Abstract
A replication-incompetent hemagglutinating virus of Japan (HVJ) envelope (HVJ-E) induces apoptosis selectively in cancer cells. Fragments of the viral RNA genome transported by HVJ-E are recognized by retinoic acid-inducible gene I (RIG-I) and mitochondrial antiviral signaling (MAVS). Specific pro-apoptotic factor are selectively upregulated in cancer cells downstream of the RIG-I/MAVS pathway.
Keywords: HVJ, MAVS, RIG-I, apoptosis, cancer, oncolytic virus, viral RNA
Since the observation that some cancer patients underwent remission in response to viral infection, cancer virotherapy has attracted increasing attention.1 Marked progress has been made in this field following the development of oncolytic viruses, i.e., viruses (including attenuated strains) that selectively replicate in cancer cells.1,2 Of note, the oncolytic activity of these viruses is generally lost following irradiation.3 Thus, replication appears to be required for oncolytic viruses, be they virulent or attenuated strains, to selectively kill cancer cells, although various cellular targets that may mediate this function have been identified.2 Therefore, inactivated viral particles that fail to replicate and generate viral proteins so far have not been considered for the development of oncolytic viruses.
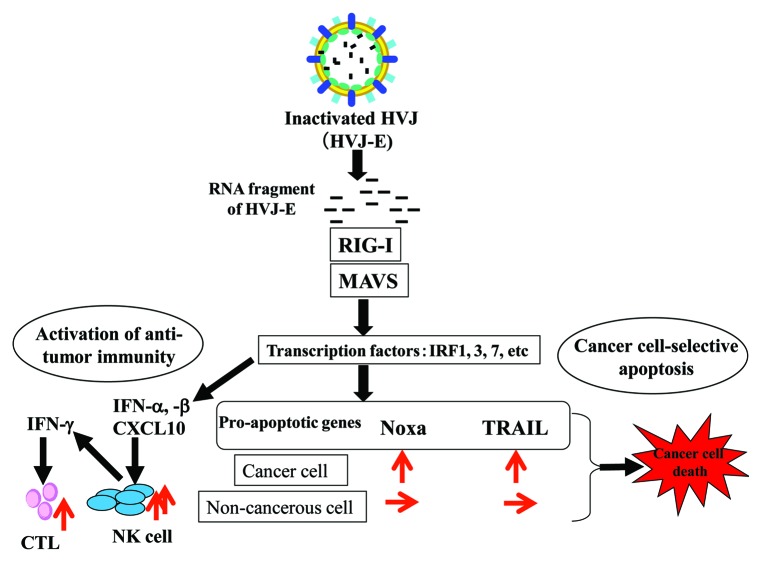
However, we have recently discovered that a replication-incompetent hemagglutinating virus of Japan (HVJ, also known as Sendai virus) envelope (HVJ-E) induces the apoptotic demise of human cancer cell lines, including prostate cancer PC3 and DU145 cells, mammary carcinoma MDA-MB-231 cells and lung cancer A549 cells, but not of non-transformed cells such as prostate epithelial PNT1 and PNT2 cells as well as primary human fibroblasts.4 We have also found that fragments of the viral RNA genome selectively promote apoptosis in cancer cells via the upregulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and NOXA downstream of the retinoic acid-inducible gene I (RIG-I)/mitochondrial antiviral signaling (MAVS) pathway (Fig. 1).
Figure 1. Signaling pathway for HVJ-E-mediated anti-tumor effects. Upon fusion with the plasma membrane, the hemagglutinating virus of Japan envelope (HVJ-E) introduces fragments of the viral RNA genome into the cytoplasm, which are recognized by retinoic acid-inducible gene I (RIG-I). The RIG-I/RNA complex associates with the mitochondrial antiviral signaling (MAVS) protein, which in turn promotes the activation of several transcription factors. In cells from the immune system, the RIG-I/MAVS pathway stimulate antitumor immunity via the production of some cytokines such as interferon (IFN)α, IFNβ and CXCL10. In cancer cells, this signaling pathway induces apoptosis upon the activation of pro-apoptotic factors, such as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and NOXA.
The most striking finding of our recent research is that RIG-I/MAVS signaling can selectively apoptosis in cancer cells, suggesting this may constitute an ideal target for cancer therapy. The RIG-I/MAVS signaling pathway has been well investigated as a protection system that elicits innate immunity upon viral infection.5 Indeed, when dendritic cells are treated with HVJ-E, type I interferon (IFN) and CXCL10 are upregulated upon the activation of the RIG-I/MAVS signaling pathway by viral RNA fragments.6,7 These factors promote in the activation of natural killer (NK) cells and, subsequently, cytotoxic T lymphocytes (CTLs) (Fig. 1). Based on these findings, we first reported that HVJ-E activates antitumor immunity.6 Thus, in immune cells, the RIG-I/MAVS signaling pathway does not cause cell death, yet does so in cancer cells. Besch et al. have reported that synthetic RNA induces type I IFN-independent apoptosis in human melanoma cells via the activation of RIG-I and melanoma-differentiation-associated gene 5 (MDA5).8 According to their analysis, pro-apoptotic molecules including PUMA and NOXA are activated by polyinosinic:polycytidylic acid (polyI:C) or 5′-triphosphate-containing RNA transcribed in vitro in both melanoma cells and non-malignant skin cells, while the expression of the anti-apoptotic molecule BCL-XL is induced in non-malignant cells only. Therefore, they conclude that melanoma-cell specific apoptosis as triggered by the activation of RIG-I and MDA5 occurs via a cytoplasmic pathway regulated by the balance between pro- and anti-apoptotic members of the BCL-2 protein family. In our experiments, the expression of anti-apoptotic proteins, including Bcl-XL and pro-apoptotic molecules such as PUMA and BAX was unchanged upon the administration of HVJ-E to both PC3 and PNT2 cells. Instead, the expression of TRAIL and NOXA was selectively activated in prostate cancer cells, lung cancer cells and breast cancer cells downstream of RIG-I and MAVS. The factors that are responsible for cancer cell-specific apoptosis downstream of the RIG-I/MAVS pathway may therefore vary among cancers.
However, it is still unclear why pro-apoptotic genes are upregulated in cancer cells but not in normal cells downstream of the RIG-I/MAVS signaling pathway. Cancer cells differ from their normal counterparts relative to gene expression pattern, resulting from alterations in chromatin status as imposed by DNA methylation and histone modifications.9 Our findings suggest that the epigenetic regulation of the loci coding for TRAIL and NOXA may vary between cancer cells and their normal counterparts. Based on preliminary experiments, we hypothesize that those loci may be silenced in cancer cells due to DNA methylation and/or histone deacetylation. Signaling via RIG-I/MAVS may therefore successfully relieve such a silencing in malignant cells, but not in normal cells. A detailed analysis of the transcriptional regulation of these (and other) genetic loci will allows us to better understand the mechanisms underlying tumorigenesis,.
Regardless of the molecular mechanism, HVJ-E can induce both anti-tumor immunity and cancer cell-selective apoptosis via the RIG-I/MAVS signaling pathway. As shown in Figure 1, when HVJ-E fuses with the membrane of cells from the immune system, such as dendritic cells and macrophages, antitumor immunity is induced. Conversely, when HVJ-E enters cancer cells, apoptotic cell death is induced. Although the effects of HVJ-E are limited in time due to its inability to replicate, antitumor immune response as induced by HVJ-E may eradicate the residual cancer cells that escape HVJ-E-induced apoptosis. In fact, we have demonstrate the antineoplastic potential of HVJ-E in various animal models.4,7
It is surprising that HVJ-E induces the apoptotic demise of cancer cells more effectively than live, replication-competent HVJ, with an increase in the multiplicity of infection (moi).4 One of the reasons that underlie this observation may be that HVJ-infected, but not HVJ-E-treated, cells produce the viral C protein, which inhibits apoptosis in infected cells by modulating the activity of signal transducer and activator of transcription 1 and 2 (STAT1 and STAT2).10 It is also possible that the inactive form of the fusion protein F0, which is expressed on the envelope of the viral progeny, may affect negatively their infectivity.7 Indeed, HVJ infection cannot spread to other cells unless F0 is converted to F1 upon proteolytic cleavage.
Thus, HVJ-E may constitute an ideal tool for cancer therapy. Clinical-grade HVJ-E has already been generated and used in clinical trials enrolling melanoma and castration-resistant prostate cancer patients in Japan. Our recent findings provide solid evidence for the clinical application of HVJ-E.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23566
References
- 1.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15:651–9. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 2.Guo ZS, Thorne SH, Bartlett DL. Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim Biophys Acta. 2008;1785:217–31. doi: 10.1016/j.bbcan.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel B, Dey A, Ghorani E, Kumar S, Malam Y, Rai L, et al. Differential cytopathology and kinetics of measles oncolysis in two primary B-cell malignancies provides mechanistic insights. Mol Ther. 2011;19:1034–40. doi: 10.1038/mt.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsushima-Miyagi T, Hatano K, Nomura M, Li-Wen L, Nishikawa T, Saga K, et al. TRAIL and Noxa are selectively upregulated in prostate cancer cells downstream of the RIG-I/MAVS signaling pathway by nonreplicating Sendai virus particles. Clin Cancer Res. 2012;18:6271–83. doi: 10.1158/1078-0432.CCR-12-1595. [DOI] [PubMed] [Google Scholar]
- 5.Yoneyama M, Fujita T. Recognition of viral nucleic acids in innate immunity. Rev Med Virol. 2010;20:4–22. doi: 10.1002/rmv.633. [DOI] [PubMed] [Google Scholar]
- 6.Kurooka M, Kaneda Y. Inactivated Sendai virus particles eradicate tumors by inducing immune responses through blocking regulatory T cells. Cancer Res. 2007;67:227–36. doi: 10.1158/0008-5472.CAN-06-1615. [DOI] [PubMed] [Google Scholar]
- 7.Kaneda Y. Virosome: a novel vector to enable multi-modal strategies for cancer therapy. Adv Drug Deliv Rev. 2012;64:730–8. doi: 10.1016/j.addr.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Besch R, Poeck H, Hohenauer T, Senft D, Häcker G, Berking C, et al. Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. J Clin Invest. 2009;119:2399–411. doi: 10.1172/JCI37155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11:726–34. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato A, Ohnishi Y, Kohase M, Saito S, Tashiro M, Nagai Y. Y2, the smallest of the Sendai virus C proteins, is fully capable of both counteracting the antiviral action of interferons and inhibiting viral RNA synthesis. J Virol. 2001;75:3802–10. doi: 10.1128/JVI.75.8.3802-3810.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]