Abstract
The blockade of immune regulatory checkpoints is emerging as a powerful anticancer strategy. We recently reported long-term results from the first-in-human clinical trial of anti-PD1 antibody-based immunotherapy, demonstrating durable tumor control off-therapy in subjects affected by colorectal and kidney cancer, as well as successful re-induction therapy in a melanoma patient.
Keywords: anti-PD1, cancer immunotherapy, immune checkpoint blockade, re-induction, nivolumab
The blockade of immune checkpoints, in particular of the pathway involving programmed death 1 (PD1) and its ligands, CD274 (PD-L1) and CD273 (PD-L2), is emerging as a promising strategy for cancer therapy. As data from clinical trials evaluating immune checkpoint-targeted drugs emerge, the possibilities offered by these agents and the challenges associated with their use are becoming increasingly clear.1,2 Recently, our group has published the results of a long-term follow-up study from the first clinical trial based on the PD1-targeting monoclonal antibody nivolumab (BMS-936558/MDX-1106/ONO-4538; Bristol-Myers Squibb).3 Preliminary results from this trial suggested favorable safety and tolerability profiles among 39 patients affected by advanced, treatment-refractory malignancies.4 Of note, the patients enrolled in the study included individuals affected by colorectal carcinoma (CRC), kidney cancer or melanoma, all of which experienced an objective response to the experimental therapy. The long-term follow-up of each patient demonstrates important principles regarding the advantages and pitfalls of immune checkpoint-blocking drugs in the treatment of advanced cancer.
The first patient we described was a 71-year-old man affected by CRC who was initially treated with surgery plus chemotherapy and, upon disease progression, received nivolumab. A partial response (PR) was observed on CT scan after only one dose of drug. The patient received four more courses of nivolumab over the following 6 months, during which he attained a complete response (CR). Therapy was discontinued and radiologic evaluation was performed 4 years after the initiation of nivolumab-based therapy, demonstrating no evidence of residual disease.
The second patient was a 76-year-old man affected by metastatic clear cell kidney cancer, whose disease progressed despite multiple prior systemic anticancer regimens. Eight weeks after a single dose of nivolumab, some lesions of this patient were regressing while others were growing, as demonstrated by CT scan. After two additional doses of nivolumab, growing lesions resolved. The patient received no further antineoplastic therapy and achieved a CR, which is ongoing more than 4 years after the discontinuation of nivolumab.
The third patient was a 55-year-old woman bearing metastatic melanoma, whose disease had progressed in spite of standard melanoma therapy. After the first nivolumab dose, like the patient described above, radiologic restaging showed a mixed response. Nivolumab was discontinued after several more doses of the drug resulted in a PR. The disease progressed 16 months later and the biopsy of one recently developed lesion confirmed the presence of melanoma expressing cell-surface PD-L1. The patient received re-induction nivolumab under a patient-specific protocol, after which repeat scans demonstrated a decreased size and avidity for fluorodeoxyglucose (FDG) of these lesions. An ongoing PR was documented 16 months after the initiation of re-induction therapy.
Taken together, the long-term outcomes of these patients illustrate several important concepts. First, PD1-blockade can induce long-lasting antitumor responses that can persist off-therapy. Additional evidence in support of this notion has been provided by Topalian and colleagues, who reported results from a Phase I clinical trial on nivolumab involving about 300 patients.2,5 In this setting, among 54 patients who achieved a PR or CR and who were not lost early at follow-up, 28 responses (52%) were observed to last for at least 1 year.
Second, the success of re-induction therapy in a melanoma patient suggests that, in the case of disease progression upon nivolumab discontinuation, the re-administration of the same anti-PD1 antibody can swing the immunological pendulum back in favor of the host, redirecting the immune system to mediate antineoplastic effects. On the contrary, tumor growth following the administration of small molecule inhibitors and chemotherapy is generally due to the development of drug resistance.
Third, the clinical evaluation of patients receiving anti-PD1 antibodies or similar immune checkpoint blocking agents requires appropriate response criteria.6 Indeed, as observed in our cohort of patients, the standard evaluation of clinical response patterns, including mixed responses, prolonged stable disease and pseudoprogression (the appearance of progressive disease on conventional radiologic imaging followed by tumor regression) may not be fully appropriate for the assessment of efficacy and for guiding therapeutic choices.
Finally, results from two recent clinical trials testing nivolumab4,5 suggest that the presence of PD-L1 on the surface of tumor cells, or of other cells residing in the tumor microenvironment, may correlate with the propensity of patients to respond to nivolumab. This and other biomarkers of response should be evaluated in larger studies, both during induction therapy and in re-induction settings, as suggested by the melanoma case described above.
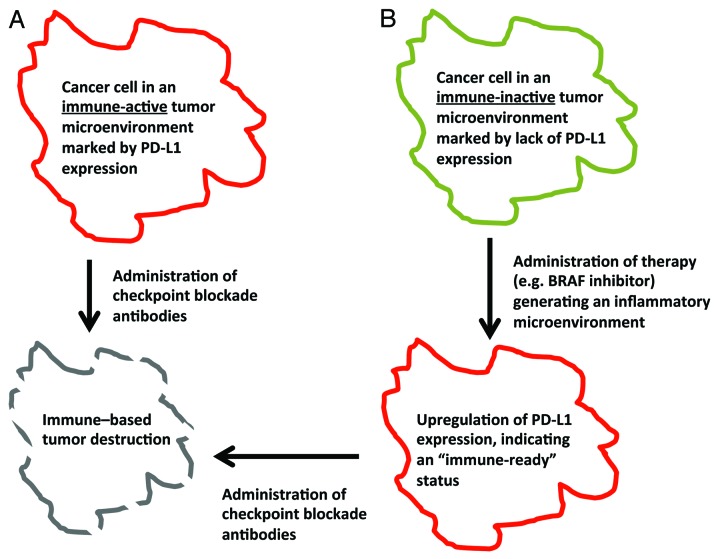
Results from previous gene expression studies conducted on tumor samples from melanoma patients receiving the anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4) antibody ipilimumab suggest that the expression of immune-related genes at baseline (before the initiation of therapy) increases the likelihood of patients to benefit from CTLA4-blocking agents.7 Along similar lines, the expression of PD-L1 in the tumor microenvironment may represent a pre-activated state of the immune system, awaiting full activation upon blockade of the PD1/PD-L1 pathway, resulting in antitumor immune responses.8 Conversely, tumors that lack an inflammatory background, and therefore do not express PD-L1, may require exogenous immunostimulatory signals to enter such a pre-activated status, which can then be leveraged with the administration of anti-PD1 antibodies (Fig. 1). For example, melanoma patients that received specific inhibitors of BRAF exhibited increased levels of tumor-infiltrating CD8+ T cells and expressed both common melanoma antigens and PD-L1.9 Additional data indicate that the antitumor effects of BRAF inhibitors may be mediated—at least in part—by the immune system.10 Hence, a potentially synergistic therapeutic regimen against melanoma may involve a targeted agent such as the BRAF inhibitor vemurafenib and an immune checkpoint blocking-agent such as nivolumab. Additional forthcoming trials testing the therapeutic potential of anti-PD1 agents, some of which are already underway, will investigate the utility of inhibiting more than one checkpoint-relevant molecule, or combining checkpoint blockade with the engagement of immune co-stimulatory receptors. Rational treatment combinations based on preclinical evidence are expected to expand the applicability and effectiveness of anti-PD1 therapies.
Figure 1. A proposed algorithm for achieving effective antitumor immune responses. (A) The blockade of the programmed death 1 (PD1)/PD-L1 pathway may be effective against tumors in which PD-L1 is expressed at the cell surface, reflecting a baseline, “pre-activated” state of the immune system. (B) The therapy of tumors in which the immune system is not in such a “pre-activated” state may require additional interventions to generate an inflammatory microenvironment, hence priming immune effector cells for mediating antineoplastic functions once the immune checkpoints are relieved (for instance with anti-PD1 antibodies).
Glossary
Abbreviations:
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- CR
complete response
- CRC
colorectal carcinoma
- mAb
monoclonal antibody
- PD1
programmed death 1
- PR
partial response
- TIL
tumor-infiltrating lymphocyte
Disclosure of Potential Conflicts of Interest
No conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23661
References
- 1.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Brahmer JR, Hodi FS, McDermott DF, Smith DC, Gettinger S, et al. Anti-programmed death-1 (PD-1) (BMS-936558/MDX-1106/ONO-4538) in patients (PTS) with advanced solid tumors: Clinical activity, safety, and molecular markers. Ann Oncol. 2012;23(suppl 9):ix152–74. [Google Scholar]
- 3.Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462–8. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 7.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:1019–31. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:27ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18:1386–94. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]