Abstract
Toll-like receptor 5 (TLR5) is an pattern recognition receptor expressed by several cells of the immune system that recognizes bacterial flagellin. We studied the influence of TLR5 in oral tongue squamous carcinoma and found that its expression levels predict patient survival and recurrence. Considering the role of microbiome in oral cancer, TLR5 may represent an important link between bacteria and oral oncogenesis.
Keywords: Toll-like receptor 5, carcinoma, oral tongue squamous cell carcinoma
Bacteria play a prominent role in immunoregulation but also can cause local and systemic inflammation. Chronic inflammation as well as certain bacteria such as Helicobacter pylori have been shown to promote oncogenesis. The oral mucosa and gastrointestinal tract are highly colonized by commensal bacteria, helping to regulate the immune system, preventing allergies and fighting off virulent bacteria. The discrimination between commensal and pathogenic bacteria is difficult, especially if the species does not cause short-term problems but facilitates carcinogenesis over long periods of time. Among thousands of different bacterial species constituting the oral flora, there are only a few with known healthy or pathogenic effects.1
Toll-like receptor 5 (TLR5) is an pattern recognition receptor recognizing flagellin, a component of bacterial flagella. TLR5 is expressed by multiple cells of the immune system, a setting in which it stimulates inflammatory responses, but also by epithelial and cancer cells. In malignant cells, TLR5 not only promotes inflammatory responses but also stimulates invasion, migration and chemokine secretion.2-4
We have recently sought to evaluate the significance of TLR5 in oral carcinoma by assessing TLR5 expression in a cohort of 119 oral tongue squamous cell carcinoma (OTSCC) patients.5 We found out that the expression of TLR5 was more abundant and widespread in cancer cells than in the adjacent healthy epithelium, where TLR5 is mainly expressed by squamous cells of the basal layer. Importantly, TLR5 expression levels predicted patient prognosis. Multivariate analyses showed indeed that high TLR5 levels are a predictor of cancer-related death (hazard ratio = ~3.5) as well as cancer recurrence (hazard ratio = ~4.4).
Our results indicate that TLR5 plays a role in the progression of oral carcinoma and favor a contribution for TLR5 in oral carcinogenesis as well.5 As a poor oral hygiene is a known risk factor for OTSCC and no endogenous ligands for TLR5 are known to date, our findings point to a bacterial activation of TLR5 exerting pathophysiologically relevant effects. A few bacterial species including Streptococcus mitis, Prevotella melaninogenica and Capnocytophaga gingivalis have been associated with oral cancer, but their actual role in the oncogenetic process remain unknown.1
During carcinogenesis, the expression pattern of TLR5 and other TLRs changes from a basolateral to diffuse, and in layered epithelia expression extends to the upper cell layers. Such an abnormal expression of TLR5 has been proposed as a biomarker for epithelial dysplasia in gastric and cervical epithelia.6,7 Interestingly, these changes lead to a situation in which TLR5 is expressed at the luminal surface, increasing the possibility for the recognition of bacterial components.6-8 Several reports on the effects of TLR5 activation on different cancers have been published, with contradictory results. Indeed, TLR5 has been suggested to exert anticancer effects as well as to promote invasiveness.3,4,9 The effects of TLR5 activation seem to vary with cancer type and anatomical localization, similar to what reported for TLR9.10
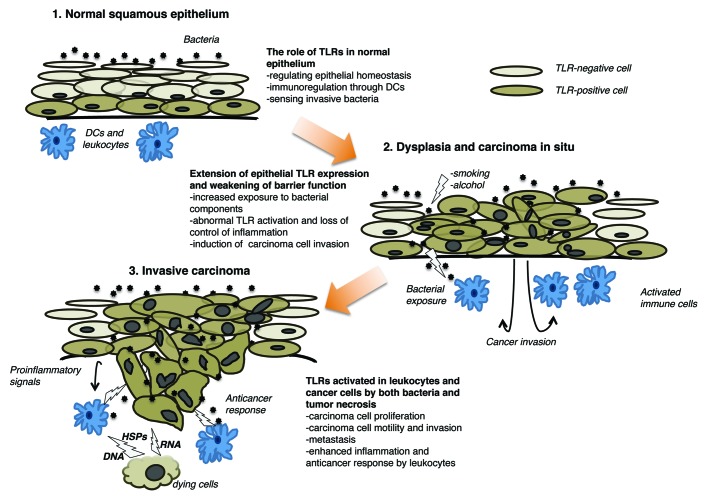
In general, TLRs appear to operate in two different ways, depending on the cell type. Cancer cells are more aggressive in response to TLR activation, whereas immune cells often respond to TLR agonist by exerting antitumor effects. Increased TLR expression levels and the structural aberrations that characterize malignant epithelia, such as the loss of cell polarity and abnormal intercellular junctions, might allow bacteria and their components to activate TLRs, hence contributing to disease progression. Other endogenous and exogenous TLR ligands as well as the existence of functionally different TLR isoforms further add to the complexity of this setting. Dying cells (be they malignant or not) release DNA fragments, heat-shock proteins and several other intracellular factors that are sensed by both immune cells and living cancer cells, hence mediating either anti-carcinogenic or pro-carcinogenic effects, possibly depending on the presence of specific TLR isoforms. This might explain the discrepancies between preclinical results and results from clinical studies, as the effects of TLR agonists on cancer cells and immune cells may counteract each other (Fig. 1).2,10
Figure 1. Effects of Toll-like receptors in the squamous epithelium. Bacteria do not penetrate the normal epithelium and contribute to epithelial homeostasis. In this setting, bacterial components are recognized by dendritic cells (DCs), which can limit inflammatory response. Conversely, invasive bacteria are sensed by Toll-like receptors (TLRs) expressed on both basal cells of the epithelium and inflammatory cells, hence inducing an inflammatory reaction. In the presence of known carcinogens such as tobacco and alcohol, the barrier function of epithelium is compromised and bacteria can penetrate. This is facilitated in the setting of carcinoma in situ, as the cellular and epithelial polarity are compromised and TLR expression extends to the whole epithelium. Such alterations allow for the activation of subepithelial cells, favoring invasiveness. In invasive carcinoma, bacterial components as well as endogenous TLR ligands released by dying cells activate both leukocytes and tumor cells. Carcinoma cells respond to TLR ligands by secreting pro-inflammatory cytokines, proliferating and exhibiting an invasive phenotype, whereas leukocytes do so by stimulating inflammation and killing malignant cells. DC, dendritic cell; HSP, heat-shock protein.
In summary, we demonstrated a prognostic value for TLR5 in OTSCC patients. Hence, TLR5 expression levels might constitute a useful tool to recognize OTSCC patients at increased risk for recurrence and death, especially in the early stages of the disease. In particular, TLR5 could be detected by immunohistochemistry in primary biopsies to help clinicians in the decision on whether or not to proceed with extensive resection, neck dissection or start post-operative radio- or chemotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23620
References
- 1.Meurman JH. Oral microbiota and cancer. J Oral Microbiol 2010; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basith S, Manavalan B, Yoo TH, Kim SG, Choi S. Roles of toll-like receptors in cancer: a double-edged sword for defense and offense. Arch Pharm Res. 2012;35:1297–316. doi: 10.1007/s12272-012-0802-7. [DOI] [PubMed] [Google Scholar]
- 3.Park JH, Yoon HE, Jeon DI, Ahn SG, Yoon JH. Activation of TLR2 and TLR5 did not affect tumor progression of an oral squamous cell carcinoma, YD-10B cells. J Oral Pathol Med. 2010;39:781–5. doi: 10.1111/j.1600-0714.2010.00900.x. [DOI] [PubMed] [Google Scholar]
- 4.Park JH, Yoon HE, Kim DJ, Kim SA, Ahn SG, Yoon JH. Toll-like receptor 5 activation promotes migration and invasion of salivary gland adenocarcinoma. J Oral Pathol Med. 2011;40:187–93. doi: 10.1111/j.1600-0714.2010.00929.x. [DOI] [PubMed] [Google Scholar]
- 5.Kauppila JH, Mattila AE, Karttunen TJ, Salo T. Toll-like receptor 5 (TLR5) expression is a novel predictive marker for recurrence and survival in squamous cell carcinoma of the tongue. Br J Cancer. 2013 doi: 10.1038/bjc.2012.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim WY, Lee JW, Choi JJ, Choi CH, Kim TJ, Kim BG, et al. Increased expression of Toll-like receptor 5 during progression of cervical neoplasia. Int J Gynecol Cancer. 2008;18:300–5. doi: 10.1111/j.1525-1438.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- 7.Pimentel-Nunes P, Afonso L, Lopes P, Roncon-Albuquerque R, Jr., Gonçalves N, Henrique R, et al. Increased expression of toll-like receptors (TLR) 2, 4 and 5 in gastric dysplasia. Pathol Oncol Res. 2011;17:677–83. doi: 10.1007/s12253-011-9368-9. [DOI] [PubMed] [Google Scholar]
- 8.Takala H, Kauppila JH, Soini Y, Selander KS, Vuopala KS, Lehenkari PP, et al. Toll-like receptor 9 is a novel biomarker for esophageal squamous cell dysplasia and squamous cell carcinoma progression. J Innate Immun. 2011;3:631–8. doi: 10.1159/000329115. [DOI] [PubMed] [Google Scholar]
- 9.Cai Z, Sanchez A, Shi Z, Zhang T, Liu M, Zhang D. Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res. 2011;71:2466–75. doi: 10.1158/0008-5472.CAN-10-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kauppila JH, Karttunen TJ, Saarnio J, Nyberg P, Salo T, Graves DE, et al. Short DNA sequences and bacterial DNA induce esophageal, gastric, and colorectal cancer cell invasion. APMIS. 2012 doi: 10.1111/apm.12016. [DOI] [PubMed] [Google Scholar]