Abstract
Widespread antimicrobial use has compromised its value, leading to a crisis of antimicrobial resistance. A major cause of misuse is insufficient knowledge of prescribing of antimicrobials in many categories of professionals. An important principle of antimicrobial stewardship is avoiding selection pressure in the patient, both on pathogen and commensal by avoiding unnecessary use, choosing the least broad-spectrum antibiotic, adequate doses, a good timing and the shortest possible duration. Up to now, most educational efforts have been targeted at professionals (mostly medical doctors) after their training and at the adult public. In the past few years, progress has been made in educating children. It is now crucial that academia and ministries of Health and Education jointly focus on an adapted undergraduate medical/professional curriculum that teaches all necessary principles of microbiology, infectious diseases and clinical pharmacology, with emphasis on the principles of prudent prescribing.
Keywords: antibiotic prescribing, antimicrobial stewardship, antibiotic policies, undergraduate curriculum, postgraduate education, clinical practice guidelines, intervention strategies, implementation
Background
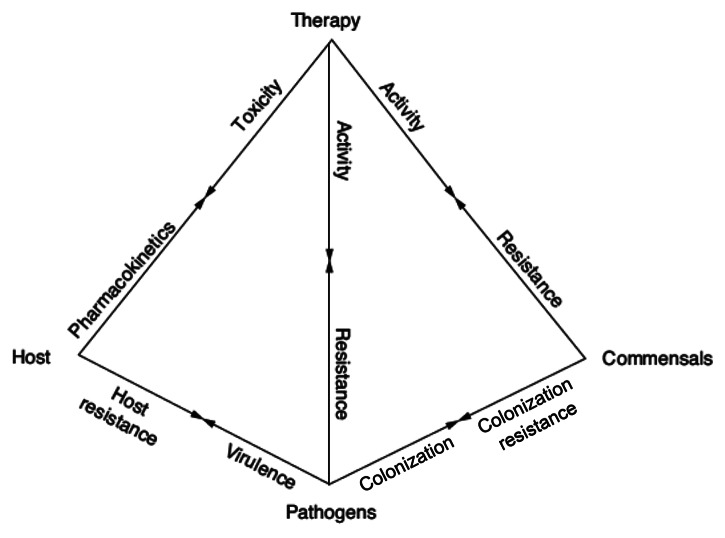
Antimicrobial drugs are a precious but finite resource, different from other drugs. They are the only drugs that do not directly affect the patient but instead affect the growth, and ecology of invading pathogens and commensal microorganisms. Antimicrobial therapy is not only based on the characteristics of a patient and a drug, but also on the characteristics of the microorganisms causing the infection and the colonizing flora. A useful didactic tool that describes the complex interrelationship between humans, microorganisms and antimicrobial drugs is the pyramid of infectious diseases (Fig. 1). The arrows in the pyramid visually illustrate the multiple interactions between the patient, the drug, the pathogen(s) and colonizing microflora. Activity of the antimicrobial drug is obtained at the cost of the development of resistance by the pathogen, but also the colonizing flora. As is shown in the pyramid (Fig. 1), the choice of the appropriate antimicrobial therapy is a complex decision, depending on the knowledge of many different aspects of infectious diseases: immunological and genetic host factors, microbial virulence, pharmacokinetics and -dynamics (PK/PD) of drugs.
Figure 1. The pyramid of infectious diseases. It describes the complex interrelationship between humans, microorganisms and antimicrobial drugs. The arrows in the pyramid illustrate the multiple interactions between the patient, the drug, the pathogen(s) and colonizing microflora. Activity of the antimicrobial drug is obtained at the cost of the development of resistance by the pathogen, but also the colonizing flora.
Prescribers of antimicrobial drugs have dual, somewhat contradictory responsibilities. On the one hand they want to offer optimal therapy for the individual patient under their care; on the other hand they have a responsibility to the same and other patients in the future and to public health to preserve the efficacy of antibiotics and minimize the development of resistance. The former responsibility tends to promote overtreatment; the latter is usually overlooked. Among antimicrobial drugs, those targeting bacteria, i.e., antibiotics, are most extensively developed and prescribed. Misuse of antibiotics, i.e., unnecessary prescriptions as well as inappropriate use (inadequate dosing, wrong duration) is frequent; up to half of the antibiotic prescriptions both in the community and in hospitals are considered unjustified.1,2 Bacterial resistance to antibiotics is a serious threat to patients that is increasing rapidly. With few new antibiotics in the research and development pipeline, particularly in the Gram-negative spectrum,3 and “old” useful antibiotics, which are not marketed anymore in some countries,4 prudent antibiotic use is the only option to delay the emergence of resistance.
The quantity and quality of antibiotic prescribing differs greatly between countries. This has become clear in international comparative studies on antibiotic prescribing in a quantitative and qualitative fashion.5,6 For instance, the amount of antibiotics used in southern European countries is about three times higher than in Scandinavian countries or the Netherlands.7 In general, these differences in use are reflected by the magnitude of the antimicrobial resistance problems: the more (broad-spectrum) antibiotics are used, the greater the prevalence of resistant microorganisms leading to a spiral of escalating broad-spectrum use.
As early as in 1993, the British Society of Antimicrobial Chemotherapy (BSAC) Working Party on Antimicrobial Use considered training in infectious diseases (ID) and knowledge of prescribing of antimicrobial drugs insufficient in clinicians, which is one of the major causes of misuse.8 According to a recent policy paper by the Infectious Diseases Society of America (IDSA), clinician training and continuing education in appropriate antimicrobial use in the United States (US) is “highly variable, non standardized, infrequent, and highly prone to bias, especially when conveyed or sponsored by pharmaceutical firms or their agents. Apart from initial training and, to a limited extent, in preparation for board recertification examinations, there is little if any compulsory training or education of physicians in antimicrobial stewardship.”9 Conversely, focus on prescribing older, narrow-spectrum drugs in targeted therapy has been taught in medical schools and has been common practice in northern European countries such as Scandinavia and the Netherlands for several decades.10
National and international authorities have repeatedly recommended multifaceted strategies to promote prudent antibiotic use for the control of antibiotic resistance.11,12 One of these strategies is to optimize education of all healthcare professionals who prescribe antibiotics. Because patients have an increasingly participative role in their treatment, education of the public is also of the utmost importance. Until recently the public’s knowledge on antibiotics—appropriateness, side effects and limitations—was poor in the European Union (EU).13 In the last decade, national and regional campaigns have been conducted to educate the public worldwide; e.g., in the US the Centers for Disease Control and prevention (CDC)’s Get smart about Antibiotics (www.cdc.gov/getsmart/), in Canada Do bugs need drugs? (www.dobugsneeddrugs.org) and across Europe the European Centre for Disease Prevention and Control (ECDC)’s annual European Antibiotic Awareness Day (http://ecdc.europa.eu/en/eaad/Pages/Home.aspx), launched on 18 November 2008. Huttner et al. identified and reviewed the characteristics and outcomes of 22 campaigns done at a national or regional level in high-income countries between 1990 and 2007.14 The intensity of the campaigns varied widely, from simple internet to expensive mass-media campaigns. All but one campaign targeted the public and physicians simultaneously. The authors conclude that public campaigns can probably contribute to more careful use of antibiotics in outpatients, at least in high-prescribing countries.14 In France yearly public antibiotic campaigns have been conducted since 2002. During its first 3 years, the French public campaign accelerated a pre-existing decrease in ambulatory antibiotic prescriptions. However, the decrease in consultation rates suggests that altered illness behavior of patients may have contributed to the observed decline.15
Because of the major deficiencies found in elementary knowledge on antibiotics in surveys of adults throughout Europe (e.g., “antibiotics are useful for colds”), educating children on this topic might be a good idea. Three major initiatives have been directed to the education of children. The first program, Do Bugs Need Drugs? started as a small six-month pilot in Alberta, Canada in 1998. It contained a kids’ section dealing with handwashing and responsible use of antibiotics in a playful way. The program evolved into a larger provincial program in Alberta and British Columbia (www.dobugsneeddrugs.org). Second, the Microbes en question mobile children’s health education campaign, supported by the French Caisse Nationale d’Assurance Maladie and the Pasteur Institute, was started as an innovative program in 2004. Finally, the European Union-funded antibiotic and hygiene teaching resource e-Bug was developed in the UK as an initiative of the Health Protection Agency. In preparation of the program, Lecky et al. examined the educational structure and educational resources or campaigns currently available by questionnaire in 10 European countries.16 It appeared that the curricula in all countries covered the topic of human health and hygiene, but limited information was provided on antibiotics and their prudent use. The European educational structure from pre-primary through upper secondary indicated that the 9- to 11-year-old and 12- to 15-year-old age groups were the most appropriate to aim the e-Bug resource at, in order to teach all children within compulsory education.16 In conclusion, such programs constitute an opportunity for instructing children and their parents on the use and non-use of antibiotics. Further details on education of the public are beyond the scope of this article.
In this article we will focus on education of the prescribers of antibiotics, and analyze and discuss all relevant aspects. We will review the principles of antimicrobial stewardship, the timeline and setting for this educational process and learning outcomes, the background of the teachers, the format of education, its evaluation and show the need for early and integrated education of all medical professionals.
Antimicrobial Stewardship Principles
According to the IDSA definition of antimicrobial stewardship it includes: optimizing the indication, selection, dosing, route of administration and duration of antimicrobial therapy to maximize clinical cure or prevention of infection while limiting the collateral damage of antimicrobial use, including toxicity, selection of pathogenic organisms (such as Clostridium difficile) and emergence of resistance.1 An important principle is avoiding selection pressure of the antibiotic in the patient, both on pathogen and commensal (Fig. 1) by choosing the least broad-spectrum antibiotic, adequate doses, a good timing and the shortest possible duration. With resistance increasing worldwide, additional uncertainty arises on the optimal broadness of spectrum needed for empirical therapy. The level of in vitro resistance that should guide the abandonment of an antibiotic and the shift to a broad-spectrum agent is not known. Antibiotic choices for empirical therapy should be decided upon at local level, guided by local antibiograms and patient outcome data. However, in settings with a well-developed microbiology laboratory system, it is possible to adapt the empirical therapy to a targeted therapy when culture results become available; streamlining or de-escalating antimicrobial therapy has become more widely accepted since intensive care physicians have adopted it as their strategy. Targeted therapy decreases unnecessary antimicrobial exposure and contains costs. De-escalation may also include discontinuation of empirical antimicrobial therapy based on clinical criteria and negative culture results.1
A lack of knowledge of infectious disease and antibiotics may seriously hamper the quality of prescription. In this situation, the prescribing physician may prefer to err on the safe side, i.e., prescribing maximal broad-spectrum treatment, instead of making a well-informed guess. A negative attitude, based on a lack of agreement with protocols or guidelines, will also affect prescribing. Likewise, a lack of self-efficacy, a lack of outcome expectancy and inertia may lead to poor prescribing.17 Based on the available international recommendations for antimicrobial stewardship policies and on the literature,1,18Table 1 presents the main principles for education in prudent antibiotic prescribing. These elements have to be translated into topics, concepts, disciplines, learning outcomes and competencies both for the undergraduate core curriculum of medical doctors and other healthcare professionals, the internship/foundation year and specialty/professional training.
Table 1. Elements of education on prudent antibiotic prescribing.
| Topic | Concept, understanding | Field, discipline | Principles, learning outcomes, competencies* |
|---|---|---|---|
|
Bacterial resistance |
Selection, mutation |
(Micro) biology, genetics |
• Extent, causes of bacterial resistance in pathogens (low antibiotic concentration, longtime exposure of microorganisms to antibiotics is driving resistance) • Extent, causes of bacterial resistance in commensals and the phenomenon of overgrowth (e.g., Clostridium difficile infection, yeast infection) |
| Epidemiology |
• Epidemiology of resistance, accounting for local variations and importance of surveillance (differences between wards, countries...) |
||
| Hygiene |
Infection control—mostly microbiology |
• Spread of resistant organisms |
|
|
Antibiotics |
Mechanisms of action of antibiotics/resistance Toxicity |
Pharmacology |
• Broad vs. narrow-spectrum antibiotics, preferred choice of narrow-spectrum drugs • Combination therapy (synergy, limiting emergence of resistance, broaden the spectrum) |
| Costs |
Ethics, public health, pharmacology |
• Collateral damage of antibiotic use (toxicity, cost) • Consequences of bacterial resistance • Lack of development of new antibiotics (limited arsenal) |
|
|
Diagnosis of infection |
Infection/inflammation |
Physiology/microbiology/immunology/infectious diseases |
• Interpretation of clinical and laboratory biological markers • Fever and C-Reactive Protein (CRP) elevation are also a sign of inflammation, not per se of an infection |
| Isolation, identification of bacteria, viruses and fungi |
(Micro) biology |
• Practical use of point-of-care tests (e.g., urine dipstick, streptococcal rapid antigen diagnostic test in tonsillitis...) • Importance of taking microbiological samples for culture before starting antibiotic therapy |
|
| Susceptibility to antibiotics |
Microbiology/infectious diseases |
• Interpretation of basic microbiological investigations (Gram stain, culture, PCR, serology...) |
|
|
Treatment of infection |
Indication for antimicrobials |
Clinical microbiology/infectious diseases organ specialty |
• Definitions and indications of empiric/directed therapy vs. prophylaxis • Clinical situations when not to prescribe an antibiotic: ○ Colonization vs. infection (e.g., asymptomatic bacteriuria) ○ Viral infections (e.g., acute bronchitis) ○ Inflammation vs. infection (e.g., fever without a definite diagnosis in a patient with no severity criteria) |
|
Prevention of infection |
|
Pharmacotherapy, surgery, anesthesiology, clinical microbiology/infectious Diseases |
• Surgical antibiotic prophylaxis: indication, choice, duration (short), timing |
|
Medical record keeping |
Choice Duration Timing |
Clinical medicine |
• Documentation of antimicrobial indication in clinical notes • Recording (planned) duration or stop date |
|
Prescribing antibiotics: initially |
Empiric therapy (local guide, antibiotic booklet...) Diagnostic uncertainty |
Clinical microbiology/infectious diseases/organ specialists Clinical pharmacology |
• Best bacteriological guess for empiric therapy • Choice in case of prior use of antibiotics when selecting an antibiotic for empiric therapy • Choosing the dose and interval of administration (basic principles of PK/PD) • Estimating the shortest possible adequate duration |
|
Prescribing antibiotics: targeted therapy |
Communication with the microbiology laboratory Value of specialist consultation in infectious diseases or microbiology |
Clinical microbiology/infectious diseases/organ specialists Hospital pharmacy |
• Reassessment of the antibiotic prescription around day 3 • Streamlining/de-escalation once microbiological results are known • IV-oral switch (bioavailability of antibiotics) • Therapeutic drug monitoring to ensure adequate drug levels (e.g., vancomycin) |
|
Prescribing antibiotics: standard of care |
The importance of guidelines in clinical practice |
Clinical medicine, organ specialists |
• Prescribing antibiotic therapy according to national/local practice guidelines |
| Quality indicators of antibiotic use |
Quality institute |
• Audit and feedback assessing prescribing practice using quality indicators |
|
| Communication skills | Discussion techniques | Psychology, clinical medicine | • Explaining to the patient the absence of an antibiotic prescription • Education of patients regarding prudent antibiotic use (comply with the doctors’ prescription, no self-medication...) |
A competency is a quality or characteristic of a person that is related to effective performance. Competencies can be described as a combination of knowledge skills, motives and personal traits.22
Who are the Prescribers of Antibiotics?
Unlike many other drugs, for which prescribing is kept within a specialty (for example neuroleptic drugs), antibiotics are prescribed universally by all medical doctors and dentists in the community. In Europe, over the counter use by the public is low except for a few southern countries (e.g., Spain and Greece).19 But even in the case of over the counter purchases, health professionals have a major responsibility as patients copy their prescribing habits.20 In some countries [for example the United Kingdom (UK) or France], midwives, clinical pharmacists or nurses (“physician assistants,” in the UK, Netherlands and Belgium) can also prescribe some antibiotics in selected clinical situations.21,22 Pharmacists also play a key role in the process, by dispensing the drugs and giving advice to the patients. Therefore, all healthcare professionals in contact with the patient must be educated with respect to knowledge on antimicrobial resistance, (lack of) evidence of benefit of antibiotics in different conditions and related beliefs, knowledge of management of symptoms and the use of microbiology laboratory tests to guide antibiotic treatment. Education on management of demanding patients is also required.23
Antibiotic management requires effective teamwork between all health professions, regardless of who writes the prescription. It is therefore crucial to educate not only prescribers, but all other healthcare professionals in contact with the patients who are prescribed an antibiotic (e.g., nurses, midwives and pharmacists), since patients should receive consistent messages on correct and prudent antibiotic use when taking antibiotics.24 Therefore, all these healthcare professionals must receive continuous training in prudent antibiotic prescribing.24,25
In hospitals, only patients with complicated or severe infections will be referred to infectious diseases departments or medical microbiologists or infectious diseases teams for consultation, the majority being treated for their infection by other physicians, mostly organ specialists. On average, one patient out of three is treated with antibiotics during his hospital stay. In the recent national English Point Prevalence Survey on healthcare-associated infections and antimicrobial use report, the prevalence of national antimicrobial use was 34.7%.26 It was greatest in the intensive care units (ICUs) (60.8%) and antimicrobial use prevalence in surgery was 36%.26 Junior doctors of all specialties often prescribe the antibiotics under the supervision of their seniors. Therefore, both senior and junior doctors must be educated in order to change practice.27-29
Finally, we must also consider the antibiotic prescriptions for animals and agriculture, made by the veterinarians. Although there are similarities of antimicrobial stewardship for companion animals and the human sector,30 antibiotic use in livestock animals is further complicated by economic issues.31 An enormous challenge is awaiting those wanting to introduce antibiotic stewardship in the undergraduate veterinarian curriculum.32
When Should Antimicrobial Stewardship Education Start?
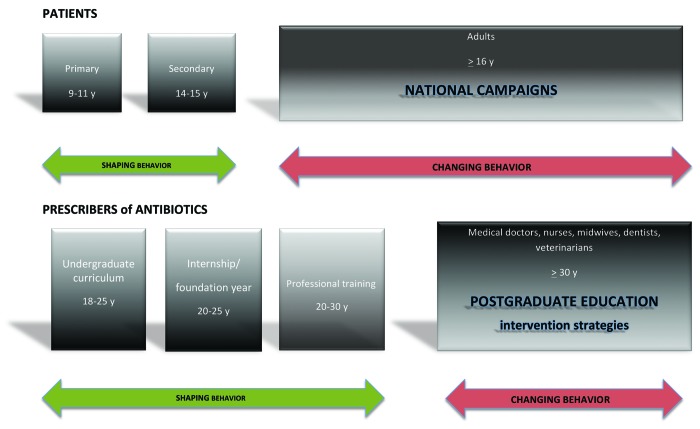
Figure 2 shows the timeline of education on prudent antibiotic use of both the patients and the prescribers of antimicrobials. As mentioned above, in the past few years a lot of progress has been made in teaching of children. Education at the primary and secondary level will prepare the individual who later might become a patient or more importantly, the anxious parent of a sick toddler with, for example, otitis media. In addition, it provides the basic education of the future professional. Not much is known about the content, volume and quality of medical curricula teaching antimicrobial stewardship principles and resistance in terms of knowledge, attitude and behavior to medical students, and more specifically, their timeline in Europe. One survey was done by the British Society for Antimicrobial Chemotherapy.8 The authors pleaded for inclusion of antimicrobial chemotherapy in the undergraduate curriculum, as most doctors regularly treat infections. European national medical undergraduate curricula can either be quite detailed such as the Raamplan Artsopleiding 2009 in the Netherlands in which general learning outcomes with keywords are specified such as: prevention of infections and spread, aspects of guidelines and protocols and development of antibiotics. Other learning outcomes of university curricula such as the University of Leuven on websites (http://med.kuleuven.be/Faculteit_Geneeskunde/english/studies/medicine) are very general and do not provide information on the topics covered. Indirect information is available from a few surveys of medical students or junior doctors.29,33 Minen et al. surveyed medical students’ perceptions and attitudes about their training on antimicrobial use to identify gaps in medical education in a US university hospital.33 Thirty percent of medical students responded. The majority of third- and fourth-year medical students believed that antibiotics were overused in the hospital and in outpatient areas. Medical students recognized the importance of judicious antibiotic use and would like greater instruction on how to choose antibiotics appropriately. Over three-quarters of the students would like more education on antibiotic selection, and 83% wanted this education to be during the third year of medical school. The resources they used the most for antibiotic selection included other physicians and handheld programs such as Epocrates, but no clear resource emerged as the dominant preference.33 Pulcini et al. conducted a survey on doctors after qualification from medical school who were still in their training years in a French and Scottish university hospital.29 In both countries, doctors were first-year trainees at the same stage in their medical training, after 5 and 6 years of undergraduate training in Dundee and Nice, respectively. Overall, 30% of those surveyed stated that they had had no training in antibiotic prescribing in the past year, although 99% had prescribed an antibiotic within the last 6 mo. A reassuring feature was that 91% (France) and 97% (Scotland) cited guidelines as a factor that influenced their prescribing, respectively.29 One of the striking differences is that US students did not mention local guidelines as resource,33 whereas British students are explicitly stimulated to prescribe according to local guidelines.22
Figure 2. Timeline of education on prudent antibiotic use. Different time periods which offer opportunities to shape or change the behavior of the public and the prescribers of antibiotics
Recently, the importance of undergraduate training in prudent prescribing of antibiotics has become increasingly recognized.12,23 In the UK and Scotland in particular, major efforts were made to adapt and revise undergraduate education on antibiotics. In the UK, the Specialist Advisory Committee on Antimicrobial Resistance (SACAR) has proposed to undertake the development of learning outcomes, i.e., statements that indicate what a student should know, understand and be able to do by the end of an educational program. This would provide a robust and transparent framework for curriculum development at all stages. Subsequently, the learning outcomes could be translated into competencies by the appropriate bodies.25 Prescribing is included as a component of the undergraduate program in the UK, and the importance of undergraduate training in prescribing is reflected in aspects of the General Medical Council’s (GMC’s) Tomorrows’ Doctors.34 The GMC has now endorsed the report entitled A single competency framework for all prescribers, as in the UK nurses are allowed to prescribe antibiotics.22 Doctors should be able to prescribe on registration. In the UK, this competency is subjected to the standards for supervision for doctors in training set out in The Trainee Doctor report.35 Statement competency examples are: (1) Competency 1: Knowledge. Has up-to-date clinical, pharmacological and pharmaceutical knowledge relevant to own area of practice, Nr 11: Understands antimicrobial resistance and the roles of infection prevention, control and antimicrobial stewardship measures; (2) Competency 7: The Healthcare system. Understands and works within local and national policies, processes and systems that impact on prescribing practice. Sees how own prescribing impacts on the wider healthcare community, Nr 59: Understands and works within local frameworks for medicines use as appropriate (e.g., local formularies, care pathways, protocols and guidelines). More specific learning outcomes or competencies can be developed departing from this list.
Re-examining the principles and learning outcomes in Table 1, it is clear that much emphasis is needed on the transfer of basic science knowledge at an early stage. Just as optimal periods and subjects have been identified to guarantee maximal exposure to children,16 the undergraduate curriculum and internship/foundation year seem optimal stages to build a solid knowledge base for later practice. For example: surgeons will have a much higher acceptance of prophylaxis guidelines if they have been exposed to the principles of guideline development and antibiotic prophylaxis taught as core competencies in the third year of medical school. Conversely, a plethora of literature is available on the difficult task of changing the behavior of trained medical practitioners, the barriers being multiple, cultural among others.17,36
Who Should Educate/Teach/Train?
As all doctors prescribe antibiotics, a strong input is needed from academia to transfer the knowledge in the undergraduate curriculum. As depicted in Table 1, the curriculum is expected to deliver knowledge and shape the right attitude and behavior regarding the basic principles of antimicrobial stewardship. In most settings the organ specialist hardly covers this topic. A wide variety of disciplines must be involved, including epidemiology, ethics and communication skills (working with guidelines, communicating with the patient). To link the undergraduate and postgraduate programs, in particular in the period of internship/foundation training, close collaboration between healthcare providers and academicians and between hospitals and medical schools is needed.
In the postgraduate training track most medical and surgical specialties are anatomically defined, but all have to deal with infections. In practice, each specialty thus has a certain degree of “claim” over antimicrobial prescribing in their field. Again, as depicted in Table 1, the input of many disciplines is required to train a junior prescriber at the bedside, and the organ specialist (e.g., urologist) may not have the full required background for implementing the general principles of prudent antibiotic prescribing in the microbiological diagnostic and therapeutic management of urological patients. With worldwide increasing resistance data, multidisciplinary guideline development of national and local guidelines becomes of the utmost importance. The ADAPTE framework (www.adapte.org) can be used to minimize barriers to the development and acceptance of guidelines.17 In particular, guidelines must be evidence-based and developed by a multidisciplinary group, involving all key stakeholders to foster acceptance and ownership. National or international guidelines should be adapted to the local context to ensure relevance for local practice and policies. Transparent reporting is essential to promote confidence in the recommendations of the adapted guideline and flexible, easily accessible formats must be used (booklet, poster, smartphone applications...). In Canada, the CanMEDS Physician Competency framework describes the knowledge, skills and abilities that specialist physicians need for better patient outcomes.37 This model has been adapted around the world in the health profession and other professions.
In the hospitals, a multidisciplinary core group, including infectious diseases specialists, microbiologists, (clinical) pharmacists and/or an antibiotic stewardship team must be involved in the development and implementation of a local educational program on prudent antibiotic prescribing. In the Netherlands, general practitioners (GPs) organize regional therapy focus groups where they discuss prescribing practices with pharmacists.
The teachers delivering the educational sessions must also be trained, both regarding the available educational strategies and the current literature on antimicrobial stewardship. As an example, the project “ABS International” implemented a training program for national antibiotic stewardship trainers in nine European countries, and offered them standard tools for implementing an antibiotic stewardship program in their hospitals (e.g., guidelines for treatment and surgical prophylaxis, organizational measures, tools to analyze consumption data...).38 Learning societies such as the ESCMID (European Society of Clinical Microbiology and Infectious Diseases) Study Group for Antibiotic Policies (ESGAP) have been organizing educational workshops on antibiotic stewardship for more than 10 years. Table 2 presents a selection of the main learning outcomes for such workshops. The format of these postgraduate educational courses is discussed in the next paragraph.
Table 2. Main learning outcomes used to design antibiotic stewardship workshops.
|
Measuring antibiotic use |
• Identify sources of data and understand how to measure antimicrobial use in the community and in hospitals • Select proper measurement units to describe the volume of antimicrobial use • Interpret antimicrobial use data locally and within a multicenter network (benchmarking) • Choose and apply a method to study the relationship between antimicrobial prescribing and bacterial resistance |
|
Auditing antibiotic use |
• Choose and apply an audit methodology for monitoring the quality of antimicrobial prescriptions |
| Improving antibiotic use | • Identify the steps and sources for evidence-based guideline development • Describe the elements needed to launch a stewardship program in hospitals Identify barriers encountered in Antimicrobial Stewardship programs and how to overcome them • Make sense of interpersonal aspects of implementing change • Identify possible intervention strategies (and their relative advantages and disadvantages) which could be implemented in a hospital • Identify the electronic antimicrobial drug prescribing aids and their advantages and disadvantages • Build national and international support for Antimicrobial Stewardship programs • Select a proper method to study the effect of interventions in hospitals • Describe how an individual hospital can determine if its antimicrobial management program was economically successful and if it had an impact on bacterial resistance |
How to Educate? Formats of Educational Curricula
Undergraduate
In the undergraduate curriculum, classical formal lectures are seldom considered as a successful means of transferring knowledge. Over the past decade, problem-based learning has been introduced in many universities. This type of education allows for alternative formats of interactive learning in smaller student groups. It is important to identify the topics or concepts that benefit from a disease- (e.g., acute bronchitis) or problem- (e.g., antimicrobial resistance) oriented rather than a pathogen- (e.g., MRSA, methicillin-resistant Staphylococcus aureus) oriented or a drug- (e.g., antibiotic classes) oriented approach.
Microbial resistance can be part of microbiology teaching, information on antibiotics part of pharmacology and managing the demands of patients in particular parents of young children integrated in communication skills sessions. However, targeted “antibiotic” sessions in the format of problem-based learning are absolutely necessary to integrate all aspects of the topic.23 Apart from formal lectures, interactive learning with case vignettes, PowerPoint presentations and role play can be particularly appropriate for this topic. Elective rather than core modules are particularly suitable for discussions in small groups. Potential topics are case studies, e.g., with questions and answer sessions, illustrating the evidence base of surgical prophylaxis. As an example, the University of Rotterdam (The Netherlands) has included a one-week module on several concepts of antimicrobial resistance, hygiene and prudent antibiotic prescribing in the core curriculum of the second year of medical school. The University of Nijmegen offers an additional elective, problem-based module on antibiotic policy for third year students, treating the history of infectious diseases, hygiene and infection control, antibiotic guidelines, principles of prophylaxis and laboratory techniques among others.
In Scotland, for example, an extensive range of e-learning resources have been developed to train both undergraduate and postgraduate healthcare professionals on prudent antibiotic prescribing.39 Such training is mandatory for junior doctors. Also in the UK, the Prudent Antibiotic User (PAUSE, www.pause-online.org.uk) is a website of shared standardized teaching materials for prudent antimicrobial prescribing for use in the undergraduate medical curriculum. PAUSE provides standardized teaching aides for all educators of antibiotic prescribing based on patient-focused, reflective learning. Resources are designed to enable students to prepare for interactive sessions to compare standard patient vignettes with their own clinical experience. The structured preparation required of the students is a key to success. The interactive discussion sessions focus on learning prudent antibiotic prescribing through reflective practice. Prepared materials in relation to structure and content for each interactive session are available for tutors to use (in a PowerPoint format). These resources include patient histories, clinical signs, investigations and questions on diagnosis, assessing severity, appropriate prescribing, public health issues and patient management. Best practice statements and core resource lists are also available. In addition guideline answers to the questions with feedback are provided, including inappropriate responses and the corresponding reasons. The materials may be shared, reproduced and modified as necessary. Another example is the educational work conducted by the Scottish Antimicrobial Prescribing Group (SAPG), hosted by the Scottish Medicines Consortium (SMC) in Scotland. The educational program is led by NHS Education for Scotland (NES) and involves scoping and development of training materials on antimicrobial stewardship both for undergraduate and postgraduate healthcare professionals. A range of online resources are available on the website (www.nes.scot.nhs.uk/education-and-training/by-theme-initiative/healthcare-associated-infections/online-short-courses.aspx). A framework of learning outcomes for antimicrobial stewardship that aligns with The Scottish Doctor report has been developed after broad consultation, and SAPG has recommended its adoption into the curricula of the five Scottish medical schools. The framework is also being evaluated by the two Schools of Pharmacy in Scotland to ensure that those learning outcomes applicable to pharmacists are covered by their undergraduate curricula. Such training is mandatory for junior doctors.39
Internship, foundation year training (UK)
Recently, the training programs of graduated doctors into primary care or specialty have been the subjects of reforms in many countries. In Scotland, the Doctors Online Training System (DOTS), a mandatory web-based education resource for all foundation training doctors, was revised to highlight current issues in prudent antibiotic prescribing in 2009.39 Access to the DOTS program has been extended to allow other medical staff, pharmacists and non-medical prescribers to participate in the training, and a primary care module has also been added. An induction pack for junior doctors and other new clinical staff has been produced and made available for antimicrobial management teams and other staff involved in training. This endeavor has been developed and taught by NES (www.nes.scot.nhs.uk/education-and-training/by-theme-initiative/healthcare-associated-infections/training-resources/antimicrobials-in-clinical-practice.aspx).
Specialist training
The format of internship/specialist training of medical doctors is very variable both regarding the onset of exposure to patients, the duration, type of training and responsibilities within Europe and the world which renders standardization of learning outcomes very difficult. This period is extremely crucial for shaping behavior, as juniors start to copy the behavior of their supervisors within the first weeks in the hospital.27 The same principles used for undergraduates apply, and competencies and learning outcomes must be clearly defined. The impact of learning sessions can be enhanced by measurement of current practice, and the use of quality improvement strategies.25,40,41
Strong political support is necessary for a curriculum program to be successfully implemented. As an example, in the UK, the General Medical Council requested in 2009 that all postgraduate deans and Royal Colleges ensure infection prevention and control and antimicrobial prescribing become standard practice implemented in all clinical settings, and that they are strongly emphasized in undergraduate and postgraduate medical training.40
Postgraduate level
Up to now, most initiatives on education in antimicrobial stewardship have been deployed in the postgraduate setting. A considerable effort has been put into education in hospitals. Many interventional programs to optimize antibiotic use have been conducted worldwide, and to a lesser extent in primary care.42,43 Intervention strategies have been categorized as educational, restrictive and supportive (Table 3). A multifaceted approach is favored to improve antibiotic use.1 Education is an essential element of any hospital program designed to influence antibiotic prescribing behavior. Educational measures are usually more popular among clinicians than restrictive measures.29,44 However, passive education alone (lectures, educational events, leaflets and handouts), without incorporation of active intervention, has been shown only marginally effective in changing antimicrobial prescribing practices and has not demonstrated a sustained impact.1,43 In many places the limited success of in-hospital education may be partly due to the rapid turnover of junior staff and the difficulty in maintaining a local continuous educational program. Printed educational materials and educational conferences alone also have had little effect on changing prescribing practices for antibiotics or other medications in the outpatient setting. Face-to-face and one-to-one educational sessions provided by physicians are based on established principles of behavioral science and market research and communications theory. This type of education has been used intensively and successfully by the pharmaceutical industry. The approach in antimicrobial stewardship has proved to be a practical, effective and safe method for reducing excessive broad-spectrum antibiotic use, but it is costly and labor-intensive.45 Clinical pathways have successfully been used to implement prudent antibiotic strategies, such as the de-escalation pathway described by Singh et al. to curb inappropriate antibiotic use for pulmonary infiltrates in the intensive care unit in their hospital.46 Clinical pathways, audit and feedback and the development of practice guidelines are discussed more extensively in another part of this issue. In conclusion, according to the Cochrane review, a wide variety of interventions have been proven successful in changing antibiotic prescribing to hospital inpatients.43 Any interventions can work some of the time.41 Hospitals are complex institutions and what is effective in one setting may not be effective in another.
Table 3. Main antimicrobial stewardship strategies recommended in the international literature to improve antibiotic use at the hospital level.1,49.
| Educational measures and active interventions | |
|---|---|
| Passive educational measures |
• Developing/updating local antibiotic guidelines • Educational sessions, workshops, local conferences |
| Active interventions |
• Clinical rounds discussing cases • Prospective audit with intervention and feedback • Reassessment of antibiotic prescriptions, with streamlining and de-escalation of therapy • Academic detailing, educational outreach visits |
| Restrictive measures |
• Limiting number of antibiotics on the hospital formulary • Antibiotic order form (compulsory) • Automatic stop order • Formulary restriction and preauthorization • Limiting reporting of susceptibilities by the microbiology laboratory • Regulating contacts with the pharmaceutical industry |
| Supportive/supplemental measures | • Multidisciplinary antimicrobial stewardship team • Consultancy service (infectious diseases, pharmacy, microbiology) • Computer-assisted management program • Parenteral to oral conversion • Therapeutic drug monitoring service |
An open access curriculum has been developed in the context of the European Union funded research project “Genomics to combat resistance against antibiotics in community-acquired lower respiratory tract infections in Europe” (GRACE). It contains a series of postgraduate courses and workshops and permitted the creation of an open access e-Learning portal. A total of 153 presentations matching the topics within the curriculum, slide material, handouts and 104 webcasts are available through this portal. The website is a considerable source of knowledge, mainly on diagnostics and much less on therapy (www.grace-edut.org/pages/default.aspx?id=1617).47
Also on a European level, the European Centre for Disease Prevention and Control (ECDC) chose the hospital prescribers as target for their European Antibiotic Awareness Day campaign in 2010. The aim of the toolkit was to support efforts at a national level to increase prudent use of antibiotics in hospitals through dissemination of evidence-based educational and information materials. The toolkit contains template materials and evidence-based key messages which may be adapted for use at national level, and suggests tactics for getting the messages regarding prudent use of antibiotics through to the target audiences. The template toolkit materials and more information about the European Antibiotic Awareness Day are available on the European Antibiotic Awareness Day website (http://ecdc.europa.eu/en/EAAD/Pages/Home.aspx/).
In the US, the CDC started its campaign “Get Smart for Healthcare” in the same year. It focuses on improving antibiotic use in inpatient healthcare facilities starting with hospitals and then expanding to long-term care facilities. The goal of the campaign is to optimize the use of antimicrobial agents in inpatient healthcare settings by focusing on strategies to help hospitals and other inpatient facilities implement interventions to improve antibiotic use. The CDC provides slides, fact sheets, and an annotated bibliography on the evidence base of outcomes among other tools on its website (www.cdc.gov/getsmart/healthcare/). The CDC also collaborates with the Society for Healthcare Epidemiology of America (SHEA) to develop simple implementation tools, and with the Institute for Healthcare Improvement (IHI) and SHEA to develop a driver diagram with practical antibiotic stewardship implementation strategies.
In view of training the trainers, multiple educational postgraduate courses and workshops have been organized in the past decade, targeting a multidisciplinary group of clinicians including infectious diseases specialists, clinical microbiologists, (ID) hospital pharmacists and hospital epidemiologists.
The ESCMID study group for Antibiotic Policies (ESGAP, available at www.escmid.org/esgap) has been conducting postgraduate international education courses “Antimicrobial Stewardship: Measuring, auditing and improving,” held bi-annually before the European Conference on Microbiology and Infectious Diseases (ECCMID). Up to now, seven courses have been organized, training over 400 medical doctors, scientists, and clinical pharmacists over the past decade. Another one-day course is conducted as a pre-ICAAC workshop (www.icaac.org/workshops). ESGAP has also published its efforts in making an inventory of antimicrobial stewardship websites.48
Conclusion
After 30 years of antibiotic policy activities trying to curb antibiotic resistance, some reflection is appropriate. Antimicrobial stewardship interventions have mainly been conducted at the postgraduate level, aiming at changing the behavior of professionals. This has proven extremely difficult and frustrating. Quite paradoxically, although a thorough analysis is of the situation is lacking, only minimal investment has been put in the antimicrobial stewardship education in the undergraduate curriculum in most countries. This is, in our opinion, a missed opportunity for the future. It seems obvious that antimicrobial stewardship is likely to be more successful when started much earlier, at the time when knowledge, attitude and behavior of professionals are being shaped.
Therefore, it is now crucial to focus on an adapted undergraduate medical/professional curriculum that teaches all necessary principles of microbiology, infectious diseases and clinical pharmacology, with emphasis on the principles of prudent prescribing in an adequate format. Appropriate curricula on antimicrobial stewardship are a joint responsibility of the academia and the ministries of Health and Education.
Recommendations
• Education on prudent antimicrobial prescribing should start early in the undergraduate curriculum, preferably in the third year of undergraduate training in medicine and correspondent level in non-medical curricula of pharmacy, dentistry, midwifery, nursing and veterinary medicine to reach all health professionals.
• This requires commitment from medical schools on a national level to agree that antimicrobial stewardship is among the necessary skills to practice.
• The teaching of principles preparing for antimicrobial stewardship should be guaranteed by the development of learning outcomes and competencies and the appropriate evaluation.
• Postgraduate education should then focus on implementation and measurement of practice, with additional supportive and restrictive measures.
Glossary
Abbreviations:
- BSAC
British Society of Antimicrobial Chemotherapy
- CDC
Centers for Disease Control and Prevention
- DOTS
Doctors Online Training System
- ECCMID
European Congress of Clinical Microbiology and Infectious Diseases
- ECDC
European Centre for Disease Prevention and Control
- ESCMID
European Society of Clinical Microbiology and Infectious Diseases
- ESGAP
European Society of Clinical Microbiology and Infectious Diseases Study Group for Antibiotic Policies
- EU
European Union
- GMC
General Medical Council
- GPs
general practitioners
- GRACE
Genomics to combat resistance against antibiotics in community-acquired lower respiratory tract infections in Europe
- ICAAC
Interscience Conference on Antimicrobial Agents and Chemotherapy
- ICU
intensive care unit
- ID
infectious diseases
- IDSA
Infectious Diseases Society of America
- IHI
Institute for Healthcare Improvement
- MRSA
methicillin-resistant Staphylococcus aureus
- NES
NHS Education for Scotland
- NHS
National Health Service
- PAUSE
Prudent Antibiotic User website
- PK/PD
pharmacokinetics and -dynamics
- SACAR
Specialist Advisory Committee on Antimicrobial Resistance
- SAPG
Scottish Antimicrobial Prescribing Group
- SHEA
Society for Healthcare Epidemiology of America
- SMC
Scottish Medicines Consortium
- UK
United Kingdom
- US
United States of America
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/23706
References
- 1.Dellit TH, Owens RC, McGowan JE, Jr., Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America. Society for Healthcare Epidemiology of America Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–77. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 2.Pulcini C, Cua E, Lieutier F, Landraud L, Dellamonica P, Roger PM. Antibiotic misuse: a prospective clinical audit in a French university hospital. Eur J Clin Microbiol Infect Dis. 2007;26:277–80. doi: 10.1007/s10096-007-0277-5. [DOI] [PubMed] [Google Scholar]
- 3.Freire-Moran L, Aronsson B, Manz C, Gyssens IC, So AD, Monnet DL, et al. ECDC-EMA Working Group Critical shortage of new antibiotics in development against multidrug-resistant bacteria-Time to react is now. Drug Resist Updat. 2011;14:118–24. doi: 10.1016/j.drup.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Pulcini C, Bush K, Craig WA, Frimodt-Møller N, Grayson ML, Mouton JW, et al. ESCMID Study Group for Antibiotic Policies Forgotten antibiotics: an inventory in Europe, the United States, Canada, and Australia. Clin Infect Dis. 2012;54:268–74. doi: 10.1093/cid/cir838. [DOI] [PubMed] [Google Scholar]
- 5.Ansari F, Erntell M, Goossens H, Davey P. The European surveillance of antimicrobial consumption (ESAC) point-prevalence survey of antibacterial use in 20 European hospitals in 2006. Clin Infect Dis. 2009;49:1496–504. doi: 10.1086/644617. [DOI] [PubMed] [Google Scholar]
- 6.Bruce J, MacKenzie FM, Cookson B, Mollison J, van der Meer JW, Krcmery V, et al. ARPAC Steering Group Antibiotic stewardship and consumption: findings from a pan-European hospital study. J Antimicrob Chemother. 2009;64:853–60. doi: 10.1093/jac/dkp268. [DOI] [PubMed] [Google Scholar]
- 7.Adriaenssens N, Coenen S, Versporten A, Muller A, Minalu G, Faes C, et al. ESAC Project Group European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe (1997-2009) J Antimicrob Chemother. 2011;66(Suppl 6):vi3–12. doi: 10.1093/jac/dkr453. [DOI] [PubMed] [Google Scholar]
- 8.Davey P, Hudson S, Ridgway G, Reeves D, British Society of Antimicrobial Chemotherapy Working Party on Antimicrobial Use A survey of undergraduate and continuing medical education about antimicrobial chemotherapy in the United Kingdom. Br J Clin Pharmacol. 1993;36:511–9. doi: 10.1111/j.1365-2125.1993.tb00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spellberg B, Blaser M, Guidos RJ, Boucher HW, Bradley JS, Eisenstein BI, et al. Infectious Diseases Society of America (IDSA) Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis. 2011;52(Suppl 5):S397–428. doi: 10.1093/cid/cir153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halls GA. The management of infections and antibiotic therapy: a European survey. J Antimicrob Chemother. 1993;31:985–1000. doi: 10.1093/jac/31.6.985. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. WHO Global Strategy for Containment of Antimicrobial Resistance. 2001. Available at: http://whqlibdoc.who.int/hq/2001/WHO_CDS_CSR_DRS_2001.2.pdf (date last accessed: 1st October 2012).
- 12.World Health Organization. The evolving threat of antimicrobial resistance: options for action. 2012. Available at: http://whqlibdoc.who.int/publications/2012/9789241503181_eng.pdf (date last accessed: 1st October 2012).
- 13.European Commission. Antimicrobial Resistance. Eurobarometer 338/Wave 72.5 2010. Available at: http://ec.europa.eu/health/antimicrobial_resistance/docs/ebs_338_en.pdf (date last accessed: 1st October 2012).
- 14.Huttner B, Goossens H, Verheij T, Harbarth S, CHAMP consortium Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis. 2010;10:17–31. doi: 10.1016/S1473-3099(09)70305-6. [DOI] [PubMed] [Google Scholar]
- 15.Chahwakilian P, Huttner B, Schlemmer B, Harbarth S. Impact of the French campaign to reduce inappropriate ambulatory antibiotic use on the prescription and consultation rates for respiratory tract infections. J Antimicrob Chemother. 2011;66:2872–9. doi: 10.1093/jac/dkr387. [DOI] [PubMed] [Google Scholar]
- 16.Lecky DM, McNulty CA, Adriaenssens N, Koprivová Herotová T, Holt J, Touboul P, et al. e-Bug Working Group What are school children in Europe being taught about hygiene and antibiotic use? J Antimicrob Chemother. 2011;66(Suppl 5):v13–21. doi: 10.1093/jac/dkr120. [DOI] [PubMed] [Google Scholar]
- 17.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 18.Davenport LA, Davey PG, Ker JS, BSAC Undergraduate Education Working Party An outcome-based approach for teaching prudent antimicrobial prescribing to undergraduate medical students: report of a Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother. 2005;56:196–203. doi: 10.1093/jac/dki126. [DOI] [PubMed] [Google Scholar]
- 19.Grigoryan L, Monnet DL, Haaijer-Ruskamp FM, Bonten MJ, Lundborg S, Verheij TJ. Self-medication with antibiotics in Europe: a case for action. Curr Drug Saf. 2010;5:329–32. doi: 10.2174/157488610792246046. [DOI] [PubMed] [Google Scholar]
- 20.Hadi U, Duerink DO, Lestari ES, Nagelkerke NJ, Werter S, Keuter M, et al. Antimicrobial Resistance in Indonesia ‘Prevalence and Prevention’ study group Survey of antibiotic use of individuals visiting public healthcare facilities in Indonesia. Int J Infect Dis. 2008;12:622–9. doi: 10.1016/j.ijid.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Dryden MS, Cooke J, Davey P. Antibiotic stewardship--more education and regulation not more availability? J Antimicrob Chemother. 2009;64:885–8. doi: 10.1093/jac/dkp305. [DOI] [PubMed] [Google Scholar]
- 22.The National Prescribing Centre (NPC) - National institute for Health and Clinical Excellence. (NICE). A single Competency Framework for all prescribers. 2012. Available at: http://www.npc.co.uk/improving_safety/improving_quality/resources/single_comp_framework.pdf (date last accessed: 1st October 2012).
- 23.Bond C. Education of patients and professionals. In: Antibiotic Policies: theory and practice. Ed IM Gould & JWM van der Meer. Kluwer Academic/Plenum Publishers New York, 2005. [Google Scholar]
- 24.Finch RG, Metlay JP, Davey PG, Baker LJ, International Forum on Antibiotic Resistance colloquium Educational interventions to improve antibiotic use in the community: report from the International Forum on Antibiotic Resistance (IFAR) colloquium, 2002. Lancet Infect Dis. 2004;4:44–53. doi: 10.1016/S1473-3099(03)00860-0. [DOI] [PubMed] [Google Scholar]
- 25.Davey P, Garner S, Professional Education Subgroup of SACAR Professional education on antimicrobial prescribing: a report from the Specialist Advisory Committee on Antimicrobial Resistance (SACAR) Professional Education Subgroup. J Antimicrob Chemother. 2007;60(Suppl 1):i27–32. doi: 10.1093/jac/dkm154. [DOI] [PubMed] [Google Scholar]
- 26.Health Protection Agency. English National Point Prevalence Survey on healthcare-associated infections and antimicrobial use. 2011. Available at: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317134304594 (date last accessed: 1st October 2012).
- 27.De Souza V, MacFarlane A, Murphy AW, Hanahoe B, Barber A, Cormican M. A qualitative study of factors influencing antimicrobial prescribing by non-consultant hospital doctors. J Antimicrob Chemother. 2006;58:840–3. doi: 10.1093/jac/dkl323. [DOI] [PubMed] [Google Scholar]
- 28.Naqvi A, Pulcini C. [Bacterial resistance and antibiotic prescription: a survey of hospital physician perception, attitude, and knowledge] Med Mal Infect. 2010;40:625–31. doi: 10.1016/j.medmal.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Pulcini C, Williams F, Molinari N, Davey P, Nathwani D. Junior doctors’ knowledge and perceptions of antibiotic resistance and prescribing: a survey in France and Scotland. Clin Microbiol Infect. 2011;17:80–7. doi: 10.1111/j.1469-0691.2010.03179.x. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Alvarez L, Dawson S, Cookson B, Hawkey P. Working across the veterinary and human health sectors. J Antimicrob Chemother. 2012;67(Suppl 1):i37–49. doi: 10.1093/jac/dks206. [DOI] [PubMed] [Google Scholar]
- 31.Aarestrup FM. Occurrence, selection and spread of resistance to antimicrobial agents used for growth promotion for food animals in Denmark. APMIS Suppl. 2000;101:1–48. [PubMed] [Google Scholar]
- 32.Fanning S, Whyte P, O’Mahony M. Essential veterinary education on the development of antimicrobial and antiparasitic resistance: consequences for animal health and food safety and the need for vigilance. Rev Sci Tech. 2009;28:575–82. doi: 10.20506/rst.28.2.1905. [DOI] [PubMed] [Google Scholar]
- 33.Minen MT, Duquaine D, Marx MA, Weiss D. A survey of knowledge, attitudes, and beliefs of medical students concerning antimicrobial use and resistance. Microb Drug Resist. 2010;16:285–9. doi: 10.1089/mdr.2010.0009. [DOI] [PubMed] [Google Scholar]
- 34.General Medical Council. Tomorrow's Doctors: outcomes and standards for undergraduate medical education. 2009. Available at: http://www.gmc-uk.org/TomorrowsDoctors_2009.pdf_39260971.pdf (date last accessed: 1st October 2012).
- 35.General Medical Council. The Trainee Doctor: foundation and specialty, including GP training. 2011. Available at: http://www.gmc-uk.org/Trainee_Doctor.pdf_39274940.pdf (date last accessed: 1st October 2012).
- 36.Hulscher ME, Grol RP, van der Meer JW. Antibiotic prescribing in hospitals: a social and behavioural scientific approach. Lancet Infect Dis. 2010;10:167–75. doi: 10.1016/S1473-3099(10)70027-X. [DOI] [PubMed] [Google Scholar]
- 37.Royal College of Physicians and Surgeons of Canada. CanMEDS Framework. 2005. Available at: http://www.royalcollege.ca/portal/page/portal/rc/common/documents/canmeds/framework/the_7_canmeds_roles_e.pdf (date last accessed: 1st October 2012).
- 38.Allerberger F, Lechner A, Wechsler-Fördös A, Gareis R. Optimization of antibiotic use in hospitals--antimicrobial stewardship and the EU project ABS international. Chemotherapy. 2008;54:260–7. doi: 10.1159/000149716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nathwani D, Sneddon J, Malcolm W, Wiuff C, Patton A, Hurding S, et al. Scottish Antimicrobial Prescribing Group Scottish Antimicrobial Prescribing Group (SAPG): development and impact of the Scottish National Antimicrobial Stewardship Programme. Int J Antimicrob Agents. 2011;38:16–26. doi: 10.1016/j.ijantimicag.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 40.McNulty CA, Cookson BD, Lewis MA. Education of healthcare professionals and the public. J Antimicrob Chemother. 2012;67(Suppl 1):i11–8. doi: 10.1093/jac/dks199. [DOI] [PubMed] [Google Scholar]
- 41.Pulcini C, Crofts S, Campbell D, Davey P. Design, Measurement, and Evaluation of an Education Strategy in the Hospital Setting to Combat Antimicrobial Resistance: Theoretical Considerations and a Practical Example. Dis Manag Health Outcomes. 2007;15:151–63. doi: 10.2165/00115677-200715030-00004. [DOI] [Google Scholar]
- 42.Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev. 2005:CD003539. doi: 10.1002/14651858.CD003539.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davey P, Brown E, Fenelon L, Finch R, Gould I, Hartman G, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2005:CD003543. doi: 10.1002/14651858.CD003543.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Pulcini C, Naqvi A, Gardella F, Dellamonica P, Sotto A. [Bacterial resistance and antibiotic prescriptions: perceptions, attitudes and knowledge of a sample of French GPs] Med Mal Infect. 2010;40:703–9. doi: 10.1016/j.medmal.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Solomon DH, Van Houten L, Glynn RJ, Baden L, Curtis K, Schrager H, et al. Academic detailing to improve use of broad-spectrum antibiotics at an academic medical center. Arch Intern Med. 2001;161:1897–902. doi: 10.1001/archinte.161.15.1897. [DOI] [PubMed] [Google Scholar]
- 46.Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505–11. doi: 10.1164/ajrccm.162.2.9909095. [DOI] [PubMed] [Google Scholar]
- 47.Finch RG, Blasi FB, Verheij TJ, Goossens H, Coenen S, Loens K, et al. GRACE and the development of an education and training curriculum. Clin Microbiol Infect. 2012;18:E308–13. doi: 10.1111/j.1469-0691.2012.03909.x. [DOI] [PubMed] [Google Scholar]
- 48.Pagani L, Gyssens IC, Huttner B, Nathwani D, Harbarth S. Navigating the Web in search of resources on antimicrobial stewardship in health care institutions. Clin Infect Dis. 2009;48:626–32. doi: 10.1086/596762. [DOI] [PubMed] [Google Scholar]
- 49.Gyssens IC. Antibiotic policy. Int J Antimicrob Agents. 2011;38(Suppl):11–20. doi: 10.1016/j.ijantimicag.2011.09.002. [DOI] [PubMed] [Google Scholar]