Abstract
Bispecific T-cell engagers (BiTEs) may break multiple barriers that currently limit the use of immunotherapy in glioblastoma patients. We have recently described a novel BiTE specific for a mutated form of the epidermal growth factor receptor, EGFRvIII, that exerts potent antineoplastic effects against established invasive tumors of the brain.
Keywords: bispecific antibodies, central nervous system neoplasms, epidermal growth factor receptor, immunotherapy, T lymphocytes
Glioblastoma (GBM) is the most common and most aggressive primary malignant tumor of the brain. Despite advances in surgical resection, radiation and chemotherapy, the prognosis of GBM patients remains dismal, with an expected overall survival at diagnosis of less than 15 mo.1 Although current therapies provide modest benefits, they are frequently associated with incapacitating toxicity, owing to their collateral effects on normal healthy tissues. Thus, there is great need for the development of safer, more effective treatments for patients affected by GBM.
Immunotherapy has emerged as an innovative approach that promises to eliminate tumor cells with unparalleled potency and precision. In particular, T cells have been shown to mediate successful antitumor immune responses.2 However, while promising, current T cell-based approaches rely on the adoptive transfer of lymphocytes expanded ex vivo, a process that is laborious, inconsistent, and in some cases complicated by the need for retroviral transduction.
In contrast, by using recombinant technologies, it has become possible to create highly specific antibody-based molecules that activate T cells against tumors, without the complexities associated with cell-based therapy. Through a divalent, “bispecific” design, these constructs tether T lymphocytes to tumor cells, resulting in a highly localized and specific T-cell activation with concomitant tumor cell lysis. A leading format of such molecules is known as “bispecific T-cell engager” (BiTE). Unlike other bispecific antibodies, BiTEs are able to activate even formerly unresponsive lymphocytes against tumor cells without the need for additional immunostimulatory signals or conventional antigen presentation via MHC molecules.3
In a recent manuscript published in the Proceedings of the National Academy of Sciences,4 we have reported the development of a novel BiTE called bscEGFRvIIIxCD3, which was designed to specifically redirect T cells against tumors expressing a well-characterized, mutated form of the epidermal growth factor receptor (EGFR), EGFRvIII. EGFRvIII is frequently expressed on the surface of GBM cells as well as by many other neoplasms, but not by normal healthy tissues.5 Because of its exclusive tumor-specific expression pattern, EGFRvIII represents an ideal target for immunotherapy. In our hands, the EGFRvIII-targeting BiTE, bscEGFRvIIIxCD3, successfully activated human T cells against EGFRvIII-expressing target cells, in the absence of any additional immunostimulatory signal, resulting in the secretion of TH1-associated cytokines and tumor-cell lysis. This EGFRvIII-targeting BiTE was similarly effective in vivo. Thus, the intravenous administration of bscEGFRvIIIxCD3 induced consistent antitumor responses in mice bearing established, late-stage intracerebral gliomas, rapidly achieving complete remission rates as high as 75% in the absence of apparent toxicity.4
Given the exquisite tumor-specificity of EGFRvIII, we believe that our EGFRvIII-targeting BiTE represents a critical conceptual advance in the safety profile of the BiTE therapeutic platform. On the contrary, BiTEs that target antigens characterized by a promiscuous systemic expression pattern have been shown to elicit unintended autoimmune responses.6 The most recent example of this problem was recorded in clinical trials testing a BiTE specific for the pan-B-cell marker CD19, wherein patients affected by B-cell malignancies experienced not only dramatic disease regression but also an unwarranted ablation of healthy circulating B cells. Thus, perhaps the most obvious drawback of the BiTE technology unveiled to date is represented by the activation of immune responses against antigens that are also expressed by non-malignant cells. To the best of our knowledge, our work focused is the first example of a BiTE that targets a truly tumor-specific antigen like EGFRvIII.
One unexpected finding of our preclinical study was the ability of our EGFRvIII-targeting BiTE administered i.v. to treat established invasive tumors located beyond the blood-brain barrier (BBB). In order for BiTEs to exert antineoplastic affects against brain tumors, both BiTEs and T cells need to efficiently access areas that have long been considered as immunoprivileged (Fig. 1). While circulating naïve T cells do not typically penetrate the central nervous system (CNS), activated T cells are known to traffic frequently across the BBB and into the CNS.7 Moreover, particles bound to the surface of antigen-specific T cells have been shown to localize to neoplastic lesions.8 However, whether this mechanism contributes in any way to the intracerebral accumulation of macromolecules like BiTEs has not been elucidated to date. Another leading hypothesis is that in the complete absence of cross-reactivity with systemic antigens, highly specific antibodies targeting brain tumors may penetrate the CNS and accumulate over time at therapeutically relevant amounts, simply owing to their relative affinity for different tissues.9 Previous research suggests that BiTEs may actually promote the localization and retention of effector T cells at intracerebral sites. This concept is supported by clinical studies on a CD19-targeting BiTE, reporting that the peripheral activation of circulating effector memory T cells is temporally associated with unexplained, but transient, CNS side effects in multiple patients.6,10 Consistent with this, we observed that the intravenous administration of our EGFRvIII-targeting BiTE promotes the diffuse infiltration of peripheral lymphocytes within EGFRvIII-expressing brain tumors. Further studies are underway to investigate these mechanisms, which have critical implications in multiple fields of medical research for which the physiology of the BBB and the delivery of therapeutic agents into the CNS are relevant.
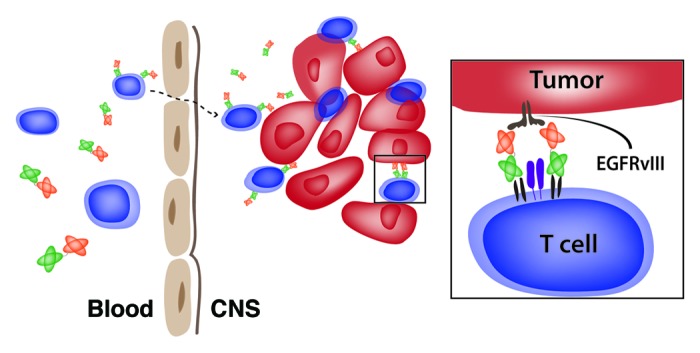
Figure 1. A bispecific T-cell engager targeting EGFRvIII triggers immune responses against brain tumors. The systemic administration of an EGFRvIII bispecific T-cell engager (BiTE) results in its successful localization to EGFRvIII-expressing brain tumors and in the activation of T cells to sustain a potent antitumor immune response. The EGFRvIII-targeting BiTE may gain access to the central nervous system (CNS) on the surface of activated T cells or may accumulate independently, owing to specific interactions with the cognate EGFRvIII antigen on brain tumor cells.
In summary, the results of our preclinical study demonstrate that the EGFRvIII-targeting BiTEs may provide a safe, highly effective therapueutic option for GBM patients. Future studies will determine whether these results can be recapitulated in the clinical setting and whether BiTEs favorably interact with other therapies that are currently employed as a standard-of-care for GBM patients.
Disclosure of Potential Conflicts of Interest
The authors have a patent pending for EGFRvIII as a tumor-specific target for bispecific antibody therapy.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23639
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi BD, Cai M, Bigner DD, Mehta AI, Kuan CT, Sampson JH. Bispecific antibodies engage T cells for antitumor immunotherapy. Expert Opin Biol Ther. 2011;11:843–53. doi: 10.1517/14712598.2011.572874. [DOI] [PubMed] [Google Scholar]
- 4.Choi BD, Kuan CT, Cai M, Archer GE, Mitchell DA, Gedeon PC, et al. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proc Natl Acad Sci U S A. 2013;110:270–5. doi: 10.1073/pnas.1219817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi BD, Archer GE, Mitchell DA, Heimberger AB, McLendon RE, Bigner DD, et al. EGFRvIII-targeted vaccination therapy of malignant glioma. Brain Pathol. 2009;19:713–23. doi: 10.1111/j.1750-3639.2009.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–7. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 7.Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schläger C, Lodygin D, et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–9. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 8.Cole C, Qiao J, Kottke T, Diaz RM, Ahmed A, Sanchez-Perez L, et al. Tumor-targeted, systemic delivery of therapeutic viral vectors using hitchhiking on antigen-specific T cells. Nat Med. 2005;11:1073–81. doi: 10.1038/nm1297. [DOI] [PubMed] [Google Scholar]
- 9.Scott AM, Lee FT, Tebbutt N, Herbertson R, Gill SS, Liu Z, et al. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc Natl Acad Sci U S A. 2007;104:4071–6. doi: 10.1073/pnas.0611693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klinger M, Brandl C, Zugmaier G, Hijazi Y, Bargou RC, Topp MS, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119:6226–33. doi: 10.1182/blood-2012-01-400515. [DOI] [PubMed] [Google Scholar]


