Abstract
Recently, we have shown that the CCL5/CCR5 axis is active in patients affected by an aggressive basal subtype of breast cancer. Using preclinical models, we have demonstrated that CCR5 promotes breast cancer invasiveness and metastatic potential, while CCR5 inhibition abrogates them. Thus, CCR5 antagonists may constitute an alternative therapeutic approach for patients affected by metastatic basal breast cancer.
Keywords: basal breast cancer, CCL5, CCR5, Maraviroc, metastasis, Vicriviroc
We have recently analyzed the expression of the chemokine CCL5 and its receptors in human breast cancer samples as well as in the normal breast epithelium.1 We found that CCL5/CCR5 signaling is preferentially active in basal and ERBB2+ disease subtypes. Analyzing a combined microarray database including 2,254 samples from 27 independent studies,2 we demonstrated that 58% or more patients affected by the basal and ERBB2+ subtype of breast cancer significantly overexpress both CCL5 and CCR5. In contrast, when a similar analysis was performed in non-neoplastic breast samples, no correlation between CCL5 and CCR5 expression levels was found, indicating that CCL5/CCR5 signaling may be preferentially activated during the development of specific breast cancer subtypes. In line with this notion, the invasiveness of human MCF-10A breast cancer cells transduced with Neu-T, H-RAS or c-SRC oncogenes increased in response to CCL5, suggesting that CCL5/CCR5 signaling is activated by oncogenic events to promote an aggressive phenotype.
Patients affected by basal breast carcinomas are at increased risk of metastasis and exhibit poor survival rates.3,4 Thus, we evaluated the importance of the CCL5/CCR5 axis in invasion and metastasis. By using human breast cancer cell lines exhibiting a basal phenotype and molecular signature, we demonstrated that the increased expression of CCR5 we had observed in tissue microarray might be caused by the existence of a CCR5+ cell population. Phenotypic analyses demonstrated that a small fraction of cells within breast cancer cell lines express CCR5 and functional analyses showed that such CCR5+ cells respond to CCL5. Furthermore, basal breast cancer cells expressing CCR5 display an increased invasiveness and metastatic potential. Metastatic tumors generated by the intravenous injection of breast cancer MDA-MB-231 cells transduced with a lentiviral vector coding for luciferase fused to an enhanced variant of the green-fluorescent protein (Luc2-eGFP) displayed an 8-fold increase in the proportion of CCR5+ cells as compared with the same cell line maintained in vitro. This indicates either that the invasive population is enriched in CCR5+ cells or that the microenviroment at metastatic sites promotes a CCR5+ phenotype. In an additional series of experiments, we showed that CCR5+ cells isolated from basal breast cancer cell lines are approximately 40-fold more invasive in vitro than their CCR5− counterparts, indicating that CCR5 expression correlates with a pro-invasive phenotype.
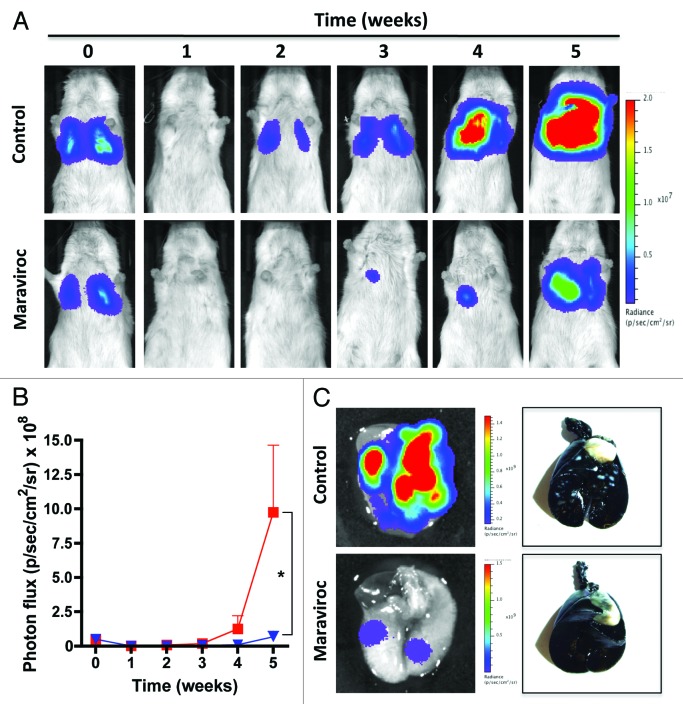
The importance of CCR5 in HIV infection led to the development of drugs that specifically target this receptor. CCR5 antagonists, which were originally developed to prevent the interaction between CCR5 and the gp120 glycoprotein of HIV-1, can efficiently block CCR5 activity at nanomolar concentrations.5,6 We therefore examined the possibility that CCR5 inhibition may reduce the invasive and metastatic potential of breast cancer cells. The CCR5 antagonists Maraviroc and Vicriviroc (both employed at a concentration of 100 nM) impaired CCL5-stimulated cytoplasmic calcium waves and reduced the invasiveness of basal breast cancer cells. We also evaluated Maraviroc effects in vivo, using a mouse model of metastatic breast cancer. The intravenous injection of Luc2-eGFP-expressing MDA-MB-231 breast cancer cells indeed generates pulmonary metastasis that can be easily followed by bioluminescence imaging. In this setting, the administration of Maraviroc at the clinically relevant dose of 8 mg/kg (twice daily) efficiently reduced pulmonary tumor burden (Fig. 1). Histological analyses performed on animals treated with Maraviroc for five weeks confirmed a significant reduction of both the number and the size of pulmonary metastases (Fig. 1). Only 50% of Maraviroc-treated mice exhibited detectable neoplastic lesions and mean tumor size was reduced by > 65% as compared with vehicle-treated mice. These results demonstrate (1) that basal breast cancer cells are sensitive to pharmacological CCR5 inhibition, and (2) that the pro-invasive and pro-metastatic activities of CCR5 can be abrogated by using specific antagonists.
Figure 1. The CCR5 antagonist Maraviroc inhibits lung metastases in vivo. MDA-MB-231 cells expressing luciferase fused to an enhanced variant of the green-fluorescent protein (Luc2-eGFP) were injected into the tail vein of NOD/SCID mice and the bioluminescent signal was quantified weekly in vivo. Representative images of mice receiving vehicle or 8 mg/Kg Maraviroc (every 12 h) are shown in (A) Quantitative data on bioluminescence imaging (means ± SEM, n = 6) are reported in panel (B) (control mice = red line; treated mice = blue line). Statistical comparisons (*p = 0.048) were by means of Student's t-tests coupled to Welch’s correction for heterogeneous variances. (C) The presence of pulmonary tumors and the differences between experimental groups were corroborated by ex vivo imaging (left panels) and India ink staining (right panels).
CCR5 has also been shown to alter the proliferation of luminal breast cancer7,8 and prostate cancer cell lines.9 However, the impact of CCR5 on the proliferation of basal cancer cells is controversial. The results obtained with our model of metastatic breast cancer did not allow us to understand whether CCR5 influences cell proliferation or tissue colonization. We therefore analyzed the role of CCR5 on tumor cell proliferation by three different strategies. We observed that (1) Maraviroc as well as Vicriviroc do not affect the viability and proliferative potential of MDA-MB-231 cells in vitro; (2) CCR5 overexpressing cells display the same proliferation rate as cells transfected with an empty vector; and (3) the administration of Maraviroc does not alter the growth of pre-established pulmonary metastases in vivo. Taken together, these results indicate that CCR5 activation does not promote the proliferation of basal breast cancer cells and that the anti-metastatic effect of CCR5 antagonists does not stem from effects on tumor growth.
To further clarify the mechanisms by which CCR5 promotes metastasis, we evaluated the effects of Maraviroc on the homing of breast cancer cells to the lungs. Of note, Maraviroc reduced the number of cancer cells that could be detected in the lungs of mice 24 h after intravenous injection by 40%. Our results are in agreement with previous findings demonstrating that the CCL5/CCR5 signaling axis plays a crucial role in the metastatic cascade as it is involved in cancer cell extravasation.10 Blocking the homing of cancer cells to metastatic sites is a highly desirable feature for a bona fide anti-metastatic drug.
Our recent paper1 demonstrates for the first time that the expression of CCR5 and CCL5 correlates with a metastatic phenotype of basal breast cancer, both in clinical samples and in cell lines. We were also the first to report the anti-metastatic effects of a CCR5 antagonist that has already been licensed by FDA for use in humans.
Our data suggest that CCR5 antagonists may be used to reduce the risk of metastasis in patients bearing basal breast cancer. Given the aggressive course of the disease and the lack of targeted therapies, the use of CCR5 antagonists as an adjuvant therapy may constitute a major improvement in the clinical management of basal breast cancer patients.
Acknowledgments
This work was supported in part by PAPIIT-UNAM IN219613 (M.V-V.), a grant from “Instituto Científico Pfizer, México” (M.V-V.), NIH grants R01CA070896, R01CA075503, R01CA132115, R01CA107382, R01CA086072 (R.G.P.), the Kimmel Cancer Center NIH Cancer Center Core grant P30CA056036 (R.G.P.), generous grants from the Dr. Ralph and Marian C. Falk Medical Research Trust and the Margaret Q. Landenberger Research Foundation and a grant from Pennsylvania Department of Health (R.G.P.)
Disclosure of Potential Conflicts of Interest
R.G.P. holds minor (< $10,000) ownership interests in, and serves as CSO/Founder of the biopharmaceutical companies ProstaGene, LLC and AAA Phoenix, Inc. R.G.P. additionally holds ownership interests (value unknown) for several submitted patent applications.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23660
References
- 1.Velasco-Velázquez M, Jiao X, De La Fuente M, Pestell TG, Ertel A, Lisanti MP, et al. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res. 2012;72:3839–50. doi: 10.1158/0008-5472.CAN-11-3917. [DOI] [PubMed] [Google Scholar]
- 2.Ertel A, Dean JL, Rui H, Liu C, Witkiewicz AK, Knudsen KE, et al. RB-pathway disruption in breast cancer: differential association with disease subtypes, disease-specific prognosis and therapeutic response. Cell Cycle. 2010;9:4153–63. doi: 10.4161/cc.9.20.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyers MO, Klauber-Demore N, Ollila DW, Amos KD, Moore DT, Drobish AA, et al. Impact of breast cancer molecular subtypes on locoregional recurrence in patients treated with neoadjuvant chemotherapy for locally advanced breast cancer. Ann Surg Oncol. 2011;18:2851–7. doi: 10.1245/s10434-011-1665-8. [DOI] [PubMed] [Google Scholar]
- 4.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–7. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 5.Wilkin TJ, Su Z, Krambrink A, Long J, Greaves W, Gross R, et al. Three-year safety and efficacy of vicriviroc, a CCR5 antagonist, in HIV-1-infected treatment-experienced patients. J Acquir Immune Defic Syndr. 2010;54:470–6. doi: 10.1097/QAI.0b013e3181e2cba0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49:4721–32. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mañes S, Mira E, Colomer R, Montero S, Real LM, Gómez-Moutón C, et al. CCR5 expression influences the progression of human breast cancer in a p53-dependent manner. J Exp Med. 2003;198:1381–9. doi: 10.1084/jem.20030580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murooka TT, Rahbar R, Fish EN. CCL5 promotes proliferation of MCF-7 cells through mTOR-dependent mRNA translation. Biochem Biophys Res Commun. 2009;387:381–6. doi: 10.1016/j.bbrc.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Vaday GG, Peehl DM, Kadam PA, Lawrence DM. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate. 2006;66:124–34. doi: 10.1002/pros.20306. [DOI] [PubMed] [Google Scholar]
- 10.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]