Abstract
The mitochondria of slowly aging Mclk1+/− mutant mice produce high levels of reactive oxygen species (ROS). These animals display enhanced immune reactivity in response to lipopolysaccharide, Salmonella, and tumor-cell grafts, experience limited damage from these treatments and are partially protected from infection and tumorigenesis. We propose that the activation of the immune system by mitochondrial ROS reduces the rate of aging.
Keywords: aging, biomarkers of aging, Mclk1, mitochondria, reactive oxygen species, T-cell cytotoxicity, xenografts
In the wild, the intensity of immune responses to pathogens or pre-malignant cells has to be balanced by the need to maintain other activities that are required for survival, such as foraging for food and escaping predators. Caged animals, however, live sheltered lives and might benefit from an enhanced immune reaction. It is interesting to consider this aspect in relationship to the biology of aging. In this context, the immune system has often been considered as the “bad guy.” In particular, it is widely believed that inflammation can exacerbate the development of chronic aging-related disorders. Moreover, it has been suggested that episodes of strong inflammation during infancy might lead to detrimental long-term effects because they enhance inflammatory processes later in life.1 This is based on the observation that, historically, declines in old-age mortality were preceded by declines in early-age mortality due to fewer episodes of severe infection and, thus, inflammation.
MCLK1 is a conserved mitochondrial hydroxylase that is necessary for the biosynthesis of ubiquinone (UQ), also known as coenzyme Q, an electron transporter and mitochondrial membrane antioxidant. The homozygous loss of Mclk1 is lethal, but the loss of a single copy of the gene leads to reduced levels of UQ in the inner mitochondrial membrane coupled to an unexpected increase of UQ in the outer mitochondrial membrane.2 This results in reduced rates of electron transport and elevated levels of mitochondrial, but not cytoplasmic, oxidative stress.3 Surprisingly, this primary phenotype is paralleled by beneficial, rather than detrimental, whole-animal phenotypes, including increased longevity. Mclk1+/− mice appear indeed to age slowly as they show a significantly slower increase in biomarkers of aging.4,5 In addition, Mclk1+/− mice are partially protected from the neurotoxic effects of cerebral ischemia-reperfusion and from premature death following spontaneous tumor development in a Tp53+/− genetic background.5,6 These findings, together with cognate observations on the lifespan of Caenorhabditis elegans, led us to hypothesize that an increase in the generation of mitochondrial reactive oxygen species (ROS) might accompany aging not because ROS play a causal role in this process but rather because ROS stimulate protective and restorative processes that help to counteract age-dependent damage.7
In our search for the mitochondrial ROS-sensitive pathways that might operate in Mclk1+/− mice we focused first on the stabilization of the transcription factor hypoxia-inducible factor 1α (HIF-1α), mediating a well-known cytoprotective signal transduction pathway that is regulated by mitochondrial ROS generation.8,9 Our in vitro, ex vivo, and (limited) in vivo evidence suggests that this pathway is indeed upregulated in Mclk1+/− mice.10
Among multiple activities, HIF-1α operates as a modulator of the immune response in both the innate and adaptive immune systems. In Mclk1+/− mice, peritoneal macrophages were activated along the inflammatory pathway, an effect that depended on mitochondrial ROS as it was suppressed by a mitochondrially-targeted antioxidant peptide.10 We also found that the elevation of circulating cytokines that accompanies the administration of lipopolysaccharide or infection with Salmonella is greatly exaggerated in Mclk1+/− animals as compared with their wild-type counterparts.5,10
We wondered whether such an altered immune response was in fact beneficial and could thus contribute to the slowly aging phenotype of Mclk1+/− mice. Three experimental approaches suggested that this is indeed the case. First, we administered Salmonella typhimurium to 129S6 mice, generating a chronic liver infection. We found that 40 days after infection, the liver bacterial load was slightly lower in Mclk1+/− mice than in wild-type animals and, interestingly, the molecular and tissue markers of damage that accompany infection and the immune response were strikingly reduced. In particular, we observed reduced levels of oxidative damage to proteins and to DNA as well as reduced level of hepatic fibrosis and of circulating alanine transferase (ALT), a marker of hepatotoxicity. Second, we administered Salmonella enteritidis to C57BL/6J mice, which produces a transient liver infection that is fully cleared after ~40 days. We observed no difference in the rate of clearance between Mclk1+/− and wild-type mice, but the former manifested lower elevations in plasma ALT, and reduced levels of hepatic fibrosis than the latter, even after three consecutive rounds of infection and clearance. Third, we subcutaneously grafted isogenic 3LL tumor cells into Mclk1+/− mice and wild-type littermate controls. The latency before any visible tumor growth was significantly longer in Mclk1+/− animals than in wild-type mice, and the cytotoxicity of the splenic T cells of the former against 3LL cells in vitro was significantly enhanced, suggesting that such an increased latency stemmed from a more robust immune response against tumor cells.
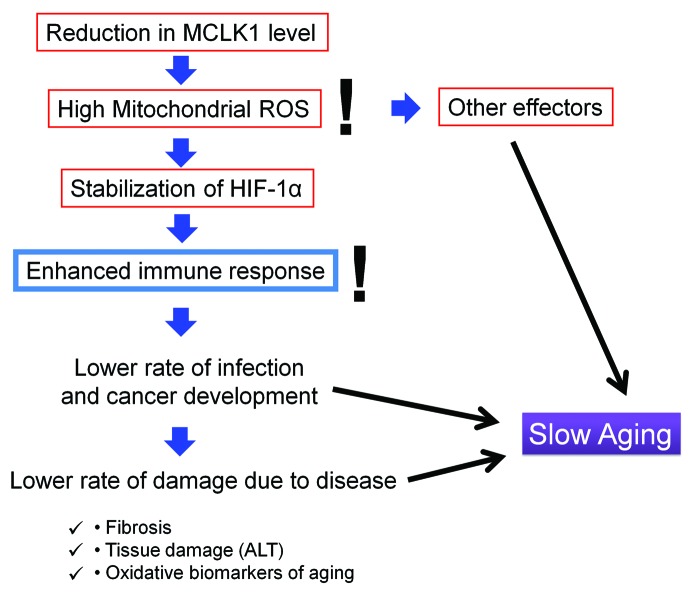
These observations are compatible with a model according to which one of the mechanisms by which elevated mitochondrial ROS can exert beneficial effects is by triggering HIF-1α signaling, which in turn stimulates the immune system to provide an improved protection against infective and neoplastic challenges thus reducing molecular damage (Fig. 1). Thus, we propose that an enhanced activation of the immune system may have long-term effects that are beneficial for lifespan. This provides an alternative interpretation of the observation that people that have not been sick as children live longer.1 Our findings suggest that rather than being protected from the long-term consequences of early inflammation, individuals with strong immune systems, perhaps due to an optimal nutrition or other favorable environmental factors, are protected from both sickness in childhood and from some of the deleterious consequences of aging later in life. We hope to test this model further by learning to stimulate mitochondrial ROS generation or the immune function by means other than Mclk1 heterozygosity and to determine whether these interventions also slow down aging.
Figure 1. Beneficial impact of mitochondrial ROS on aging. ALT, alanine transferase; HIF-1α, hypoxia-inducible factor 1α; ROS, reactive oxygen species.
Disclosure of Potential Conflicts of Interest
No conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23793
References
- 1.Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305:1736–9. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- 2.Lapointe J, Wang Y, Bigras E, Hekimi S. The submitochondrial distribution of ubiquinone affects respiration in long-lived Mclk1+/- mice. J Cell Biol. 2012;199:215–24. doi: 10.1083/jcb.201203090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lapointe J, Hekimi S. Early mitochondrial dysfunction in long-lived Mclk1+/- mice. J Biol Chem. 2008;283:26217–27. doi: 10.1074/jbc.M803287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lapointe J, Stepanyan Z, Bigras E, Hekimi S. Reversal of the mitochondrial phenotype and slow development of oxidative biomarkers of aging in long-lived Mclk1+/- mice. J Biol Chem. 2009;284:20364–74. doi: 10.1074/jbc.M109.006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Wang Y, Argyriou C, Carrière A, Malo D, Hekimi S. An enhanced immune response of Mclk1⁺/⁻ mutant mice is associated with partial protection from fibrosis, cancer and the development of biomarkers of aging. PLoS One. 2012;7:e49606. doi: 10.1371/journal.pone.0049606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng H, Lapointe J, Hekimi S. Lifelong protection from global cerebral ischemia and reperfusion in long-lived Mclk1(+/)(-) mutants. Exp Neurol. 2010;223:557–65. doi: 10.1016/j.expneurol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hekimi S, Lapointe J, Wen Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011;21:569–76. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–8. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, et al. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–14. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Malo D, Hekimi S. Elevated mitochondrial reactive oxygen species generation affects the immune response via hypoxia-inducible factor-1alpha in long-lived Mclk1+/- mouse mutants. J Immunol. 2010;184:582–90. doi: 10.4049/jimmunol.0902352. [DOI] [PubMed] [Google Scholar]