Abstract
By means of well-characterized autoimmunity models, we comparatively probed the “selfness” of malignant cells and their normal counterparts. We found that tumors activate self-tolerance mechanisms much more efficiently than normal tissues, reflecting a status of immunoprivileged “self.” Our findings indicate that potent autoimmune responses can eradicate established malignancies, yet the collateral destruction of healthy tissues may prove difficult to circumvent.
Keywords: altered self, autoimmunity, cancer, immunoprivileged self, regulatory T cell (Treg)
Recent clinical trials testing immunotherapeutic anticancer regimens have generated exciting results.1-3 The ultimate success of such interventions, however, will likely depend on the immunological identity of tumors. Adaptive immunity is characterized by fine specificities, owing to a lymphocyte repertoire that is capable of discriminating the “self” from “non-self” tissues. Tumors represent a dilemma to this dichotomy. Cancer cells originate indeed from the malignant transformation of healthy cells, i.e., they have a self origin. However, neoplastic cells are also characterized by genomic instability4 and hence presumably generates an array of new antigens (neoantigens) that may not be perceived as self by the immune system. A long-standing premise of tumors as “altered self” entities posits that malignant cell bear sufficient antigenic changes to elicit immunosurveillance.5 However, the identification of bona fide tumor-specific antigens (TSAs) in humans is difficult, and the clinical benefits of anticancer immunotherapy are often paralleled by robust autoimmune reactions,6 suggesting that tumor cells, no matter how malignant they are, remain for the most part self entities.
To examine how immune effectors specific for self antigens deal with tumors, we used CD4+ or CD8+ effector T (Teff) cell clones that are fully capable to drive spontaneous autoimmune responses.7 These CD4+ and CD8+ autoimmune Teff cells were tested in vivo for their efficacy against insulinoma or lymphoma cells as well as against normal cells expressing the same antigens within the same animals. A few observations from this study have profound implications for anticancer immunotherapy. First, autoimmune Teff cell clones were able to eradicate established tumors even in the presence of myeloid-derived suppressor cells (MDSCs), provided that immunosuppressive cells of the adaptive immune system were absent. Second, a suboptimal fraction of self antigen-specific, FOXP3+ regulatory T (Treg) cells that failed to protect normal tissues from autoimmune Teff cells was sufficient to exert prominent immunosuppressive effects to block tumor-targeting immune responses, in both adoptive T-cell transfer and acute Treg depletion experiments. Third, in an adoptive T-cell transfer setting, the depletion of cytotoxic lymphocyte antigen 4 (CTLA4) by RNA interference (RNAi) could substantially boost the efficacy of autoimmune Teff cells against tumors.7
We concluded that tumor represents an immunoprivileged self entity, based on the observation that malignant cells could employ self tolerance mechanisms more efficiently than their normal counterparts to avoid autoimmune responses.7 The concept of immunoprivilege has long been used to explain the status of increased protection from immune responses exhibited by a few critical organs, such as the brain, eyes and testes. The traditional view of immunoprivilege involved the exclusion of immune cells from the privilegedsites. However, recent studies have demonstrated that immunoprivileged tissues rather exhibit increased levels of immune regulation.8 Along similar lines, it would be tempting to speculated the existence of an exclusion-based immunoprivilege for some types of cancer, e.g., lung carcinoma, and an immunoprivilege mainly mediated by in situ immune regulation for other neoplasms, e.g., melanoma.
Of note, a large body of evidence from experimental tumor models indicates that cancer-specific immunity can be readily achieved, and that antitumor immune responses can eradicate neoplasms in the absence of prominent autoimmune reactions (reviewed in ref. 9). Our study does not contradict these findings.7 Its focus was indeed to test how potent autoimmune T cells respond to an established tumor, beginning from when the tumor size is very small, and our experiments did not address the potential role of autoimmune Teff cells in immunosurveillance at oncogenesis. Thus, the study was not a direct refutation of the “altered self” view or the immunosurveillance hypothesis.5 Likely, both a situation of “altered self” and one of “immunoprivileged self” could be represented in the natural history of spontaneous tumors.
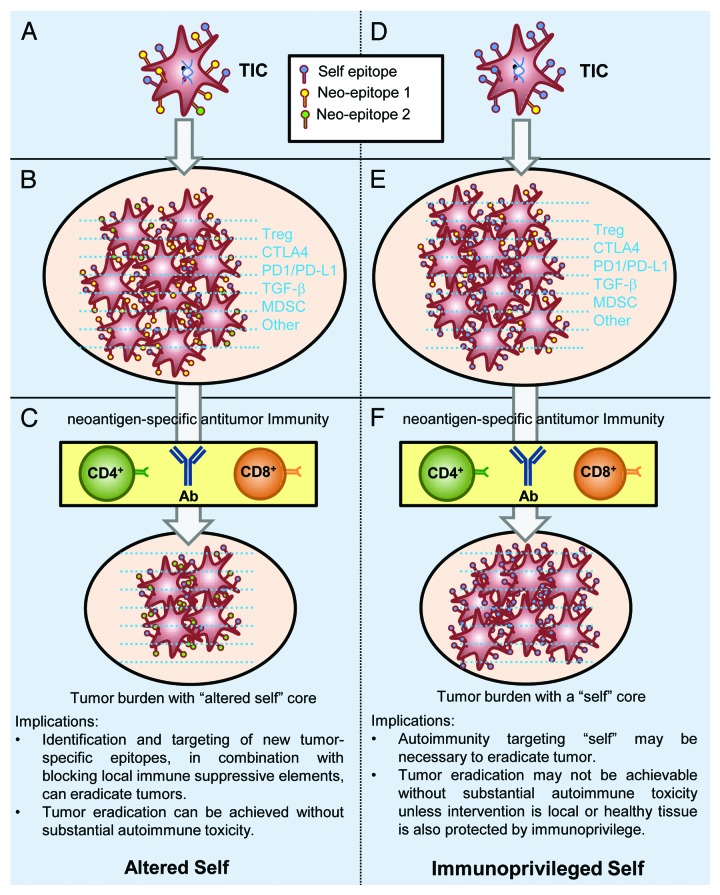
Nevertheless, the premises of tumor as an “altered self” or an “immunoprivileged self” entity have distinct implications for antitumor immunity and immunotherapy (Fig. 1). On one hand, according to the “altered self” view, genetic changes in tumor-initiating cells (TICs) generate an array of neoantigenic epitopes. Tumors evade the attack of the immune system by establishing a microenvironment constituted by immunosuppressive cells and factors. Targeting tumor-specific antigens while blocking immunosuppressive factors can reduce the tumor burden and eventually eradicate neoplastic lesions. On the other hand, according to the “immunoprivileged self” view, despite substantial genetic and epigenetic changes, neoantigens would account for a minimal fraction of the antigenic repertoire of TICs as compared with self antigens. Thus, established tumors are largely “self” in their immunological identity. Furthermore, immunosuppressive elements orchestrated by self antigen-specific Treg cells form a local microenvironment that can inhibit even potent autoimmune responses. In this setting, neoantigen-specific antitumor immunity edits the antigenic identity of neoplasms to limited extents, leaving untouched the tumor immune privileges.
Figure 1. Tumor as an “altered self” or “immunoprivileged self” entity. The hypothesis that self epitopes are abundant in the antigenic repertoire of tumor cells is based on the facts that tumor-specific antigens (TSAs) are difficult to identify and that antitumor immune responses often target self antigens. Blue dashes depict the immunosuppressive microenvironment that is often associated with tumors. Oval areas reflect overall tumor burdens and do not necessarily represent individual tumor sites. Ab, antibody; TIC, tumor-initiating cell.
Is cancer an immunological problem or an oncological one?10 The “immunoprivileged self” hypothesis would suggest that cancer is an immunological problem at its root, yet the eradication of this problem would be beyond the reach of immunology in the absence of oncological interventions. “But the worst enemy you can meet will always be yourself…,” as the nineteenth century German philosopher Friedrich Nietzsche wrote in Thus Spoke Zarathustra, which also stated, “You must be ready to burn yourself in your own flame….” Tumor as an “immunoprivileged self” entity may constitute the worst possible challenge for the immune system. Autoimmune inflammatory reactions could be effective as the body’s own “flame” but only if “burns” are not life-threatening. Therefore, the impact of immunotherapy by itself may be limited, unless the tumor antigenic repertoire is substantially altered or its immunoprivilege eliminated by physical interventions such as surgical removal, radiation therapy or chemotherapeutic agents.
Disclosure of Potential Conflicts of Interest
No conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23794
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knudson AG., Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68:820–3. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 6.Caspi RR. Immunotherapy of autoimmunity and cancer: the penalty for success. Nat Rev Immunol. 2008;8:970–6. doi: 10.1038/nri2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miska J, Bas E, Devarajan P, Chen Z. Autoimmunity-mediated antitumor immunity: tumor as an immunoprivileged self. Eur J Immunol. 2012;42:2584–96. doi: 10.1002/eji.201242590. [DOI] [PubMed] [Google Scholar]
- 8.Benhar I, London A, Schwartz M. The privileged immunity of immune privileged organs: the case of the eye. Front Immunol. 2012;3:296. doi: 10.3389/fimmu.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilboa E. The risk of autoimmunity associated with tumor immunotherapy. Nat Immunol. 2001;2:789–92. doi: 10.1038/ni0901-789. [DOI] [PubMed] [Google Scholar]
- 10.Zitvogel L, Kroemer G. OncoImmunology: a new journal at the frontier between oncology and immunology. Oncoimmunology. 2012;1:1–2. doi: 10.4161/onci.1.1.17645. [DOI] [PMC free article] [PubMed] [Google Scholar]