile phase at a flow rate of 1.5 mL/min. The concentration of 2H4-SAH was estimated by LC-MS/MS. The obtained 2H4-SAH stock solution was combined with additional internal standards in eluent A. Calibrators and stable isotope internal standards were included in each analytical run for calibration. Stock standards of S-adenosylmethionine (SAMe) and S-adenosylhomocysteine (SAH) were prepared in 0.1 M PCA at a concentration of 1 mmol/L and stored at −80°C. An amino acid standard solultion (500 μmol/L in 0.1M HCL; Fluka) containing methionine (met), tryptophan (trp), phenylalanine (phe), tyrosine (tyr), isoleucine (ile), leucine (leu) and valine (val) was purchased from Sigma (St. Louis, MO, USA).
Preparation details of half-brain tissue samples obtained from Pahenu2 mice were as follows. Calibration stock solutions of each standard were diluted in Type 1 water to perform a 6-point calibration curve (Table 3). Pahenu2 half-brain tissue samples that were previously deproteinized 1:5 with ice-cold 0.1 M PCA were thawed and spun. Samples were prepared by the addition of 180 μL mobile phase A containing approximately 10 – 50 μmol/L of each internal standard to 20 μL of blank, standard, QC or brain extract and mixed by vortex. Prepared sample was loaded into a 96-well microtiter plate and 5 μl was injected for analysis.
Table 3.
Mass transitions, retention times, and specific calibration curves
| Analyte | Analyte MRM (m/z) | Labeled Isotope | Labeled Isotope MRM (m/z) | Retention time | Serum Calibration Curve | Tissue Calibration Curve |
|---|---|---|---|---|---|---|
| SAM | 399→250 | 2H3-SAMe | 402→250 | 7.1 | 400 - 12.5nmol/L | 10 - 0.31μmol/L |
| SAH | 385→136 | 2H4-SAH | 389→138 | 6.8 | 400 - 12.5nmol/L | 10 - 0.31μmol/L |
| met | 150→104 | 2H3-met | 153→107 | 6.5 | 250 - 7.8 μmol/L | 200 - 6.25 μmol/L |
| trp | 205→188 | 2H3-trp | 208→191 | 8.0 | 250 - 7.8 μmol/L | 200 - 6.25 μmol/L |
| phe | 166→120 | 2H3-phe | 171→125 | 7.6 | 250 - 7.8 μmol/L | 200 - 6.25 μmol/L |
| tyr | 182→136 | 2H7-tyr | 189→143 | 6.5 | 250 - 7.8 μmol/L | 200 - 6.25 μmol/L |
| ile | 132→86 | 13C6-ile | 138→91 | 7.3 | 250 - 7.8 μmol/L | 200 - 6.25 μmol/L |
| leu | 132→82 | 2H3-leu | 135→85 | 7.5 | 250 - 7.8 μmol/L | 200 - 6.25 μmol/L |
| val | 118→72 | 2H8-val | 126→80 | 5.9 | 250 - 7.8 μmol/L | 200 - 6.25 μmol/L |
Preparation details of serum samples obtained from Pahenu2 mice were as follows. Stock solutions of each standard were diluted in Type 1 water to perform a 6-point calibration curve (Table 3). Sample preparation utilized microcentrifugal filter units, Amicon Ultra-0.5 ml, 10 kDa NMWL (Millipore, USA). Samples were prepared by the addition of 100 μL mobile phase A containing 10 – 50 μmol/L labeled-isotope internal standards to 20 μl of blank, standard, serum, QC or unknown serum followed by vortex. Microcentrifugal filter units were centrifuged for 20 min at 14,800 × g at 4°C. Sample filtrate was transferred to a microtiter plate and 5 μl was injected for analysis.
Chromatographic separation was achieved on an EZ-faast 250 × 2.0 mm 4 μm AAA-MS analytical column (Phenomenex) maintained at 36°C at a flow of 250 μL/min with a binary gradient and a total run time of 12 minutes. Eluents for HPLC were: (A) 4 mM ammonium acetate, 0.1% formic acid, 0.1% heptafluorobutyric acid (pH=2.5); (B) 100% methanol and 0.1% formic acid. The initial gradient condition was 75% A: 25% B and was increased in a linear fashion to 100% B in 6 min and held constant for 1 min. At 7.1 min the eluents wrere reset to initial conditions for 5 minutes. The flow from the column was delivered to the ion source from the period of 3 to 8 min, otherwise the flow was diverted to waste. The compounds were detected by multiple reaction monitoring (MRM) using positive ESI with a dwell time of 30 ms. The curtain gas was set at 15 L/min, and source gas 1 and 2 were set at 60 L/min. The heater was set to 700° C with an ionspray voltage of 5000V and CAD gas (nitrogen) was set at 3.5 × 10e-5 Torr. Sample separation and injection was performed by a Shimadzu Prominence LC System interfaced with a 4000 Q TRAP® LC-MS/MS (ABSciex). All data were collected using Analyst software version 1.4.2.
Analyses of monoamines in brain
Monoamine metabolites in Pahenu2 and heterozygous Pahenu2 half-brain were quantified by reverse-phase HPLC with electrochemical detection. Flash-frozen tissues were homogenized in 0.1 M perchloric acid supplemented with dithioerythritol and diethylenetriamine pentaacetic acid (Ogburn et al 2006). A schematic diagram of the pathways of degradation for the monoamine neurotransmitters, dopamine (DA) and 5-hydroxytryptamine (5-HT; serotonin) is shown in Fig. 3. Note that the turnover of DA is described as the sum (DOPAC + HVA)/DA, while that for 5-HT is represented by 5-HIAA/5-HT. The ratio of 3-methoxytyramine (3-MT) to DA represents an estimate of the release of DA into the synaptic cleft.
Fig. 3.
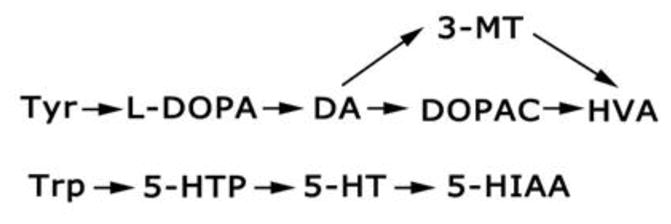
Schematic of dopamine (DA) and serotonin (5-hydroxytryptamine; 5-HT) synthesis and metabolism. Abbreviations: Tyr, tyrosine; Trp, tryptophan; L-DOPA, L-dihydroxyphenylalanine; 5-HTP, 5-hydroxytryptophan; DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid; 3-MT, 3-methoxytyramine (a DA metabolite released into the synaptic cleft); 5-HIAA, 5-hydroxyindoleacetic acid.
Blood chemistries
Comprehensive serum chemistry was obtained using the serum pathology profile provided by IDEXX RADIL (Columbia, MO, USA; http://www.idexxradil.com).
Brain estimation of NL content
During initial evaluation of NPAA administration with NL, we employed the MassTrak system (http://www.waters.com). With this system, we found perfect coelution of NL with phe, precluding accurate quantitation of either species. However, employing LC-MS/MS analysis for quantitation of phe in later studies, we could employ both systems to estimate brain/blood NL levels by differential, thus providing an estimate of NL levels during intervention. The absence of stable isotopically labeled internal standard for NL precluded our capacity to quantitate NL levels using LC-MS/MS.
Statistical analyses
Metabolite data was grouped with respect to genotype (Pahenu2 and heterozyogous Pahenu2) in order to correlate the effects of NPAAs under normo- and hyperphenylalaninemic conditions. A column statistical approach (ANOVA with Tukey post-hoc) facilitated significant reduction of data analysis with this grouping. For studies employing MAIB and those comparing metabolites for wild-type (WT) and heterozygous Pahenu2 mice, a two-way t test was employed. Analysis was performed with the Graphpad Prism V5 program, and significance set at the 95th percentile.
Results
Diet consumption and general health
Food and water consumption, and body weights, were recorded during each three-week trial. Food and water consumption, and body weights, did not differ significantly with respect to control cohorts (heterozygous Pahenu2) as a function of treatment intervention (data not shown). There were no obvious phenotypic differences for subjects consuming AIB, NB or MAIB with regard to movement. Conversely, subjects consuming 5% NL displayed significant motor impairment (also seen with 3% NL consumption; data not shown). This motor impairment is best described as impaired levation, specifically an inability to raise the abdomen from the cage bottom, inability to climb, and a slow irregular gait. These findings were prominent in Pahenu2 subjects but not observed in heterozygous Pahenu2 subjects.
LNAA levels and methionine metabolites in brain extracts
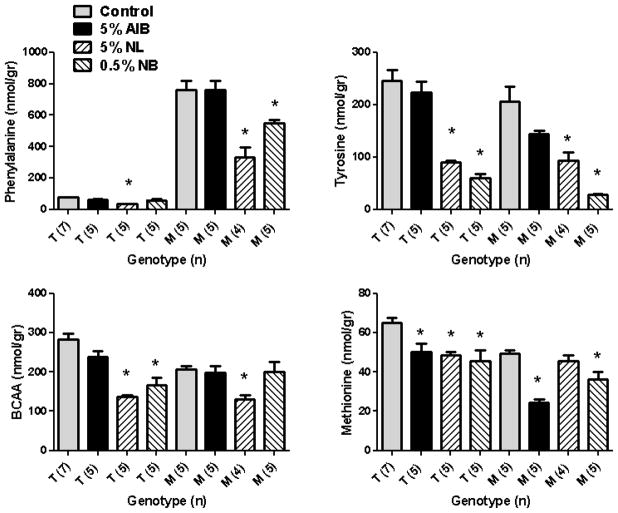
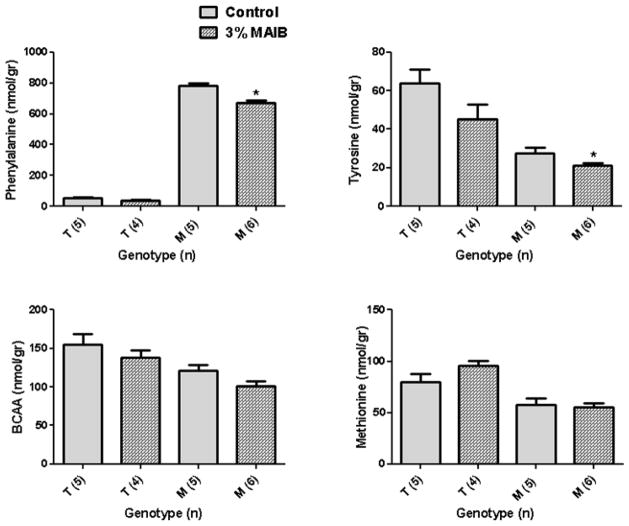
NL, NB, and MAIB intervention significantly lowered brain phe levels in Pahenu2 subjects, with NL displaying the largest reduction (56% vs. 27% for NB; Fig. 4), whereas 5% AIB intervention did not reduce brain phe. Additionally, NL feeding significantly reduced phe even in heterozygous Pahenu2 subjects. No dietary intervention significantly altered trp levels (not shown), although all diets (AIB, NL and NB) manifested pronounced effects on tyr, total BCAAs and met (Fig. 4). Tyr levels were significantly decreased in both genotypes with NL and NB feeding, and these diets had comparable effects on total BCAA levels in both genotypes. Significant effects of all dietary interventions were observed on met levels (Fig. 4), and met was the only LNAA for which 5% AIB intervention led to a significant reduction (both genotypes). Dietary intervention with 3% MAIB also significantly decreased phe in Pahenu2 mice (14% reduction; Fig. 5), while slightly (but significantly) reducing tyr levels as well. Conversely, there was no significant alteration on brain total BCAAs nor met levels (Fig. 5), and there were no changes in brain trp levels (data not shown).
Fig. 4.
Selected brain LNAAs amino acids as a function of diet and genotype (T, heterozygous Pahenu2 mice; M, Pahenu2 mice; trp levels not shown). Amino acid levels shown as nmol/gram (gr) wet weight tissue. Parenthetical values represent the number of mice studied. Total branched chain amino acids (BCAA) represent the sum of ile, val and leu. Statistical analysis (one-way ANOVA with Tukey post-hoc) compared cohorts with and without NPAAs, and within genotype only (*p<0.05 compared to control, no drug intervention).
Fig. 5.
Selected brain LNAAs as a function of dietary intervention with 3% MAIB. For abbreviations, see Fig. 4 legend. Statistical analysis employed a two-way t test, comparing dietary intervention only within the same genotype.
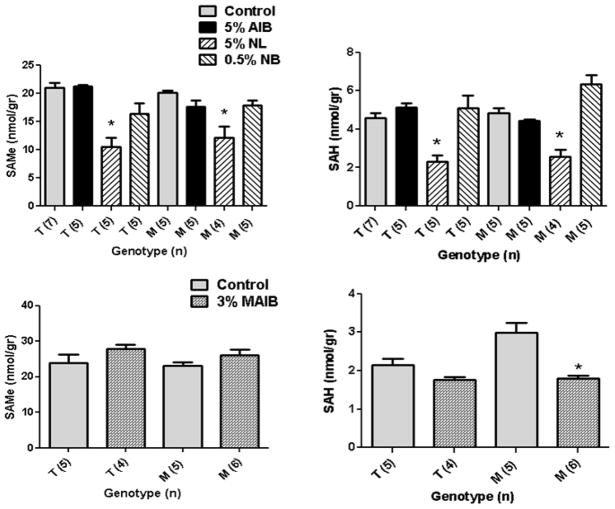
We extended brain LNAA characterization to an examination of downstream met metabolites, S-adenosylmethionine (SAMe) and S-adenosylhomocysteine (SAH) (Fig. 6). For SAMe, 5% NL feeding had a significant lowering effect in both genotypes, while 3% MAIB intervention significantly reduced SAH, but not SAMe, levels (Fig. 6).
Fig. 6.
S-adenosylmethionine (SAMe) and S-adenosylhomocysteine (SAH) levels as a function of dietary intervention. For abbreviations, see Fig. 4 legend. Statistical analysis (one way ANOVA with Tukey post-hoc) compared cohorts with and without NPAAs (for AIB, NL and NB), and within genotype only (*p<0.05 compared to control). Statistical analysis for MAIB intervention employed a two-way t test, comparing dietary intervention only within genotype.
LNAA levels in sera as a function of dietary intervention
We examined LNAA levels in sera to estimate the potential of our dietary intervention to restrict gut uptake and absorption. Dietary intervention with NL, NB and MAIB had no significant effect on sera levels of phe, tyr, met, trp or total BCAA levels (data not shown). Of interest, 5% AIB feeding significantly reduced blood trp and met levels (data not shown), the latter consistent with the isolated effect of AIB on brain met (Fig. 4).
Brain monoamines as a function of dietary intervention
Since tyr and trp represent precursors of the monoamine neurotransmitters dopamine and serotonin (Fig. 3), respectively, we characterized monoamine neurotransmitters and associated metabolites in brain extracts. For AIB, NL and NB feeding, there were no differences for DOPAC, HVA and DA release (the latter quantified as the 3-MT/DA ratio) with respect to either genotype (data not shown). The most significant effects on monoamine levels was observed with 0.5% NB intervention, which significantly decreased DA and 5-HT levels, in both genotypes, while simultaneously lowering 3-MT levels (Fig. 7). A significant effect with NL intervention was only observed in heterozygous Pahenu2 mice for serotonin and its metabolite, 5-HIAA. NB intervention drastically enhanced turnover of both DA and 5-HT, in both genotypes (Fig. 7). The significant depletion of serotonin levels in Pahenu2 mice in the absence of drug intervention, and its downstream metabolite 5-HIAA, was consistent with our previous findings (Arning et al 2009). Feeding of 5% AIB showed no effect on any monoamine metabolite.
Fig. 7.
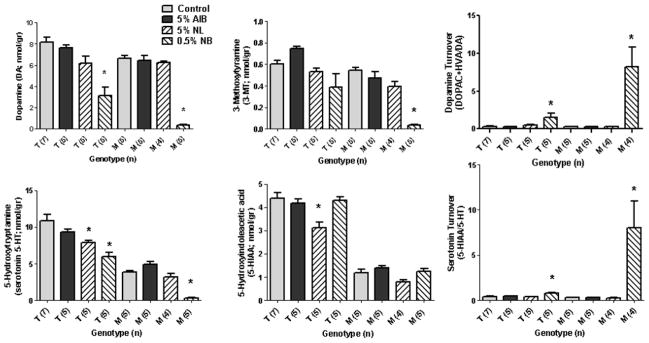
Monoamine neurotransmitters and metabolites (see Fig. 3) in brain extracts of Pahenu2 and heterozygous Pahenu2 mice as a function of diet. Statistical analyses and abbreviations as described in legends to Figs. 3 and 4. Not shown are DOPAC, HVA, and the ratio of 3-MT/DA (dopamine release), which showed no significant differences for either genotype with any diet, with the exception of MAIB (see Fig. 8).
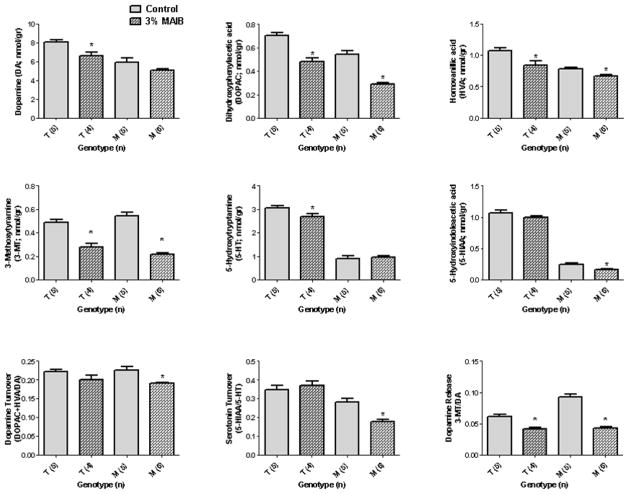
Although 3% MAIB feeding did not significantly reduce either DA or 5-HT concentrations in Pahenu2 mice (Fig. 8), significant reductions in DOPAC, HVA and 3-MT levels were seen in both genotypes. As well, there was a significant reduction in the 5-HT metabolite, 5-HIAA, in Pahenu2 subjects. Finally, the turnover of both DA and 5-HT was significantly decreased in Pahenu2 mice with MAIB feeding, while the release of DA (measured as the ratio of 3-MT/DA) was significantly decreased in both genotypes (Fig. 8). These results for DA and 5-HT were opposite to those observed for NB feeding (Fig. 7).
Fig. 8.
Monoamine neurotransmitters and associated metabolites in brain extract of Pahenu2 and heterozygous Pahenu2 mice as a function of 3% MAIB feeding. Abbreviations and statistical analyses as described in Figs. 3 and 5.
Effect of NL feeding on blood chemistries
Heterozygous Pahenu2 mice were supplemented with 5% NL (n=7) or unsupplemented chow (n=6) for 3 weeks, at which time sera was isolated for blood chemistries. The latter included glucose, blood urea nitrogen (BUN), creatinine, total protein, albumin, phosphorus, Na+, Cl−, K+, total CO2, cholesterol, triglycerides, Ca+2, total bilirubin, alkaline phosphatase (ALP), alanine aminotransferase, and gamma-glutamyltransferase. No significant differences were observed between cohorts, with two exceptions: BUN (5.43 ± 0.30 mg/dL (+NL) vs. 3.83 ± 0.17 (no NL) (p=0.001; two-tailed t test); and ALP (41.6 ± 4.0 U/L (+ NL) vs. 57.7 ± 2.3 (no NL) (p=0.0065). An increased BUN level with NL intervention suggested endogenous metabolism of NL.
Estimation of NL levels in brain and blood
To verify uptake, absorption and transport across the BBB, NL levels were estimated in terminal samples of sera and brain. In five subjects, the sera NL concentration was 3.11 ± 0.41 mM (SD; range 2.57–4.75) while that in brain was 822 ± 27 nmol/gr tissue (assuming a tissue density approximating water, ~0.82 μM). These data support absorption, transport and uptake into brain. Specific methodology has not been developed to accurately quantify NB, AIB and MAIB.
Discussion
The objective of the pilot studies presented was to provide proof-of-principle supporting the use of dietary NPAA intervention in Pahenu2 mice as a potential treatment strategy targeting brain phe levels. These initial interventional studies represent the first application of NL and AIB in Pahenu2 mice, and the first in vivo application of NB in a mammalian system. MAIB was previously employed to study the transport of amino acids across the BBB in the rat, where it was found to have an absence of inhibitory effects on phe influx using intravenous injection (Wadhwani et al 1990). Overall, the phe-reducing capacity of NL, NB and MAIB in the brain of Pahenu2 mice provides a rationale for their further characterization in this mouse model. At the concentrations employed, however, neither NL nor NB was selective for phe, with significant effects on other LNAAs, methionine analogues and monoamine metabolites. Nonetheless, the different L system transporters described in mammals (Table 1), their differential locations and amino acid specificities, suggests that appropriate NPAA concentrations can be formulated (either alone or in combination) that may maximally restrict brain phe transport with minimal effects on other LNAAs.
A novel finding in our work was the effect of 3% MAIB feeding on LNAA transport. MAIB is reported to be a specific A system inhibitor (Broer and Brookes 2001), yet its consumption in Pahenu2 mice led to significant reduction of both brain phe and tyr, and concomitant effects primarily on the dopamine system. The mild effects of 3% MAIB feeding on LNAA levels (normal BCAA, met, trp), coupled with an absence of effect on DA and 5-HT levels in Pahenu2 mice, suggests that MAIB deserves further consideration as a candidate molecule targeting the reduction of phe-specific transport into brain in Pahenu2 mice. Another interesting observation from our studies was the met-specific effect of AIB. Generally, AIB had minimal effects on most LNAAs, with the exception of a specific reduction of met in both brain and sera, and serum trp levels. Earlier studies revealed that AIB shares a transport system in common with alanine, cysteine, glycine, methionine, serine and proline (Broer and Brookes 2001; Lepley and Mukkada 1983), and other investigators have demonstrated the capacity of met to inhibit AIB transport in both rat liver and mammary gland (Crawhall and Purkiss 1973; Shennan and McNeillie 1994). In the latter study by Shennan and McNeillie, trp was also a potent inhibitor of AIB transport, consistent with our findings of reduced blood met and trp during AIB intervention.
Structural evidence suggests that AIB, NB and MAIB are unlikely to be extensively metabolized in mammals, yet systematic metabolic analyses is lacking. The use of any NPAA as a competitive inhibitor of phe transport, in either Pahenu2 or PKU patients, will be thwarted if there is a significant contribution to the nitrogen pool resulting from consumption and metabolism. Negative nitrogen balance represents one of the mitigating factors against the long term use of LNAA supplements in PKU patients as a mechanism to restrict phe transport into brain (van Spronsen et al 2010). As secondary and methylated amines, respectively, neither AIB nor MAIB are expected to contribute significantly to the nitrogen load. The bridged nature of NB also argues against a significant component of metabolism. However, NL is a primary amine that may be readily transaminated in vivo, consistent with our results. As well, NL may be metabolized by intestinal bacteria. For example, NL is transaminated by Candida in gut, and it may be a substrate for amino acid oxidase (Der Garabedian and Vermeersch 1987). AIB has been shown to be a substrate for at least one bacterial species (Aaslestad and Larson 1964). Our initial blood chemistries for NL suggest that careful consideration be given to nitrogen load during our NPAA interventions, and we are examining the use of both D- and L-norleucine individually.
We visually observed motor impairment in Pahenu2 subjects receiving NL, which was not observed in animals receiving AIB, MAIB or NB. We speculate that the movement abnormalities associated with NL are associated with depletion of SAMe and SAH (Fig. 6), which was not observed with NB and AIB feeding, although MAIB did significantly lower SAH levels. Since SAMe is the methyl donor for a number of reactions of dopamine metabolism (including catechol-O-methyltransferase and phenylethanolamine-N-methyltransferase), as well as epinephrine and norepinephrine metabolism, this may provide some insights into the motor dysfunction seen with NL feeding (Kurian et al 2011). Moreover, it remains to be explained why Pahenu2 mice have such a striking depletion of serotonin, which we have seen in both the current and previous studies (Arning et al 2009). Interventions geared to replenish 5-HT levels in Pahenu2 subjects would seem prudent (e.g., 5-hydroxytryptophan), and may have treatment relevance to patients with PKU, especially with respect to the long-term cognitive dysfunction observed (Enns et al 2010). This is supported by mounting evidence that the serotoninergic system plays an important role in cognition and learning (Geldenhuys and Van der Schyf 2011)
Utilizing NPAAs to reduce cerebral phe transport is pertinent to the long-term objective of employing oral NPAA intervention(s) in PKU patients for whom dietary adherence to low-protein intake is suboptimal, and potentially to identify NPAA interventions that would facilitate normal dietary protein intake for patients. Additional preclinical studies are required to optimize the concentration and possible combinatorial dosages of NPAAs, in order to produce a selective inhibition of phe transport into brain. Studies along these lines are actively underway in our laboratory.
Concise 1 sentence take-home message.
We have demonstrated the capacity of selected non-physiological amino acids to restrict brain phenylalanine accumulation in a murine model of PKU, Pahenu2 mice, highlighting a rationale for further preclinical development of these compounds as a potential future treatment strategy in PKU.
Acknowledgments
The authors are indebted to Dr. Cary Harding, Oregon Health Sciences University, for providing the Pahenu2 murine model, and to Dr. Kristen J. Skvorak for supplying wild-type subjects for characterization of brain amino acids and monoamines. The guidance and advice of Drs. Viktor Kozich, Harvey Mudd, and William Zinnanti are gratefully acknowledged. This work was supported by a grant from the National PKU Alliance (www.npkua.org), which is gratefully acknowledged.
List of Abbreviations
- 3-MT
3- methoxytyramine
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HT
serotonin
- 5-HTP
5-hydroxytryptophan
- AIB
aminoisobutyric acid
- BBB
blood brain barrier
- DA
dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- HVA
homovanillic acid
- Ile; I
isoleucine
- L-DOPA
L-dihydroxyphenylalanine
- LAT1
L-type amino acid transporter
- Leu; L
leucine
- LNAA
large neutral amino acid
- NL
DL-norleucine
- MAIB
methyl-aminoisobutyric acid
- Met; M
methionine
- NB
2-aminobicyclo(2,2,1)heptane-2-carboxylic acid (2-aminonorbornane)
- NPAA
non-physiological amino acid
- PAH
phenylalanine hydroxylase
- Phe; F
phenylalanine
- PKU
phenylketonuria
- SAH
S-adenosylhomocysteine
- SAMe
S-adenosylmethionine
- Trp; W
tryptophan
- Tyr; Y
tyrosine
- Val; V
valine
Literature cited
- Aaslestad HG, Larson AD. Bacterial metabolism of 2-methylalanine. J Bacteriol. 1964;88:1296–303. doi: 10.1128/jb.88.5.1296-1303.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arning E, Bottiglieri T, Sun Q, et al. Metabolic profiling in phenylalanine hydroxylase-deficient (Pah−/−) mouse brain reveals decreased amino acid neurotransmitters and preferential alterations of the serotoninergic system. Molec Genet Metab. 2009;98:21. [Google Scholar]
- Babu E, Kanai Y, Chairoungdua A, et al. Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J Biol Chem. 2003;278:43838–45. doi: 10.1074/jbc.M305221200. [DOI] [PubMed] [Google Scholar]
- Bodoy S, Martín L, Zorzano A, Palacín M, Estévez R, Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem. 2005;280:12002–11. doi: 10.1074/jbc.M408638200. [DOI] [PubMed] [Google Scholar]
- Bröer S, Brookes N. Transfer of glutamine between astrocytes and neurons. J Neurochem. 2001;77:705–19. doi: 10.1046/j.1471-4159.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- Choi TB, Pardridge WM. Phenylalanine transport at the human blood-brain barrier. Studies with isolated human brain capillaries. J Biol Chem. 1986;261:6536–41. [PubMed] [Google Scholar]
- Christensen HN, Streicher JA, Elbinger RL. Effects of feeding individual amino acids upon the distribution of other amino acids between cells and extracellular fluid. J Biol Chem. 1948;172:515–24. [PubMed] [Google Scholar]
- Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70:43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Chrostowski MK, McGonnigal BG, Stabila JP, Padbury JF. LAT-1 expression in pre- and post-implantation embryos and placenta. Placenta. 2009;30:270–6. doi: 10.1016/j.placenta.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawhall JC, Purkiss P. Transport of methionine and proline by rat liver slices and the effect of certain hormones. Biochem J. 1973;136:15–24. doi: 10.1042/bj1360015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der Garabedian PA, Vermeersch JJ. Candida L-norleucine, leucine:2-oxoglutarate aminotransferase. Purification and properties. Eur J Biochem. 1987;167:141–7. doi: 10.1111/j.1432-1033.1987.tb13315.x. [DOI] [PubMed] [Google Scholar]
- Dotremont H, Francois B, Diels M, Gillis P. Nutritional value of essential amino acids in the treatment of adults with phenylketonuria. J Inherit Metab Dis. 1995;18:127–30. doi: 10.1007/BF00711746. [DOI] [PubMed] [Google Scholar]
- Ennis SR, Ren XD, Betz AL. Transport of alpha-aminoisobutyric acid across the blood-brain barrier studied with in situ perfusion of rat brain. Brain Res. 1994;643:100–7. doi: 10.1016/0006-8993(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Enns GM, Koch R, Brumm V, Blakely E, Suter R, Jurecki E. Suboptimal outcomes in patients with PKU treated early with diet alone: revisiting the evidence. Mol Genet Metab. 2010;101:99–109. doi: 10.1016/j.ymgme.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Geldenhuys WJ, Van der Schyf CJ. Role of serotonin in Alzheimer’s disease: a new therapeutic target. CNS Drugs. 2011;25:765–81. doi: 10.2165/11590190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–32. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Knudsen GM, Hasselbalch S, Toft PB, Christensen E, Paulson OB, Lou H. Blood-brain barrier transport of amino acids in healthy controls and in patients with phenylketonuria. J Inherit Metab Dis. 1995;18:653–64. doi: 10.1007/BF02436753. [DOI] [PubMed] [Google Scholar]
- Kurian MA, Gissen P, Smith M, Heales S, Jr, Clayton PT. The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol. 2011;10:721–33. doi: 10.1016/S1474-4422(11)70141-7. [DOI] [PubMed] [Google Scholar]
- Lepley PR, Mukkada AJ. Characteristics of an uptake system for alpha-aminoisobutyric acid in Leishmania tropica promastigotes. J Protozool. 1983;30:41–6. doi: 10.1111/j.1550-7408.1983.tb01030.x. [DOI] [PubMed] [Google Scholar]
- Lin J, Raoof DA, Thomas DG, et al. L-type amino acid transporter-1 overexpression and melphalan sensitivity in Barrett’s adenocarcinoma. Neoplasia. 2004;6:74–84. doi: 10.1016/s1476-5586(04)80054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon R, Michals-Matalon K, Bhatia G, et al. Large neutral amino acids in the treatment of phenylketonuria (PKU) J Inherit Metab Dis. 2006;29:732–8. doi: 10.1007/s10545-006-0395-8. [DOI] [PubMed] [Google Scholar]
- McKean CM, Boggs DE, Peterson NA. The influence of high phenylalanine and tyrosine on the concentrations of essential amino acids in brain. J Neurochem. 1968;15:235–41. doi: 10.1111/j.1471-4159.1968.tb06202.x. [DOI] [PubMed] [Google Scholar]
- Michals-Matalon K, Bhatia G, Guttler F, Tyring SK, Matalon R. Response of phenylketonuria to tetrahydrobiopterin. J Nutr. 2007;137:1564S–1567S. doi: 10.1093/jn/137.6.1564S. [DOI] [PubMed] [Google Scholar]
- Ogburn KD, Bottiglieri T, Wang Z, Figueiredo-Pereira ME. Prostaglandin J2 reduces catechol-O-methyltransferase activity and enhances dopamine toxicity in neuronal cells. Neurobiol Dis. 2006;22:294–301. doi: 10.1016/j.nbd.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Pietz J, Kreis R, Rupp A, Mayatepek E, Rating D, Boesch C, Bremer HJ. Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J Clin Invest. 1999;103:1169–78. doi: 10.1172/JCI5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni RL, Flickinger KS, Thoene JG, Christensen HN. Characterization of carrier-mediated transport systems for small neutral amino acids in human fibroblast lysosomes. J Biol Chem. 1987;262:6010–7. [PubMed] [Google Scholar]
- Roe CR, Bottiglieri T, Wallace M, Arning E, Martin A. Adult Polyglucosan Body Disease (APBD): Anaplerotic diet therapy (Triheptanoin) and demonstration of defective methylation pathways. Mol Genet Metab. 2010;101:246–52. doi: 10.1016/j.ymgme.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745–51. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- Shennan DB, McNeillie SA. Characteristics of alpha-aminoisobutyric acid transport by lactating rat mammary gland. J Dairy Res. 1994;61:9–19. doi: 10.1017/s0022029900028016. [DOI] [PubMed] [Google Scholar]
- Skvorak KJ, Hager EJ, Arning E, et al. Hepatocyte transplantation (HTx) corrects selected neurometabolic abnormalities in murine intermediate maple syrup urine disease (iMSUD) Biochim Biophys Acta. 2009;1792:1004–10. doi: 10.1016/j.bbadis.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith QR, Momma S, Aoyagi M, Rapoport SI. Kinetics of neutral amino acid transport across the blood-brain barrier. J Neurochem. 1987;49:1651–8. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Tews JK, Harper AE. Tissue amino acids in rats fed norleucine, norvaline, homoarginine or other amino acid analogues. J Nutr. 1986;116:1464–72. doi: 10.1093/jn/116.8.1464. [DOI] [PubMed] [Google Scholar]
- Tews JK, Repa JJ, Harper AE. Norleucine: a branched-chain amino acid analog affecting feeding behavior of rats. Pharmacol Biochem Behav. 1990;35:911–21. doi: 10.1016/0091-3057(90)90379-v. [DOI] [PubMed] [Google Scholar]
- Tews JK, Repa JJ, Harper AE. Branched-chain and other amino acids in tissues of rats fed leucine-limiting amino acid diets containing norleucine. J Nutr. 1991;121:364–78. doi: 10.1093/jn/121.3.364. [DOI] [PubMed] [Google Scholar]
- Tovar A, Tews JK, Torres N, Harper AE. Some characteristics of threonine transport across the blood-brain barrier of the rat. J Neurochem. 1988;51:1285–93. doi: 10.1111/j.1471-4159.1988.tb03098.x. [DOI] [PubMed] [Google Scholar]
- van Spronsen FJ, Enns GM. Future treatment strategies in phenylketonuria. Mol Genet Metab. 2010;99:S90–5. doi: 10.1016/j.ymgme.2009.10.008. [DOI] [PubMed] [Google Scholar]
- van Spronsen FJ, de Groot MJ, Hoeksma M, Reijngoud DJ, van Rijn M. Large neutral amino acids in the treatment of PKU: from theory to practice. J Inherit Metab Dis. 2010;33:671–6. doi: 10.1007/s10545-010-9216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwani KC, Smith QR, Rapoport SI. Facilitated transport of L-phenylalanine across blood-nerve barrier of rat peripheral nerve. Am J Physiol. 1990;258:R1436–44. doi: 10.1152/ajpregu.1990.258.6.R1436. [DOI] [PubMed] [Google Scholar]
- Zagreda L, Goodman J, Druin DP, McDonald D, Diamond A. Cognitive deficits in a genetic mouse model of the most common biochemical cause of human mental retardation. J Neurosci. 1999;19:6175–82. doi: 10.1523/JNEUROSCI.19-14-06175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinnanti WJ, Lazovic J, Griffin K, et al. Dual mechanism of brain injury and novel treatment strategy in maple syrup urine disease. Brain. 2009;132:903–18. doi: 10.1093/brain/awp024. [DOI] [PMC free article] [PubMed] [Google Scholar]