Abstract
Although γδ T cells are known to exert both tumor-preventing and tumor-promoting activity, there is still little information on the functional characteristics and clinical significance of γδ T cells isolated from the tumor site. In a recent study, we have investigated the distribution of different γδ T-cell populations in melanoma and their impact on disease outcome.
Keywords: cytokines, cytotoxicity, melanoma, tumor-infiltrating lymphocytes, γδ T cells
Substantial evidence indicates that the immune system participates in cancer pathogenesis and may either contribute to disease progression or potently inhibit tumor growth. On one hand, regressing tumor lesions are often markedly infiltrated by mononuclear cells and the presence of T lymphocytes has been associated with improved prognosis in patients affected by different types of cancer.1 On the other hand, tumor infiltration by T-lymphocyte subsets endowed with immunoregulatory or suppressive potential has been associated with tumor progression and unfavorable prognosis.1 Although most studies on the role of the immune system in oncogenesis, tumor progression and response to therapy have focused on CD8+ T cells, other lymphocyte subsets may be involved. Thus, understanding the role of different subsets of tumor-infiltrating immune cells in the pathogenesis of specific tumors is critical for the development of efficient antitumor immunotherapeutic strategies.
γδ T lymphocytes are important effector cells that may play a critical role in cancer immunosurveillance.2 In line with this notion, results from recent clinical trials support the use of γδ T cells as immunotherapeutic agents, either via the adoptive transfer of ex vivo expanded γδ T cells or following the activation of γδ T cells in vivo, by means of compounds such as phosphoantigens or aminobisphosphonates.3 γδ T cells are heterogeneous and can be sub-divided into two main populations based on phenotypic and functional parameters: γδ T cells expressing the Vδ1 T-cell receptor (TCR) chain, which are mostly found in mucosal tissues, and γδ T cells expressing the Vδ2 TCR chain, which predominate in the peripheral blood and secondary lymphoid organs. γδ T cells are endowed with a variety of effector functions that contribute to antitumor immune responses. In particular, γδ T cells can mediated cytotoxic activity against cancer cells, produce TH1-associated cytokines and cross-talk with dendritic cells, macrophages and B cells.4 This said, under appropriate conditions, γδ T cells may divert from their typical TH1-like phenotype and polarize toward TH2, TH17, and regulatory T cells (Tregs),4 all of which (at least potentially) inhibit antitumor immune responses.
Driven by these observations, we have recently evaluated the representation and functional characteristics of tumor-infiltrating γδ T cells isolated from patients affected by primary melanoma and correlated the levels of infiltrating γδ T cells with common clinicopathological features.5
Immune cells were detected in 70% of 74 tissue specimens from primary melanoma patients, macrophages being the predominant cell population. γδ T cells were the major CD3+ T-lymphocyte subset and Vδ1 and Vδ2 T cells were equally represented. The vast majority of melanoma-infiltrating Vδ1 and Vδ2 T cells exhibited an effector memory (TEM) or terminally differentiated effector memory (TEMRA) phenotype, which is indicative of cells that reside at or have migrated to sites of inflammation and display immediate effector functions.6 However, because the yield of primary γδ T cells isolated from melanoma lesions was very low and did not allow for detailed functional studies, we generated a panel of Vδ1 and Vδ2 polyclonal γδ T-cell lines starting from the tumor-infiltrating immune cells of 8 patients. All tested Vδ1 and Vδ2 T-cell lines were able to produce cytokines with proven antitumor activity, such as tumor necrosis factor α (TNFα) and interferon γ (IFNγ) and to kill melanoma cells in vitro, suggesting that both resident Vδ1 and recently migrated Vδ2 T cells may contribute to tumor growth control. However, we found substantial differences in the cytotoxic activity of Vδ1 and Vδ2 T cells. In particular, while the majority of Vδ1 T-cell lines exerted potent cytotoxic effects against melanoma cell lines in vitro, only a few Vδ2 T-cell lines were able to kill such targets.
In our study, the presence of γδ T cells, and in particular of the Vδ2 subset, among tumor-infiltrating immune cells significantly correlated with early stage melanoma, a finding that may stem, at least in part, from the limited efficacy of local immune responses at advanced disease stages. Of note, higher percentages of γδ T cells were detected in patients bearing early (Stage 0–II) melanoma lesions and no metastases, whereas γδ cells were scarce in patients at an advanced disease stage (Stage III–IV) and bearing metastatic lesions.
Recent studies have revealed a high frequency of γδ T cells among tumor-infiltrating lymphocytes from patients affected by different types of cancer, but the clinical relevance of intratumoral γδ T cells remains unknown. Thus, while in one study the percentage of γδ T cells infiltrating renal cell carcinoma lesions failed to correlate with prognostic features,7 in a cohort of breast cancer patients the amount of intratumoral γδ T cells correlated positively with advanced tumor stages and lymph node metastasis, but inversely with both relapse-free and overall survival.8 Nowadays, we can only speculate on the discrepancy between results obtained in melanoma, renal cell carcinoma and breast cancer patients. The relative abundance of Vδ1 and Vδ2γδ T cells can only be discerned by the use of additional markers (cytokine production, cytotoxic activity, migratory properties), implying that a positive or negative correlation with prognosis may depend on the specific γδ T-cell subset recruited at the tumor site (Fig. 1). Furthermore, the net biological effects of γδ T cells may depend on the tumor type and its anatomical location, perhaps reflecting microenvironmental differences. For instance, transforming growth factor β (TGFβ), which is abundantly secreted at the tumor site by tumor-infiltrating macrophages or malignant cells themselves, may favor the differentiation of γδ cells with Treg-like properties, which inhibit antitumor immune responses.4
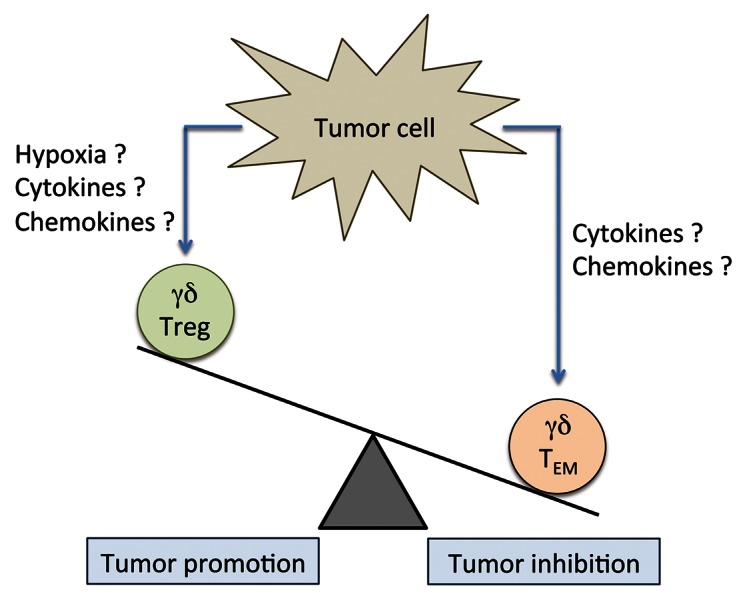
Figure 1. Potential role of γδ T cells in tumor control. TEM, effector memory T cell; Treg, regulatory T cell.
There is thus an urgent need for additional prospective investigations of the relevance of γδ T cells in different types of tumor, evaluating multiple structural and functional parameters. Such a knowledge will undoubtedly help in the determination of the most significant biomarkers to predict disease outcome and eventually guide γδ T cell-based tumor-specific immunotherapies.
Glossary
Abbreviations:
- TEM
effector memory T
- TEMRA
terminally differentiated effector memory T
- Treg
regulatory T cell
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23434
References
- 1.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–96. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Hannani D, Ma Y, Yamazaki T, Déchanet-Merville J, Kroemer G, Zitvogel L. Harnessing γδ T cells in anticancer immunotherapy. Trends Immunol. 2012;33:199–206. doi: 10.1016/j.it.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Caccamo N, Todaro M, Sireci G, Meraviglia S, Stassi G, Dieli F. Mechanisms underlying lineage commitment and plasticity of human γδ T cells. Cell Mol Immunol. 2013;10:30–4. doi: 10.1038/cmi.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordova A, Toia F, La Mendola C, Orlando V, Meraviglia S, Rinaldi G, et al. Characterization of human γδ T lymphocytes infiltrating primary malignant melanomas. PLoS One. 2012;7:e49878. doi: 10.1371/journal.pone.0049878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, et al. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–7. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inman BA, Frigola X, Harris KJ, Kuntz SM, Lohse CM, Leibovich BC, et al. Questionable relevance of γ δ T lymphocytes in renal cell carcinoma. J Immunol. 2008;180:3578–84. doi: 10.4049/jimmunol.180.5.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma C, Zhang Q, Ye J, Wang F, Zhang Y, Wevers E, et al. Tumor-infiltrating γδ T lymphocytes predict clinical outcome in human breast cancer. J Immunol. 2012;189:5029–36. doi: 10.4049/jimmunol.1201892. [DOI] [PMC free article] [PubMed] [Google Scholar]


