Abstract
Current antiangiogenic immunotherapeutic strategies mainly focus on the blockade of circulating cytokines or receptors that are overexpressed by endothelial cells. We proposed globotriaosylceramide (Gb3) as a viable alternative target for antiangiogenic therapies. In this setting, we developed an anti-Gb3 antibody and validated its therapeutic efficacy in metastatic tumor models.
Keywords: antiangiogenic therapy, antibody, Gb3, glycosphingolipid
The dependence of tumor growth on blood vessels makes tumor angiogenesis a rational target for anticancer therapy. Several antiangiogenic drugs that are currently used in clinical settings target endothelial cell growth factors or their receptors. The clinical benefits of these compounds, however, are limited by several compensatory mechanisms. Thus, the discovery of novel pro-angiogenic factors remains essential to design more efficient antiangiogenic therapies. Recent works demonstrate that the immunological or pharmacological blockage of secreted bioactive lipids such as sphingosine-1-phosphate (S1P) or lysophosphatidic acid (LPA) inhibit tumor angiogenesis.1 In addition, tumor-associated glycosphingolipids (GSLs), complex lipids composed of a backbone hydrophobic ceramide membrane anchor and a hydrophilic cell surface-exposed oligosaccharide, have recently been shown to constitute viable targets for cancer therapy.2 We hypothesized that some GSLs may be overexpressed by proliferating endothelial cells, making them promising candidate targets for the development of novel antiangiogenic therapeutic strategies.3
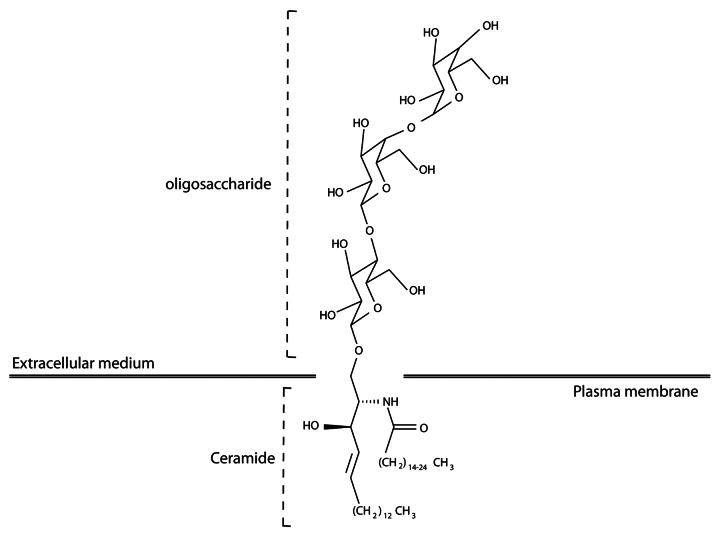
To develop monoclonal antibodies (mAbs) that would specifically recognize GSLs overexpressed by angiogenic endothelial cells, BALB/c mice were immunized with proliferating primary human microvascular endothelial cells (HMVECs) activated by colic T84 adenocarcinoma cells. Upon somatic hybridization, 13 out of 1086 hybridomas were selected by ELISA and FACS analyses to bind components of the plasma membrane of proliferating endothelial cells. Seven of these hybridomas produced mAbs that were found to bind glycoproteins, while the remaining six secreted mAbs targeting the very same glycolipid, the neutral GSL globotriaosylceramide (Gb3), also known as CD77 (Fig. 1). All selected hybridomas produced IgM mAbs. Although the majority of currently approved therapeutic antibodies are IgGs, IgMs may offer unique advantages for use in cancer patients. First, IgMs are decavalent and exhibit a high avidity for their antigenic target. Second, IgMs are mainly limited to the vascular compartment owing to their large molecular weight, representing a potential advantage for immunotherapeutic approaches.
Figure 1. Structure of globotriaosylceramide.
After selecting the Gb3-targeting mAb 3E2 based on its high affinity, we confirmed by immuno-thin layer chromatography, FACS and Scatchard analyses that the concentration of Gb3 on the plasma membrane is increased in proliferating as compared with non-proliferating endothelial cells. The overexpression of Gb3 by angiogenic endothelial cells relates to an increased transcriptional activity of the gene coding for Gb3 synthase, as quantified by quantitative RT-PCR. We confirmed these data in vivo, finding that tumor blood vessels, but not the vasculature or normal tissues, display a robust 3E2 immunostaining. In vivo single-photon emission CT (SPECT) would provide an additional validation to propose Gb3 as a marker of tumor angiogenesis. We focused, however, our attention on the therapeutic properties of the anti-Gb3 mAb 3E2. The immunotargeting of Gb3 to limit angiogenesis has been already proposed, mainly based on verotoxins,4 which bind to at least three Gb3 molecules before being internalized and hence driving the apoptotic demise of endothelial cells. Because of critical issues regarding the immunogenicity and in vivo distribution of verotoxin-based vectors, we thought that mAbs could represent a viable alternative for the therapeutic blockade of tumor angiogenesis.
We found that the administration of 3E2 to proliferating endothelial cells in vitro induces the downregulation of AKT- and ERK-driven stress response signaling pathways, correlating with an increased duration of mitosis and decreased proliferation rates. Our data were reproduced ex vivo, as 3E2 inhibited the spreading of endothelial cells from aortic rings cultured in the presence of appropriate growth factors. This said, Gb3 certainly does not constitute the only rate-limiting factor in angiogenesis and tumor progression. Indeed, vascular-endothelial growth-factor (VEGF)-induced angiogenesis also depends on lactosylceramide, a Gb3 degradation product.5 The understanding of the connections between different pro-angiogenic molecular pathways is important to define adequate antiangiogenic treatments based on one or more drugs. Contrarily to the case of verotoxin and other anti-Gb3 mAbs, we failed to observe an increase in endothelial cell death upon exposure to 3E2 by using time-lapse microscopy, Hoechst 33342 staining and sub-G1 peak analysis. Heterogeneities in the hydroxylation state, chain length, and unsaturation degree of fatty acids may influence Gb3 lateral motility and trisaccharide head orientation, thereby modulating the binding and internalization of hostile ligands, which perhaps explains the unique properties of 3E2. Further studies are required to characterize the 3E2-binding epitope on Gb3 and the discrepancy between signaling pathways activated by 3E2 and verotoxins.
The expression of Gb3 was not decreased after 3E2 administration, suggesting that 3E2 may be used sequentially and exert prolonged antitumor effects. To avoid a tumor cell response and focus our attention on the antivascular activity of 3E2, we employed A/J mice and syngenic NXS2 murine neuroblastoma cells, resulting in neoplastic lesions in which Gb3 expression was limited to the tumor microvasculature. Two models were available: NXS2 cells injected subcutaneously grow as a primary tumor mass, while injected intravenously spread as hepatic metastases. 3E2 was effective in limiting the development of tumor blood vessels and reducing vessel density, thus impacting on tumor growth and metastatic spread. The pro-apoptotic activity of verotoxin may provoke severe side effects due to the targeting of normal Gb3-expressing cells, especially those making up the kidney microvasculature. Conversely, 3E2 affects endothelial cell proliferation but it neither induces their apoptotic demise nor interferes with the recruitment of immune effectors, de facto representing a novel antiangiogenic strategy. In addition, Gb3 is overexpressed by tumors from different origins including breast, prostate, and colon carcinomas. Thus, 3E2-based immunotherapy should target and inhibit the proliferation of both endothelial and cancer cells, resulting in improved tumor control and/or regression.
In conclusion, targeting Gb3 by 3E2 inhibits tumor-induced angiogenesis, limits the growth of primary lesions and interferes with metastatic spread. A better characterization of the molecular events triggered by 3E2 will help us to understand its potential therapeutic application, either as a standalone intervention or combined with other antineoplastic agents.
Disclosure of Potential Conflicts of Interest
No conflicts of interest were disclosed.
Footnotes
These authors equally contributed to the manuscript.
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23700
References
- 1.Peyruchaud O. Novel implications for lysophospholipids, lysophosphatidic acid and sphingosine 1-phosphate, as drug targets in cancer. Anticancer Agents Med Chem. 2009;9:381–91. doi: 10.2174/1871520610909040381. [DOI] [PubMed] [Google Scholar]
- 2.Sonnino S, Prinetti A, Mauri L, Chigorno V, Tettamanti G. Dynamic and structural properties of sphingolipids as driving forces for the formation of membrane domains. Chem Rev. 2006;106:2111–25. doi: 10.1021/cr0100446. [DOI] [PubMed] [Google Scholar]
- 3.Desselle A, Chaumette T, Gaugler MH, Cochonneau D, Fleurence J, Dubois N, et al. Anti-Gb3 monoclonal antibody inhibits angiogenesis and tumor development. PLoS One. 2012;7:e45423. doi: 10.1371/journal.pone.0045423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paton JC, Paton AW. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11:450–79. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajesh M, Kolmakova A, Chatterjee S. Novel role of lactosylceramide in vascular endothelial growth factor-mediated angiogenesis in human endothelial cells. Circ Res. 2005;97:796–804. doi: 10.1161/01.RES.0000185327.45463.A8. [DOI] [PubMed] [Google Scholar]