Abstract
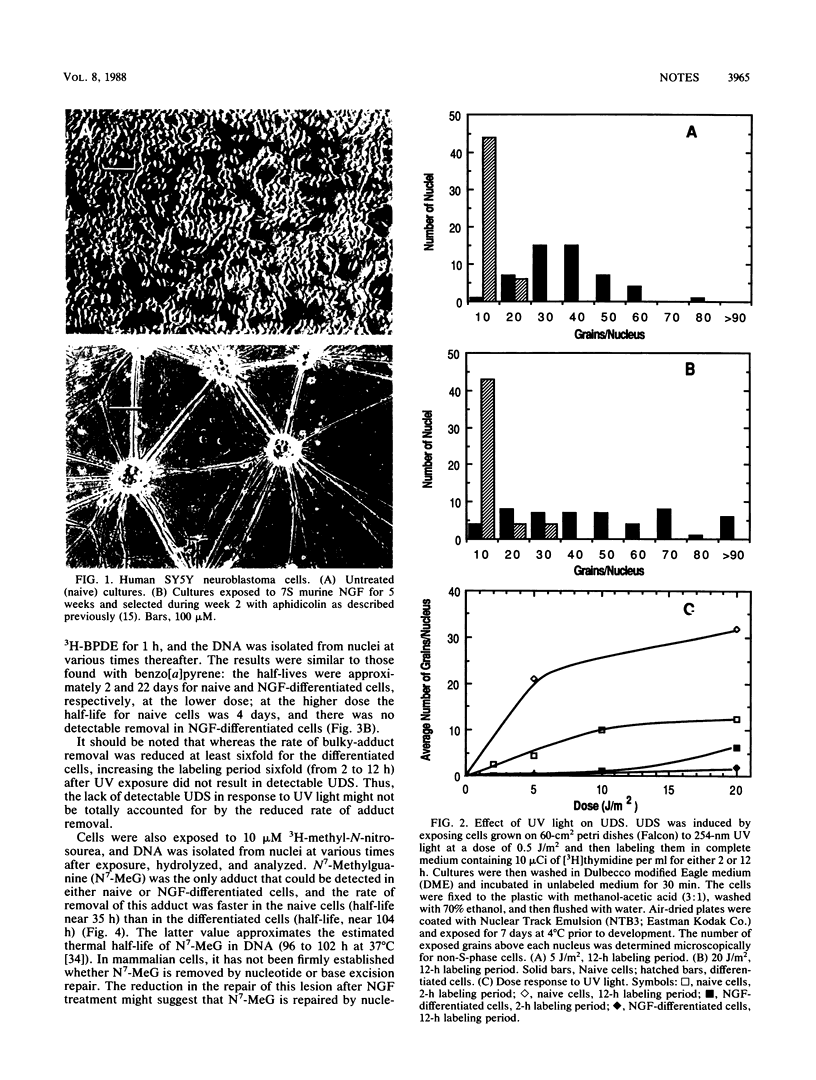
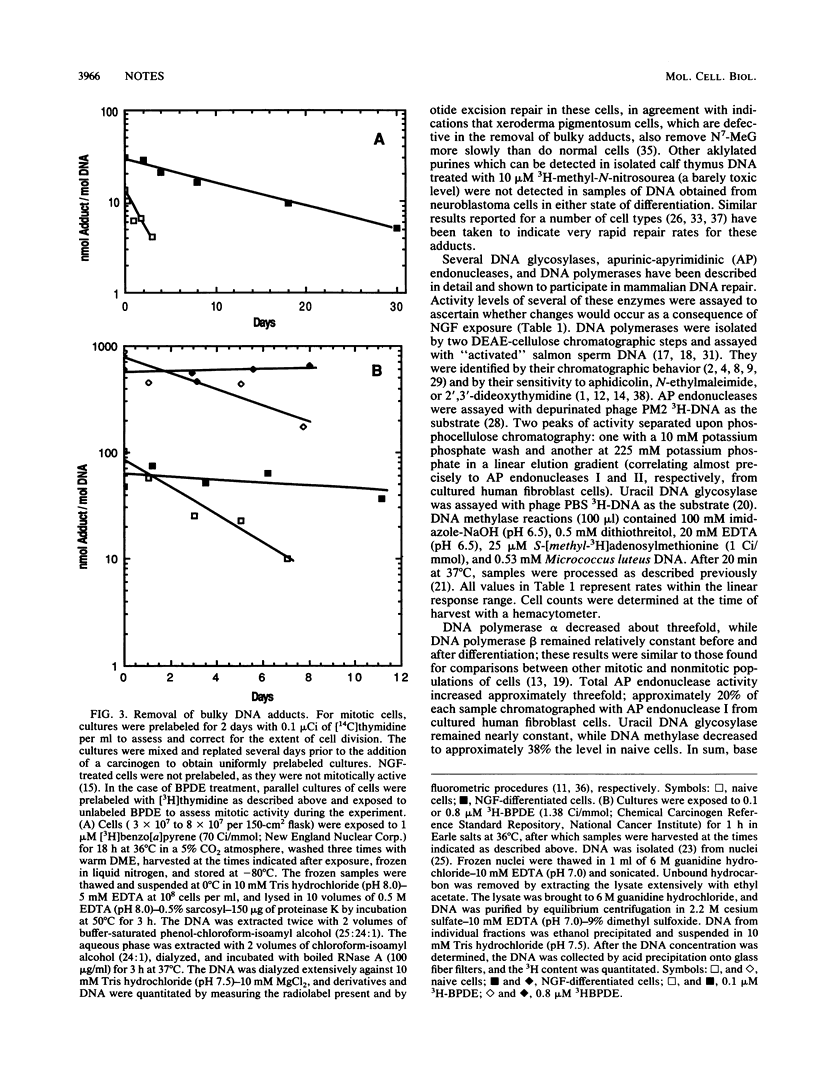
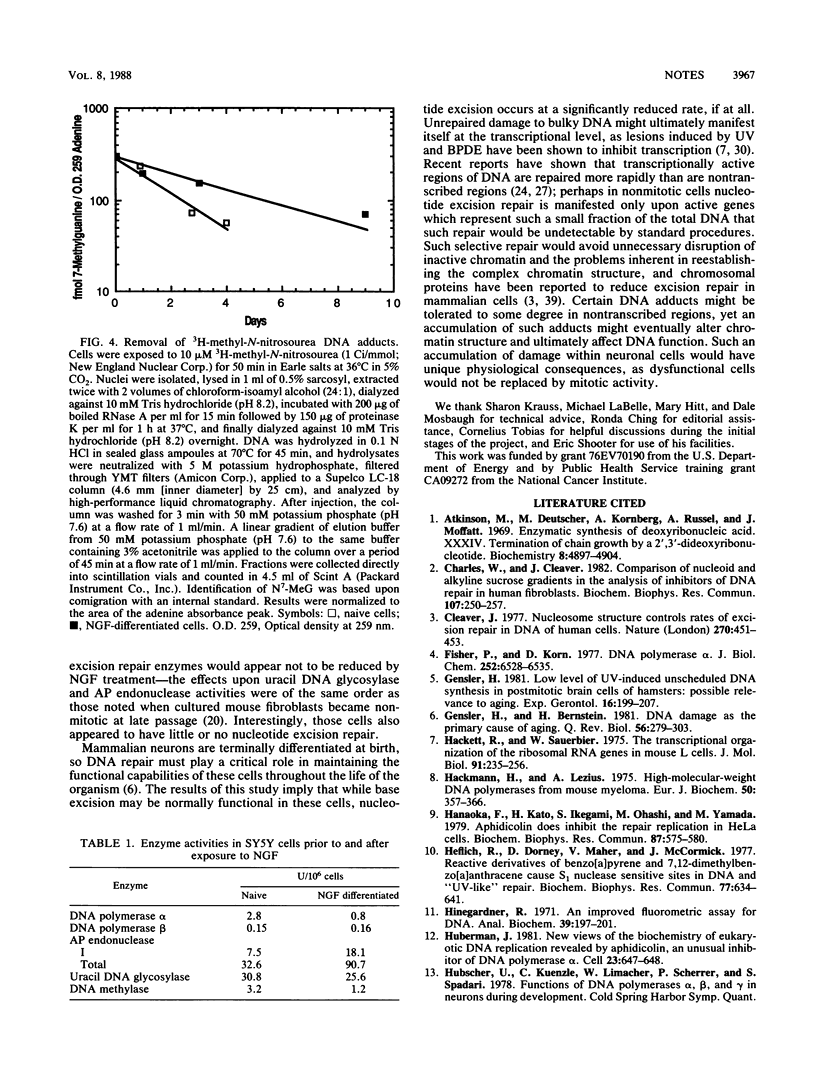
Human SY5Y neuroblastoma cells which were differentiated in culture by treatment with 7S murine nerve growth factor for 5 weeks and selection with aphidicolin (L. Jensen, Dev. Biol. 120:56-64, 1987) demonstrated a considerably slower rate of removal of DNA adducts of benzo[a]pyrene, benzo[a]pyrenediolepoxide, and N7-methylguanine than did undifferentiated mitotic cells. A dramatic decline in unscheduled DNA synthesis induced by UV radiation was similarly observed. DNA polymerase beta and uracil DNA glycosylase were unchanged after differentiation, DNA polymerase alpha and DNA methylase decreased roughly threefold, and total apurinic-apyrimidinic endonuclease activity increased roughly threefold after treatment.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. R., Deutscher M. P., Kornberg A., Russell A. F., Moffatt J. G. Enzymatic synthesis of deoxyribonucleic acid. XXXIV. Termination of chain growth by a 2',3'-dideoxyribonucleotide. Biochemistry. 1969 Dec;8(12):4897–4904. doi: 10.1021/bi00840a037. [DOI] [PubMed] [Google Scholar]
- Charles W. C., Cleaver J. E. Comparison of nucleoid and alkaline sucrose gradients in the analysis of inhibitors of DNA repair in human fibroblasts. Biochem Biophys Res Commun. 1982 Jul 16;107(1):250–257. doi: 10.1016/0006-291x(82)91697-7. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Nucleosome structure controls rates of excision repair in DNA of human cells. Nature. 1977 Dec 1;270(5636):451–453. doi: 10.1038/270451a0. [DOI] [PubMed] [Google Scholar]
- Fisher P. A., Korn D. DNA polymerase-alpha. Purification and structural characterization of the near homogeneous enzyme from human KB cells. J Biol Chem. 1977 Sep 25;252(18):6528–6535. [PubMed] [Google Scholar]
- Gensler H. L., Bernstein H. DNA damage as the primary cause of aging. Q Rev Biol. 1981 Sep;56(3):279–303. doi: 10.1086/412317. [DOI] [PubMed] [Google Scholar]
- Gensler H. L. Low level of U.V.-induced unscheduled DNA synthesis in postmitotic brain cells of hamsters: possible relevance to aging. Exp Gerontol. 1981;16(2):199–207. doi: 10.1016/0531-5565(81)90046-2. [DOI] [PubMed] [Google Scholar]
- Hachmann H. J., Lezius A. G. High-molecular-weight DNA polymerases from mouse myeloma. Purification and properties of three enzymes. Eur J Biochem. 1975 Jan 2;50(2):357–366. doi: 10.1111/j.1432-1033.1975.tb09811.x. [DOI] [PubMed] [Google Scholar]
- Hackett P. B., Sauerbier W. The transcriptional organization of the ribosomal RNA genes in mouse L cells. J Mol Biol. 1975 Jan 25;91(3):235–256. doi: 10.1016/0022-2836(75)90378-2. [DOI] [PubMed] [Google Scholar]
- Hanaoka F., Kato H., Ikegami S., Oashi M., Yamada M. Aphidicolin does inhibit repair replication in HeLa cells. Biochem Biophys Res Commun. 1979 Mar 30;87(2):575–580. doi: 10.1016/0006-291x(79)91833-3. [DOI] [PubMed] [Google Scholar]
- Heflich R. H., Dorney D. J., Maher V. M., McCormick J. J. Reactive derivatives of benzo(a)pyrene and 7,12-dimethylbenz(a)anthracene cause S1 nuclease sensitive sites in DNA and "UV-like" repair. Biochem Biophys Res Commun. 1977 Jul 25;77(2):634–641. doi: 10.1016/s0006-291x(77)80026-0. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Jensen L. M. Phenotypic differentiation of aphidicolin-selected human neuroblastoma cultures after long-term exposure to nerve growth factor. Dev Biol. 1987 Mar;120(1):56–64. doi: 10.1016/0012-1606(87)90103-5. [DOI] [PubMed] [Google Scholar]
- Karran P., Moscona A., Strauss B. Developmental decline in DNA repair in neural retina cells of chick embryos. Persistent deficiency of repair competence in a cell line derived from late embryos. J Cell Biol. 1977 Jul;74(1):274–286. doi: 10.1083/jcb.74.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S. W., Linn S. Changes in DNA polymerases alpha, beta, and gamma during the replicative life span of cultured human fibroblasts. Biochemistry. 1982 Mar 2;21(5):1002–1009. doi: 10.1021/bi00534a027. [DOI] [PubMed] [Google Scholar]
- Krauss S. W., Linn S. Fidelity of fractionated deoxyribonucleic acid polymerases from human placenta. Biochemistry. 1980 Jan 8;19(1):220–228. doi: 10.1021/bi00542a033. [DOI] [PubMed] [Google Scholar]
- Krauss S. W., Linn S. Studies of DNA polymerases alpha and beta from cultured human cells in various replicative states. J Cell Physiol. 1986 Jan;126(1):99–106. doi: 10.1002/jcp.1041260114. [DOI] [PubMed] [Google Scholar]
- La Belle M., Linn S. DNA repair in cultured mouse cells of increasing population doubling level. Mutat Res. 1984 Jul-Aug;132(1-2):51–61. doi: 10.1016/0167-8817(84)90066-x. [DOI] [PubMed] [Google Scholar]
- Lautenberger J. A., Linn S. The deoxyribonucleic acid modification and restriction enzymes of Escherichia coli B. I. Purification, subunit structure, and catalytic properties of the modification methylase. J Biol Chem. 1972 Oct 10;247(19):6176–6182. [PubMed] [Google Scholar]
- Lieberman M. W., Forbes P. D. Demonstration of DNA repair in normal and neoplastic tissues after treatment with proximate chemical carcinogens and ultraviolet radiation. Nat New Biol. 1973 Feb 14;241(111):199–201. doi: 10.1038/newbio241199a0. [DOI] [PubMed] [Google Scholar]
- MacLeod M. C., Mansfield B. K., Huff A., Selkirk J. K. Simultaneous preparation of nuclear DNA, RNA, and protein from carcinogen treated-hamster embryo fibroblasts. Anal Biochem. 1979 Sep 1;97(2):410–417. doi: 10.1016/0003-2697(79)90094-0. [DOI] [PubMed] [Google Scholar]
- Madhani H. D., Bohr V. A., Hanawalt P. C. Differential DNA repair in transcriptionally active and inactive proto-oncogenes: c-abl and c-mos. Cell. 1986 May 9;45(3):417–423. doi: 10.1016/0092-8674(86)90327-2. [DOI] [PubMed] [Google Scholar]
- Marzluff W. F., Jr, Murphy E. C., Jr, Huang R. C. Transcription of ribonucleic acid in isolated mouse myeloma nuclei. Biochemistry. 1973 Aug 28;12(18):3440–3446. doi: 10.1021/bi00742a013. [DOI] [PubMed] [Google Scholar]
- Medcalf A. S., Lawley P. D. Time course of O6-methylguanine removal from DNA of N-methyl-N-nitrosourea-treated human fibroblasts. Nature. 1981 Feb 26;289(5800):796–798. doi: 10.1038/289796a0. [DOI] [PubMed] [Google Scholar]
- Mellon I., Bohr V. A., Smith C. A., Hanawalt P. C. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosbaugh D. W., Linn S. Further characterization of human fibroblast apurinic/apyrimidinic DNA endonucleases. The definition of two mechanistic classes of enzyme. J Biol Chem. 1980 Dec 25;255(24):11743–11752. [PubMed] [Google Scholar]
- Mosbaugh D. W., Linn S. Gap-filling DNA synthesis by HeLa DNA polymerase alpha in an in vitro base excision DNA repair scheme. J Biol Chem. 1984 Aug 25;259(16):10247–10251. [PubMed] [Google Scholar]
- Nocentini S. Inhibition and recovery of ribosomal RNA synthesis in ultraviolet-irradiation mammalian cells. Biochim Biophys Acta. 1976 Nov 12;454(1):114–128. doi: 10.1016/0005-2787(76)90359-2. [DOI] [PubMed] [Google Scholar]
- Schlabach A., Fridlender B., Bolden A., Weissbach A. DNA-dependent DNA polymerases from HeLa cell nuclei. II. Template and substrate utilization. Biochem Biophys Res Commun. 1971 Aug 20;44(4):879–885. doi: 10.1016/0006-291x(71)90793-5. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Shiloh Y., Becker Y. Kinetics of O6-methylguanine repair in human normal and ataxia telangiectasia cell lines and correlation of repair capacity with cellular sensitivity to methylating agents. Cancer Res. 1981 Dec;41(12 Pt 1):5114–5120. [PubMed] [Google Scholar]
- Singer B., Brent T. P. Human lymphoblasts contain DNA glycosylase activity excising N-3 and N-7 methyl and ethyl purines but not O6-alkylguanines or 1-alkyladenines. Proc Natl Acad Sci U S A. 1981 Feb;78(2):856–860. doi: 10.1073/pnas.78.2.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar R., Strauss B. Removal of O6-methylguanine from DNA of normal and xeroderma pigmentosum-derived lymphoblastoid lines. Nature. 1981 Jan 29;289(5796):417–420. doi: 10.1038/289417a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S., Farquhar M. N. Specific measurement of DNA in nuclei and nucleic acids using diaminobenzoic acid. Anal Biochem. 1978 Aug 15;89(1):35–44. doi: 10.1016/0003-2697(78)90724-8. [DOI] [PubMed] [Google Scholar]
- Warren W., Crathorn A. R., Shooter K. V. The stability of methylated purines and of methylphosphotriesters in the DNA of V79 cells after treatment with N-methyl-N-nitrosourea. Biochim Biophys Acta. 1979 Jun 20;563(1):82–88. doi: 10.1016/0005-2787(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Baltimore D., Bollum F., Gallo R., Korn D. Nomenclature of eukaryotic DNA polymerases. Eur J Biochem. 1975 Nov 1;59(1):1–2. doi: 10.1111/j.1432-1033.1975.tb02416.x. [DOI] [PubMed] [Google Scholar]
- Wilkins R. J., Hart R. W. Preferential DNA repair in human cells. Nature. 1974 Jan 4;247(5435):35–36. doi: 10.1038/247035a0. [DOI] [PubMed] [Google Scholar]
- Yang L. L., Maher V. M., McCormick J. J. Error-free excision of the cytotoxic,mutagenic N2-deoxyguanosine DNA adduct formed in human fibroblasts by (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5933–5937. doi: 10.1073/pnas.77.10.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]



