Abstract
Invariant natural killer T (iNKT) cells have the capacity to amplify adaptive immune responses by licensing antigen-presenting cells. A simple vaccine consisting of whole tumor cells pulsed with an iNKT-cell agonist efficiently delivers antigens plus adjuvants to endogenous dendritic cells and has potential for clinical applications.
Keywords: NKT cell, cancer vaccine, dendritic cell, glioblastoma multiforme, glioma, α-galactosylceramide
Invariant natural killer T (iNKT) cells are a T-cell subset with properties of the innate immune system. The semi-invariant αβ T-cell receptor expressed by iNKT cells recognizes glycolipid antigens bound to CD1d, an MHC class I-like molecule that is expressed at high levels by dendritic cells (DCs). While the functions of iNKT cells are complex and context-dependent, it is known that iNKT cells encountering strong agonistic ligands rapidly release a wide range of cytokines, and can license DCs to initiate adaptive immune responses in a manner that is critically dependent on CD40-CD40L interactions (reviewed in ref. 1). Because the iNKT/CD1d axis is conserved in humans, there is interest in exploiting iNKT cells therapeutically, and most clinical work in this sense focuses on administering the potent synthetic iNKT-cell agonist α-galactosylceramide (α-GalCer) as a single agent, or loaded onto DCs (reviewed in ref. 2). However, animal studies have shown that the licensing functions of iNKT cells can be better exploited by the co-administration of α-GalCer and antigens, leading to potent antigen-specific responses.1 This licensing function has yet to be tested in humans, but holds promise as a strategy to enhance vaccine-induced immunotherapy in the clinic.
The design of effective immunotherapies against high-grade glioma faces numerous challenges, including a high level of heterogeneity among distinct tumors and a paucity of defined, immunogenic tumor-associated antigens. An exception is a mutant form of the epidermal growth factor receptors, EGFRvIII, which is expressed in approximately 30% of glioblastoma cases. However, the use of a peptide vaccine directed against EGFRvIII in EGFRvIII+ patients resulted in antigen loss and clinical relapse, illustrating the limitations of targeting a single antigen.3 For these reasons, there are valid grounds for the development of vaccines based on antigens derived from autologous tumor-cell preparations that are multivalent and highly personalised. The most common approach in this sense has been to load tumor-cell lysates on to autologous monocyte-derived DCs. However, the success of DC culture is variable, the optimum DC maturation protocol remains unresolved and a number of early phase clinical trials in high grade glioma patients has reported only a modest impact for this approach. An alternative strategy is to deliver antigens and an adjuvant to endogenous antigen-presenting cells (APCs). Intact tumor cells themselves provides a ready-made vehicle for this purpose, although it is crucial that APCs taking up infused tumor cells also receive strong licensing signals.
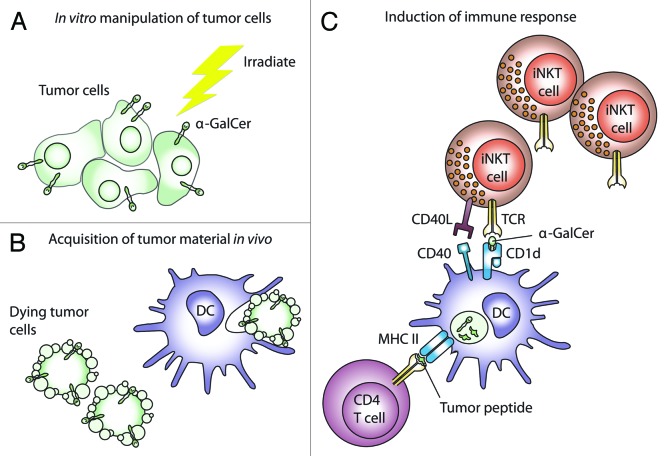
We have recently explored the potential of a vaccine consisting of irradiated glioma cells pulsed with α-GalCer and showed this approach to be effective in a prophylactic setting, as well as against established intracranial tumors if regulatory T cells were first attenuated.4 We have also demonstrated that this simple vaccine is effective in models of central nervous system (CNS) lymphoma, melanoma and acute myeloid leukemia (unpublished results). Other groups have successfully used similar vaccine designs in melanoma and lymphoma patients.5,6 The efficacy of our vaccine was not dependent on CD1d surface expression by glioma cells. This is an important translational point because human glioblastoma multiforme (GBM) cells also do not express CD1d. It also suggests that α-GalCer was not presented directly by tumor cells but must have been transferred to host cells. This is consistent with the idea that the glycolipid and tumor antigens must be presented by the same endogenous APC7,8 (Fig. 1). This method of α-GalCer delivery also avoids an indiscriminate systemic iNKT-cell activation, which is often observed upon the administration of free α-GalCer, hence minimizing the possibilities of anergy, which could limit prime-boost strategies. Exactly how α-GalCer becomes physically associated with tumor cells is unclear. In a previous study, the efficacy of a vaccine consisting of B16 melanoma cells pulsed with α-GalCer was enhanced by transfecting tumor cells with CD1d.6 In view of our results, obtained with CD1d-negative tumor cells, we speculate that the lipid domain of the α-GalCer molecule may simply insert into the plasma membrane.
Figure 1. A simple immunotherapy using α-GalCer-pulsed tumor cells. (A) Tumor material obtained from debulking surgery is loaded with α-galactosylceramide (α-GalCer) in vitro and then irradiated. (B) Upon intravenous administration, dying tumor cells are acquired in lymphoid tissues draining the lung, or in spleen. (C) The acquired tumor material is processed, and antigenic peptides are presented on MHC molecules to T cells. In our studies, the presentation of antigens to CD4+ T cells was essential for the elicitation of antitumor responses. Importantly, α-GalCer is also acquired by antigen-presenting cells (APCs) and presented on CD1d molecules. APCs therefore rapidly express immunostimulatory factors such as CD40 upon the interaction with the relatively numerous invariant natural killer T (iNKT) cells found in lymphoid tissues, thus acquiring an enhanced capacity to drive conventional T-cell responses. Subsequent antitumor effects are mediated by activated T cells; although activated iNKT cells also have the potential to exert a direct cytotoxic function. DC, dendritic cell.
The licensing function of iNKT cells may elicit adaptive responses that are qualitatively different to those induced by DC licensed via different pathways. For example, iNKT cell-licensed DCs secrete a different array of chemokines than DCs stimulated by conventional CD4+ T cells.8 Whether this explains the observation that vaccine-induced tumor immunity was completely dependent on CD4+ T cells, but did not require CD8+ T cells, is unknown; although similar findings have been reported in a model of lymphoma.5 In addition to their licensing capacity, iNKT cells may also negatively regulate the immunosuppressive functions of myeloid-derived suppressor cells,9 a cell population that is increasingly being indicated as a barrier to effective immunotherapy against glioma and other cancers.10
One concern in implementing our vaccine in the clinic is the relatively low number of iNKT cells found in humans as compared with mice. Whereas iNKT cells account for 1% of circulating T cells in C57BL/6 mice, we found that this figure is an order of magnitude lower in GBM patients and varies to consistent degrees among subjects.4 Encouragingly, the abundance of iNKT cells in GBM patients was equal to that of age-matched controls, and cells from GBM patients readily proliferated in response to α-GalCer, suggesting no major functional defects. Nevertheless, further studies are needed to determine whether some patients simply have an insufficient amount of iNKT cells to benefit from the adjuvant effects of α-GalCer. The adoptive transfer of an expanded iNKT-cell population prior to vaccination could conceivably circumvent this problem. Alternatively, this simple whole tumor-cell vaccine could be adapted to incorporate additional adjuvants that work cooperatively with α-GalCer to promote the licensing of DCs through multiple pathways.
Disclosure of Potential Conflicts of Interest
No conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23789
References
- 1.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 2.Schneiders FL, Scheper RJ, von Blomberg BME, Woltman AM, Janssen HLA, van den Eertwegh AJM, et al. Clinical experience with α-galactosylceramide (KRN7000) in patients with advanced cancer and chronic hepatitis B/C infection. Clin Immunol. 2011;140:130–41. doi: 10.1016/j.clim.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–9. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunn MK, Farrand KJ, Broadley KW, Weinkove R, Ferguson P, Miller RJ, et al. Vaccination with irradiated tumor cells pulsed with an adjuvant that stimulates NKT cells is an effective treatment for glioma. Clin Cancer Res. 2012;18:6446–59. doi: 10.1158/1078-0432.CCR-12-0704. [DOI] [PubMed] [Google Scholar]
- 5.Hong C, Lee H, Oh M, Kang CY, Hong S, Park SH. CD4+ T cells in the absence of the CD8+ cytotoxic T cells are critical and sufficient for NKT cell-dependent tumor rejection. J Immunol. 2006;177:6747–57. doi: 10.4049/jimmunol.177.10.6747. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii S. Cross-presentation of glycolipid from tumor cells loaded with alpha-galactosylceramide leads to potent and long-lived T cell mediated immunity via dendritic cells. J Exp Med. 2007;204:2641–53. doi: 10.1084/jem.20070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–7. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 8.Semmling V, Lukacs-Kornek V, Thaiss CA, Quast T, Hochheiser K, Panzer U, et al. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat Immunol. 2010;11:313–20. doi: 10.1038/ni.1848. [DOI] [PubMed] [Google Scholar]
- 9.De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–48. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13:591–9. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]