Abstract
CD137 is ectopically expressed on Hodgkin and Reed-Sternberg (HRS) cells, causing the removal of the immunostimulatory CD137 ligand from HRS cells as well as from surrounding antigen presenting cells. This inhibits T-cell co-stimulation and supports the immune evasion of Hodgkin’s lymphoma.
Keywords: CD137, Reed-Sternberg cells, classical Hodgkin’s lymphoma, immune evasion
Hodgkin’s lymphoma (HL) is a hematological cancer caused by malignant Hodgkin and Reed-Sternberg (HRS) cells. HRS cells constitute a small minority, often less than 1%, of the HL tumor mass. HRS cells recruit and attract an abundance of inflammatory cells, making up the extended tumor stroma that characterizes HL.1
The fact that HRS cells can survive for long periods and proliferate among an overwhelming majority of immune cells in the stroma of HL lesions implies that they have developed effective immunosuppressive mechanisms. It is well established that tumor cells are positively selected as they develop mechanisms that allow them to successfully escape immune responses. One of these escape mechanisms involves the ectopic expression of immunosuppressive molecules.2
We have detected the ectopic expression of CD137 on HRS cells in 86% of classical HL cases,3 and the very same frequency was observed by Anderson et al.4 In most cases, HRS cells derive from B cells, on which CD137 is not detectable. The correlation between ectopic CD137 expression and malignant transformation suggested that CD137 provides a growth and/or selection advantage to HRS cells in HL.
CD137 is a member of the tumor necrosis factor (TNF) receptor family, and is expressed by activated T cells as a co-stimulatory molecule.5 The cross-linking of CD137 boosts the activity of T cells to a level that—in mice—enables them to reject even established tumors.6 The CD137 ligand (CD137L) is constitutively expressed on the surface of antigen-presenting cells (APCs), and during cognate interactions APCs co-stimulate T cells, thus promoting immune responses, by means of the CD137/CD137L system.5
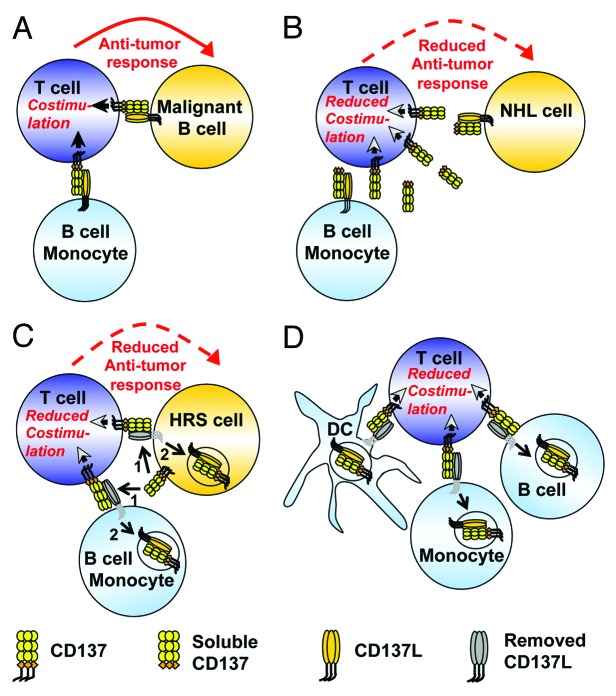
The constitutive expression of CD137L and its potent co-stimulatory activity pose a problem for APCs undergoing malignant transformation, as tumor-infiltrating T cells will receive CD137L-mediated co-stimulatory signals (Fig. 1A). Indeed, murine B-cell lymphoma cells engineered to express CD137L are promptly rejected.7 Thus, any mechanism that leads to the downregulation of CD137L on malignant APCs translates into a growth advantage for these tumors.
Figure 1. Immune regulation by trogocytic CD137 transfer. (A) The cross-linking of CD137 (on T cells) by CD137L (on B cells and monocytes) enhances antitumor T-cell responses. (B) Soluble CD137 (sCD137) binds to and hence neutralizes CD137L on non-Hodgkin's lymphoma (NHL) cells, hence impairing antitumor immune responses. (C) Hodgkin and Reed-Sternberg (HRS) cells ectopically express CD137, which can be transferred to CD137L on HRS cells and surrounding antigen-presenting cells (APCs) (1). The resulting CD137/CD137L complex gets internalized (2), resulting in reduced antitumor immune responses. (D) CD137 transfer as a physiological immunoregulatory mechanism. The trogocytic transfer of CD137 from activated T cells to APCs, including dendritic cells (DCs), monocytes and B cells, and the subsequent internalization (arrows) of the CD137/CD137L complex during cognate interactions limits T-cell co-stimulation.
One mechanism whereby cancer cells might disable the co-stimulatory activity of CD137L involves the expression of soluble CD137 (sCD137), which binds to and neutralizes CD137L (Fig. 1B).8 Indeed, increased levels of sCD137 have been detected in the serum of multiple leukemia and lymphoma patients, in particular among individuals affected by chronic lymphocytic leukemia.9 However, sCD137 has been detected in the serum of no more than 10% of HL patients, and circulating levels were rather low, suggesting that HL cells use a mechanism other than sCD137 to abrogate the co-stimulatory activity of CD137L.9
In order to study the significance of CD137 ectopically expressed on HRS cells, we engineered HRS cell lines for CD137 overexpression or silencing. We noticed that CD137 expression engenders the downregulation of constitutively expressed CD137L, while CD137 silencing allows for CD137L expression. Of note, the overexpression of CD137 did not reduce the de novo synthesis of CD137L. Rather, we observed that CD137 binds to CD137L and the resulting CD137/CD137L complex is internalized, resulting in an accelerated CD137L turnover. In turn, the disappearance of CD137L from the surface of HRS cells resulted in impaired T-cell co-stimulation, as evidenced by the reduced secretion of interferon γ, the signature cytokine of TH1 responses (Fig. 1C).
The experiments pointing to a CD137-mediated downregulation of CD137L have been conducted in vitro. However, circumstantial evidence indicates that the same occurs in vivo. In classical HL, CD137 is expressed while CD137L is absent, in spite of the fact that normal B cells constitutively express CD137L. In contrast, in many non-Hodgkin’s lymphoma (NHL) subtypes including mantle cell lymphoma and follicular lymphoma as well as chronic lymphocytic leukemia, which do not express CD137, CD137L is readily detectable.4,10 The inverse relationship between CD137 and CD137L expression in B-cell lymphomas is in line with our findings in vitro, and implies that ectopically expressed CD137 also downregulates CD137L in vivo.
The ectopic expression of CD137 affects HRS-cell functions other than CD137L expression. In particular, HRS cells were found to transfer CD137 to neighboring CD137L-expressing APCs by trogocytosis. The trogocytic transfer of CD137 is followed by the internalization of CD137L by APCs, negatively affecting T-cell co-stimulation (Fig. 1C). These findings identify a novel mechanism of immune escape that HRS cells employ to get rid of the co-stimulatory activity of CD137L.
The inhibitory effect of CD137 on the exposure and co-stimulatory activity of CD137L is unlikely to constitute a prerogative of HRS cells. More plausibly, HRS cells simply exploit an existing immunoregulatory circuitry to their own advantage. We postulate that the physiological equivalent of this immune escape mechanism is the trogocytic transfer of CD137 from activated T cells to APCs during cognate interactions, concurrently depriving T cells from CD137-mediated co-stimulation and reducing the co-stimulatory capacity of APCs (Fig. 1D). The precise characterization of the underlying mechanisms should increase our knowledge on the regulation of immune responses and may be of relevance for the treatment of a wide range of diseases, encompassing cancer and autoimmune disorders.
Altogether, our findings not only identify a new mechanism that helps HL cells to escape immune surveillance but also ignite a wave of investigation aimed at understanding other TNF family members and their ligands behave similarly and at defining the physiological correlates of this immunoregulatory circuitry.
Glossary
Abbreviations:
- APC
antigen-presenting cell
- CD137L
CD137 ligand
- HL
Hodgkin’s lymphoma
- NHL
non-Hodgkin’s lymphoma
- HRS
Hodgkin and Reed-Sternberg
- sCD137
soluble CD137
- TNF
tumor necrosis factor
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23441
References
- 1.Küppers R. Molecular biology of Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2009;1:491–6. doi: 10.1182/asheducation-2009.1.491. [DOI] [PubMed] [Google Scholar]
- 2.Pawelec G. Tumour escape from the immune response. Cancer Immunol Immunother. 2004;53:843. doi: 10.1007/s00262-004-0531-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho WT, Pang WL, Chong SM, Castella A, Al-Salam S, Tan TE, et al. Expression of CD137 on Hodgkin and Reed-Sternberg cells inhibits T-cell activation by eliminating CD137 ligand expression. Cancer Res. 2013;73:652–61. doi: 10.1158/0008-5472.CAN-12-3849. [DOI] [PubMed] [Google Scholar]
- 4.Anderson MW, Zhao S, Freud AG, Czerwinski DK, Kohrt H, Alizadeh AA, et al. CD137 is expressed in follicular dendritic cell tumors and in classical Hodgkin and T-cell lymphomas: diagnostic and therapeutic implications. Am J Pathol. 2012;181:795–803. doi: 10.1016/j.ajpath.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thum E, Shao Z, Schwarz H. CD137, implications in immunity and potential for therapy. Front Biosci. 2009;14:4173–88. doi: 10.2741/3521. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Lv J, Wang P, Yin X, Tan A, Chen Y. Recombinant human CD137L for cancer immunotherapy: effects of different fusions and linkers on its activity. Cancer Immunol Immunother. 2011;4:489–95. doi: 10.1007/s00262-011-1097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guinn BA, DeBenedette MA, Watts TH, Berinstein NL. 4-1BBL cooperates with B7-1 and B7-2 in converting a B cell lymphoma cell line into a long-lasting antitumor vaccine. J Immunol. 1999;162:5003–10. [PubMed] [Google Scholar]
- 8.Shao Z, Sun F, Koh DR, Schwarz H. Characterisation of soluble murine CD137 and its association with systemic lupus. Mol Immunol. 2008;45:3990–9. doi: 10.1016/j.molimm.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Furtner M, Straub RH, Kruger S, Schwarz H. Levels of soluble CD137 are enhanced in sera of leukemia and lymphoma patients and are strongly associated with chronic lymphocytic leukemia. Leukemia: official journal of the Leukemia Society of America. Leukemia Research Fund, UK. 2005;19:883–5. doi: 10.1038/sj.leu.2403675. [DOI] [PubMed] [Google Scholar]
- 10.Zhao S, Zhang H, Xing Y, Natkunam Y. CD137 Ligand Is expressed in primary and secondary lymphoid follicles and in B-cell lymphomas: diagnostic and therapeutic implications. Am J Surg Pathol. 2013;37:250–8. doi: 10.1097/PAS.0b013e318268c6ea. [DOI] [PubMed] [Google Scholar]