Abstract
Tumor-associated antigen (TAA) subunit-based vaccines constitute promising tools for the anticancer immunotherapy. Given the weak antigenic nature of most TAAs, however, the efficacy of TAA-based vaccines requires adjuvants exerting potent immunostimulatory functions. Co-stimulatory members of the tumor necrosis factor ligand (TNFL) family may be used in this sense due to their pleiotropic and robust effects on cells of innate, adaptive and regulatory immune responses.
Keywords: anticancer immunity, cancer vaccines, CD137, lung cancer, SA-4-1BBL, survivin
Owing to intense efforts and significant progresses in the understanding of the complex nature of anticancer immune responses and of the mechanisms employed by tumors to overcome such responses, cancer immunotherapy has come of age. Among various immunotherapeutic strategies, anticancer vaccines based on tumor-associated antigen (TAA) subunits are attractive as a standard, ready-to-use and cost-effective treatment approach. Numerous preclinical and clinical studies have demonstrated that progressing tumors evade immunity by interfering with all the three arms of the immune system: innate, adaptive and regulatory immune responses.1 By extrapolation, effective anticancer vaccines will have to target the same arms of the immune system to achieve therapeutic responses. Since most TAAs, except viral ones, are self and—as such—weakly immunogenic, the therapeutic efficacy of TAA-based vaccines requires the use of adjuvants with pleiotropic effects on the immune system. Co-stimulatory members of the tumor necrosis factor ligand (TNFL) family appear promising in this sense, as they play prominent roles in the generation and expansion of primary immune responses as well as in the establishment of long-term immunological memory,2 which is critical for the control of tumor recurrence.
We have recently proposed that 4-1BBL (CD137L), a co-stimulatory member of the TNFL family, may constitute an adjuvant of choice for the development of therapeutic vaccines, due to its pleiotropic effects on various cellular components of the innate, adaptive and regulatory immune system (Fig. 1).3-5 4-1BB is inducibly expressed by cells of the adaptive immune system, such as CD4+ and CD8+ T cells, of the innate immune system, such as natural killer (NK) and NKT cells, as well as by antigen-presenting cells (APCs), including some subsets of dendritic cells (DCs) (Fig. 1).2 The stimulation of 4-1BB with agonistic antibodies has demonstrated the importance of this receptor for the survival of effector and memory T cells, as well as for their effector functions against malignant and infected cells.6-8 However, the use of agonistic antibodies targeting co-stimulatory receptors, such as various members of the CD28 family, is associated with adverse effects. Thus, we hypothesized that natural co-stimulatory ligands may not only overcome the toxicity associated with antibodies but also result in improved therapeutic outcomes, as they deliver signals that differ qualitatively and/or quantitatively from those elicited by agonistic antibodies.9 We thus focused on the use of natural co-stimulatory ligands as adjuvants for TAA subunit-based vaccines.
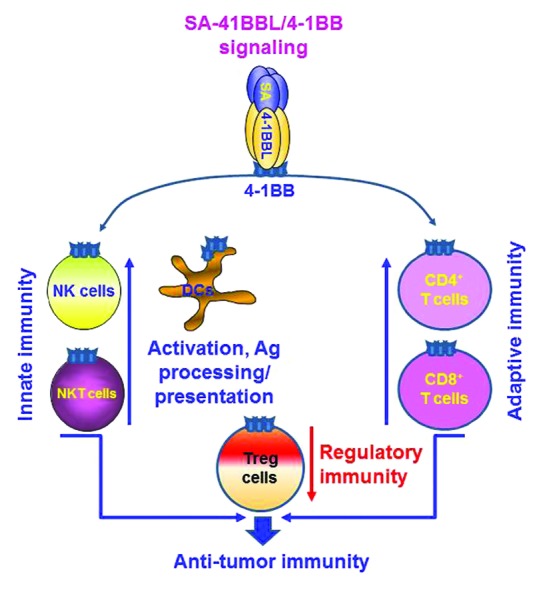
Figure 1. Pleiotropic effects of 4-1BB signaling on cells from the innate, adaptive and regulatory immune system. Streptavidin-4-1BBL (SA-4-1BBL) employed as an adjuvant for tumor-associated antigen (TAA) subunit-based anticancer vaccines positively influences the generation of effector anticancer immune responses. The engagement of SA-4-1BBL with 4-1BB on APCs enhances their activation, antigen uptake and cross-presentation to T cells. T cells. Activated T cells in turn upregulate 4-1BB and become responsive to SA-4-1BBL-mediated co-stimulation, resulting in enhanced T-cell survival and effector functions. Similar effector functions also ensue the engagement of 4-1BB on natural killer (NK) and NKT cells. Importantly, the activation of 4-1BB on effector T cells not only prevents their conversion into regulatory T cells (Tregs) in the tumor microenvironment, but also render them resistant to Treg-mediated immunosuppression. These events culminate in the generation of therapeutic innate and adaptive immune responses against malignant cells.
4-1BBL exists as a trimer and exerts no functions in a soluble form as such.5 We therefore designed a novel form of 4-1BBL by fusing the extracellular portion of murine 4-1BBL to the C-terminus of core streptavidin (SA), hence generating a chimeric SA-4–1BBL molecule. In this configuration, SA-4-1BBL molecules exist as tetramers or higher-order multimers, exerting robust co-stimulatory activity on both CD4+ and CD8+ T cells in vitro and in vivo (Fig. 1) but none of the adverse effects associated with the use of a 4-1BB-targeting agonistic antibody (3H3).9 Most importantly, SA-4-1BBL exerts better co-stimulatory activity on CD4+ T cells than 3H3.9 In a recent study published in PLoS ONE,4 we demonstrated that a vaccine formulation containing survivin (SVN) as self TAA and SA-4-1BBL as adjuvant is very efficient (~70%) against 3LL lung carcinoma in a prime-only setting. Mice in which the vaccine completely eradicated tumors effectively controlled disease recurrence as simulated by the injection of a lethal dose of live 3LL tumor cells 60 days later, demonstrating the presence of long-lasting immunological memory. The therapeutic efficacy of the vaccine—which was improved to 100% using a prime-boost regimen—was associated with increased percentages of IFNγ-secreting CD8+ T cells and improved cytotoxic T- and NK-cell responses. Importantly, the depletion of CD8+ T cells one day before vaccination completely abrogated its efficacy, while NK-cell depletion negatively affected efficacy, but did not completely abolish it. Interestingly, the depletion of CD4+ T cells had no effects on the therapeutic efficacy of the vaccine, which is surprising given that SVN is a self antigen and the generation of SVN-specific CD8+ T-cell responses, in particular memory responses, may require considerable CD4+ T-cell help. This notion is consistent with previous results indicating that CD4+ T cells are required not only for CD8+ T-cell memory responses to weak viral antigens, but also for primary responses in selected settings.10 Although our observations suggest that CD4+ T cells are dispensable for the efficacy of TAA subunit-based vaccines adjuvanted with SA-4-1BBL, they do not completely rule out a role of this cell population in the eradication of primary tumors and in the establishment of long-term immunological memory. Indeed, CD4+ T cells may only be required for priming, which can take place by the sheer exposure of the immune system to the tumor in vivo, a setting in which TAAs are efficiently taken up, processed and presented by DCs. Studies are underway in our laboratory to clarify this issue. Importantly, our vaccine formulation was efficient while failing to induce any observable toxicity or autoimmune events.
In conclusion, the results of numerous studies conducted by others with 4-1BB-targeting agonistic antibodies7,8 coupled to our observations on the efficacy of SA-4-1BBL as an adjuvant for TAA subunit-based anticancer vaccines3,5 demonstrate the potential of the 4-1BB co-stimulatory pathway as a prominent target for the development of efficient anticancer vaccines. The use of SA-4-1BBL as an adjuvant provides considerable advantages over that of agonistic antibodies, mostly due to its potential to elicit robust immune responses in the absence of detectable systemic toxicity.
Glossary
Abbreviations:
- Ab
antibody
- APC
antigen-presenting cell
- SA-4-1BBL
streptavidin-4-1BBL
- TAA
tumor-associated antigen
- TNFL
tumor necrosis factor family ligand
Disclosure of Potential Conflicts of Interest
The technology described herein is licensed from University of Louisville by ApoVax, Inc., for which Haval Shirwan serves as CSO, Member of the Board, and Haval Shirwan and Esma Yolcu have significant financial interest in the Company.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23440
References
- 1.Schabowsky RH, Madireddi S, Sharma R, Yolcu ES, Shirwan H. Targeting CD4+CD25+FoxP3+ regulatory T-cells for the augmentation of cancer immunotherapy. Curr Opin Investig Drugs. 2007;8:1002–8. [PubMed] [Google Scholar]
- 2.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–85. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madireddi S, Schabowsky RH, Srivastava AK, Sharma RK, Yolcu ES, Shirwan H. SA-4-1BBL costimulation inhibits conversion of conventional CD4+ T cells into CD4+ FoxP3+ T regulatory cells by production of IFN-γ. PLoS One. 2012;7:e42459. doi: 10.1371/journal.pone.0042459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava AK, Sharma RK, Yolcu ES, Ulker V, MacLeod K, Dinc G, et al. Prime-boost vaccination with SA-4-1BBL costimulatory molecule and survivin eradicates lung carcinoma in CD8+ T and NK cell dependent manner. PLoS One. 2012;7:e48463. doi: 10.1371/journal.pone.0048463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma RK, Schabowsky R-H, Srivastava AK, Elpek KG, Madireddi S, Zhao H, et al. 4-1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines. Cancer Res. 2010;70:3945–54. doi: 10.1158/0008-5472.CAN-09-4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat Immunol. 2002;3:536–41. doi: 10.1038/ni798. [DOI] [PubMed] [Google Scholar]
- 7.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellström KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–5. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 8.Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12:693–8. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 9.Schabowsky RH, Elpek KG, Madireddi S, Sharma RK, Yolcu ES, Bandura-Morgan L, et al. A novel form of 4-1BBL has better immunomodulatory activity than an agonistic anti-4-1BB Ab without Ab-associated severe toxicity. Vaccine. 2009;28:512–22. doi: 10.1016/j.vaccine.2009.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang JC, Livingstone AM. Cutting edge: CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J Immunol. 2003;171:6339–43. doi: 10.4049/jimmunol.171.12.6339. [DOI] [PubMed] [Google Scholar]


