Abstract
The FRAXtr algorithm uses clinical risk factors (CRF) and bone mineral density (BMD) to predict fracture risk but does not include falls history in the calculation. Using results from the Hertfordshire Cohort Study, we examined the relative contributions of CRFs, BMD and falls history to fracture prediction. We studied 2299 participants at a baseline clinic that included completion of a health questionnaire and anthropometric data. A mean of 5.5 years later (range 2.9-8.8yrs) subjects completed a postal questionnaire detailing fall and fracture history. In a subset of 368 men and 407 women, bone densitometry was performed using a Hologic QDR 4500 instrument. There was a significantly increased risk of fracture in men and women with a previous fracture. A one standard deviation drop in femoral neck BMD was associated with a hazards ratio (HR) of incident fracture (adjusted for CRFs) of 1.92 (1.04-3.54) and 1.77 (1.16-2.71) in men and women respectively. A history of any fall since the age of 45 years resulted in an unadjusted HR of fracture of 7.31(3.78-14.14) and 8.56(4.85-15.13) in men and women respectively. In a ROC curve analysis, the predictive capacity progressively increased as BMD and previous falls were added into an initial model using CRFs alone. Falls history is a further independent risk factor for fracture. Falls risk should be taken into consideration when assessing whether or not to commence medication for osteoporosis and should also alert the physician to the opportunity to target falls risk directly.
Keywords: Epidemiology, osteoporosis, BMD, fracture, fall, FRAX
INTRODUCTION
Osteoporotic fractures are common in later life and associated with considerable morbidity, mortality and economic cost [1]. One of the largest risk factors for fractures is a reduction in bone mineral density (BMD) [2]. However fracture risk ultimately depends not only on the mechanical strength of the bone but also on the forces applied to it. There is therefore a large overlap between the BMD of patients with and without a fracture [3] and approximately half of patients with hip fractures do not fall into the osteoporotic range [4-6]. Risk factors for fracture can be purely skeletal-related affecting bone mass, bone geometry, bone microarchitecture and bone turnover, or solely fall-related such as neuromuscular dysfunction, poor balance, cognitive impairment, cardiovascular instability, reduced visual acuity and sedative medications. Others risks are both skeletal and fall related such as age, genotype, family history of fracture, weight, weight change and mobility [7]. Falls occur commonly in older people with an estimated prevalence of 28-35% in those aged 65 years living in the community and this rises to 42% after the age of 75 years [8]. Following a fall-related injury, older people have a subsequent decline in functional status and an increased risk of requiring institutional care [8]. A fear of falling may also occur and cause further debility through anxiety and activity limitation.
The fracture risk assessment tool (FRAX) algorithm [9] has been developed to estimate the 10-year risk of hip and major osteoporotic fractures based on clinical risk factors (CRF), with or without BMD. The risk factors included in FRAX are age, sex, body mass index (BMI), personal history of fracture, parental history of hip fracture, current smoking, glucocorticoid use, rheumatoid arthritis (RA), alcohol intake and other causes of secondary osteoporosis. One criticism of the FRAX model by some users has been the lack of consideration of falls or falls risk in predicting fractures. Using results from the Hertfordshire Cohort Study, we examined the relative contributions of CRFs, BMD and falls history to the risk of fracture. We also investigated to what degree the inclusion of falls risk, in addition to CRFs and BMD, would improve fracture prediction.
MATERIALS AND METHODS
Participant recruitment
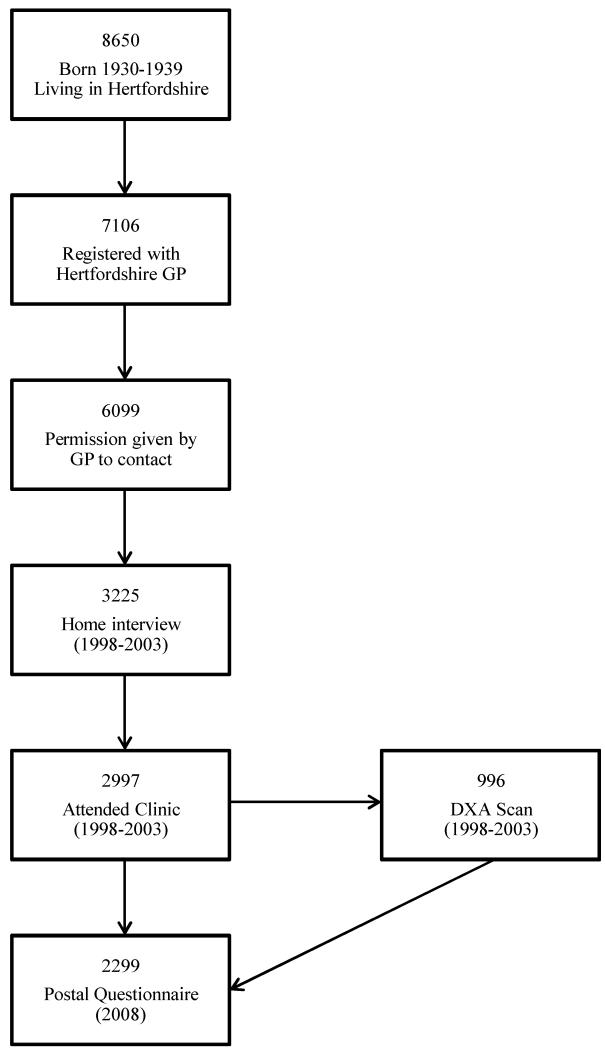
The Hertfordshire Cohort Study is a large, prospective, population-based study of men and women that were initially recruited to investigate the effects of adverse environmental influences in early life on diseases in adulthood. Individuals enrolled in this study were selected with the help of the National Health Service Central Registry at Southport, and Hertfordshire Family Health Service Association. We traced 8650 men and women who were born between 1931 and 1939 in Hertfordshire, who still lived there during the period 1998-2003. Of these, 7106 were confirmed as registered with a Hertfordshire GP. We were given written permission from the General Practitioners of 6099 subjects to contact them (Figure 1). We approached each person by letter, asking them if they would be willing to be contacted by one of our research nurses.
Figure 1.
Flowchart showing participant recruitment and follow up in the Hertfordshire Cohort Study.
Data collection
Three thousand two hundred and twenty five agreed to participate in a home interview with a trained research nurse, during which a structured questionnaire was administered. This included information on socioeconomic status, medical history, cigarette smoking, alcohol consumption, dietary calcium intake and reproductive variables in women. Physical activity was assessed by a previously validated questionnaire [10]. Of these, 2997 (93%) then attended a clinic for physiological investigations. Height was measured to the nearest 0.1cm using a Harpenden pocket stadiometer (Chasmors Ltd, London, UK) and weight to the nearest 0.1kg on a SECA floor scale (Chasmors Ltd, London, UK).
498 men and 498 women returned for a further appointment when bone mineral content (BMC), bone area and BMD were measured by dual energy X-ray absorptiometry (DXA) at the lumbar spine and proximal femur (neck, total, intertrochanteric and trochanteric regions, Wards triangle) using a Hologic QDR 4500 instrument. Those subjects who underwent DXA scans did not significantly vary from the clinic population as a whole with regard to birthweight or weight at one year, age, social class, cigarette and alcohol consumption, height, weight or body mass index [11].
Measurement precision error, expressed as coefficient of variation, was 1.55% for lumbar spine BMD, 1.45% for total femur and 1.83% for femoral neck BMD for the Hologic QDR 4500; these figures were obtained by twenty five volunteers who were not part of the study undergoing 2 scans on the same day, getting on and off the table between examinations. Short-term (2 month) precision error for the QDR 4500 was less than 1% for both sites (manufacturer’s figures). Individuals taking drugs known to alter bone metabolism (such as bisphosphonates) were excluded from this part of the study, although women taking Hormone Replacement Therapy (HRT) were allowed to participate. There were no other exclusion criteria to this part of the study, and subjects were approached for consent as they attended clinic.
Follow up
Those subjects still resident in Hertfordshire in 2008 (n=2777) were subsequently sent out a postal questionnaire. 1168 men and 1131 women completed this, a mean of 5.5 years (range 2.9 - 8.8) after their baseline clinic. Information was obtained on incident fractures through self-report alone; a method which has previously been validated [12]. We studied this group of 2299 subjects, 775 of whom had femoral neck BMD available from the DXA scan conducted during their initial assessment.
Statistical methods
Statistical analysis was conducted using STATA 11. Cox’s proportional hazards models were used to obtain hazards ratios for fracture risk associated with CRFs, BMD and falls. Based on the number of fractures available, this study has 80% power to detect a hazards ratio of 1.68 and 2.22 for a risk factor based model at the 5% significance level in women and men respectively.
We assessed the discrimination and calibration of three models in fracture risk prediction: CRFs; CRFs and BMD; and CRFs, BMD and fall-history. Discrimination (the ability to discriminate individuals at high risk from people at low risk) was assessed by receiver-operator curve (ROC) analysis, as well as the generation of Akaike Information Criteria. We then estimated the net reclassification index (NRI) for the model including CRFs and BMD, before and after fall-history was included. Finally we assessed calibration (comparing the extent to which the predicted risk corresponds to the observed risk in the population) for the three models.
Ethical permission for the study was granted by the East and North Hertfordshire Ethical Committees. All participants gave written informed consent.
RESULTS
Subjects
The characteristics of the study population at baseline are displayed in table 1. The mean age of men and women in the study population at baseline was 65.8 and 66.6 years respectively. Follow up occurred a mean (SD) of 6.0 (1.4) and 5.0 (1.1) years later for men and women respectively. Thirty seven percent of men and 63 percent of women had never smoked, while 50% of the men (29% of the women) and 13% of the men (9% of the women) were ex-smokers and current smokers respectively. Five percent of men and 19 percent of women were non-drinkers, while 22% of men and 4% of women consumed greater than the recommended number of units of alcohol per week. Two hundred and twenty two men (19.7%) and 454 women (42%) reported at least one fall after the age of 45 years. One hundred and forty eight men (12.7%) and 237 women (21.0%) reported a fracture after the age of 45 years and prior to the baseline study.
Table 1.
Summary characteristics of study participants
| Characteristic | Men | Women | ||
|---|---|---|---|---|
| Total N | Mean (SD), unless otherwise stated |
Total N | Mean (SD), unless otherwise stated |
|
| Age at baseline (yrs) | 1168 | 65.8 (2.9) | 1131 | 66.6 (2.7) |
| BMI (kg/m2)a | 1163 | 26.9 (1.1) | 1130 | 27.0 (1.2) |
| Previous fractureb [N(%)] | 1168 | 148 (12.7%) | 1131 | 237 (21.0%) |
| Family history of fracturec [N(%)] | 1159 | 268 (23.1%) | 1126 | 364 (32.3%) |
| Ever smoked [N(%)] | 1168 | 733 (62.8%) | 1129 | 423 (37.5%) |
| Alcohol consumption greater than recommended unitsd [N(%)] |
1167 | 251 (21.5%) | 1131 | 48 (4.2%) |
| Rheumatoid arthritise [N(%)] | 1168 | 51 (4.4%) | 1131 | 75 (6.6%) |
| Comorbiditiesf [N(%)] | 1116 | 1077 | ||
| - None | 539 (48.3%) | 548 (50.9%) | ||
| - 1 | 362 (32.4%) | 378 (35.1%) | ||
| - 2 or more | 215 (19.3%) | 151 (14.0%) | ||
| Femoral neck BMD (g/cm2) | 368 | 0.85 (0.12) | 407 | 0.76 (0.12) |
| Total hip BMD (g/cm2) | 368 | 1.04 (0.14) | 407 | 0.90 (0.13) |
| Lumbar spine BMD (g/cm2) | 368 | 1.07 (0.16) | 408 | 0.96 (0.17) |
| Fallen since the age of 45[N(%)] | 1128 | 222 (19.7%) | 1080 | 454 (42.0%) |
| Fallen in last year [N(%)] | 1124 | 89 (7.9%) | 1072 | 149 (13.9%) |
Geometric mean (SD)
Fracture after the age of 45 years but before baseline
Parents or siblings fracture after aged 45yrs
Recommended units per week are 21 for men and 14 for women
Diagnosed since study baseline
Out of bronchitis, diabetes, IHD, hypertension and stroke
Study Findings
In the period between baseline and follow up there were a total of 170 incident fractures with the majority occurring in women (table 2). The most common site of fracture in women was the distal radius/ulna and in men was the ribs/sternum. Table 3 shows that in women there is a significantly increased risk of fracture in older subjects and those with a previous fracture. The risk was also elevated in those who were ex-smokers or with a family history although these did not reach statistical significance. A similar pattern was seen in men however the only significant factors were previous fracture and previous smoking.
Table 2.
Distribution and incidence of fractures by gender and fracture site.
| Fracture Site | Men | Women | Total | |||
|---|---|---|---|---|---|---|
| N | Incidence rate1 | N | Incidence rate1 | N | Incidence rate1 | |
| Distal Radius/Ulna | 3 | 0.43 | 37 | 6.52 | 40 | 3.17 |
| Distal Tibia/Fibula | 3 | 0.43 | 18 | 3.17 | 21 | 1.66 |
| Ribs/Sternum | 13 | 1.87 | 6 | 1.06 | 19 | 1.50 |
| Hand | 5 | 0.72 | 14 | 2.47 | 19 | 1.50 |
| Foot | 6 | 0.86 | 13 | 2.29 | 19 | 1.50 |
| Hip | 2 | 0.29 | 5 | 0.88 | 7 | 0.55 |
|
| ||||||
| Other | 14 | 2.01 | 31 | 5.47 | 45 | 3.56 |
Incidence rates are per 1000 person years
Table 3.
Cox’s proportional hazards model with clinical risk factors (unadjusted) as explanatory variables for incident fracture.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Hazard Ratio |
95% CI | p- value |
N | Hazard Ratio |
95% CI | p- value |
|
|
|
|
|||||||
| Age at baseline (yrs) |
1161 | 0.94 | (0.84, 1.05) | 0.254 | 1115 | 1.08 | (1.01, 1.17) | 0.032 |
| Weight (kg) | 1157 | 0.98 | (0.95, 1.01) | 0.171 | 1114 | 1.01 | (0.99, 1.02) | 0.229 |
| Height (cm) | 1156 | 1.03 | (0.98, 1.09) | 0.181 | 1114 | 0.97 | (0.93, 1.00) | 0.050 |
| Previous Fracturea |
1161 | 2.75 | (1.37, 5.52) | 0.005 | 1115 | 3.40 | (2.26, 5.09) | <0.001 |
| Family history of fractureb |
1152 | 1.52 | (0.77, 3.01) | 0.224 | 1110 | 1.35 | (0.90, 2.04) | 0.151 |
| Current smokerc |
152 | 1.36 | (0.41, 4.52) | 0.615 | 95 | 0.74 | (0.32, 1.71) | 0.474 |
| Ex-smokerc | 574 | 2.54 | (1.15, 5.58) | 0.021 | 322 | 1.17 | (0.76, 1.80) | 0.481 |
| Alcohol consumptiond |
1160 | 1.01 | (0.99, 1.02) | 0.529 | 1115 | 1.02 | (0.99, 1.05) | 0.238 |
| Rheumatoid arthritise |
1161 | 1.22 | (0.29, 5.07) | 0.783 | 1115 | 0.85 | (0.37, 1.94) | 0.701 |
| Comorbiditiesf | ||||||||
| - 1 | 359 | 0.90 | (0.44, 1.84) | 0.778 | 375 | 1.26 | (0.81, 1.96) | 0.295 |
| - 2 or more | 212 | 0.80 | (0.32, 1.98) | 0.631 | 149 | 1.11 | (0.60, 2.07) | 0.742 |
Fracture after the age of 45 years but before baseline
Parents or siblings fracture after aged 45yrs
Compared to never smoked
Units per week
Diagnosed since baseline
Out of bronchitis, diabetes, IHD, hypertension and stroke
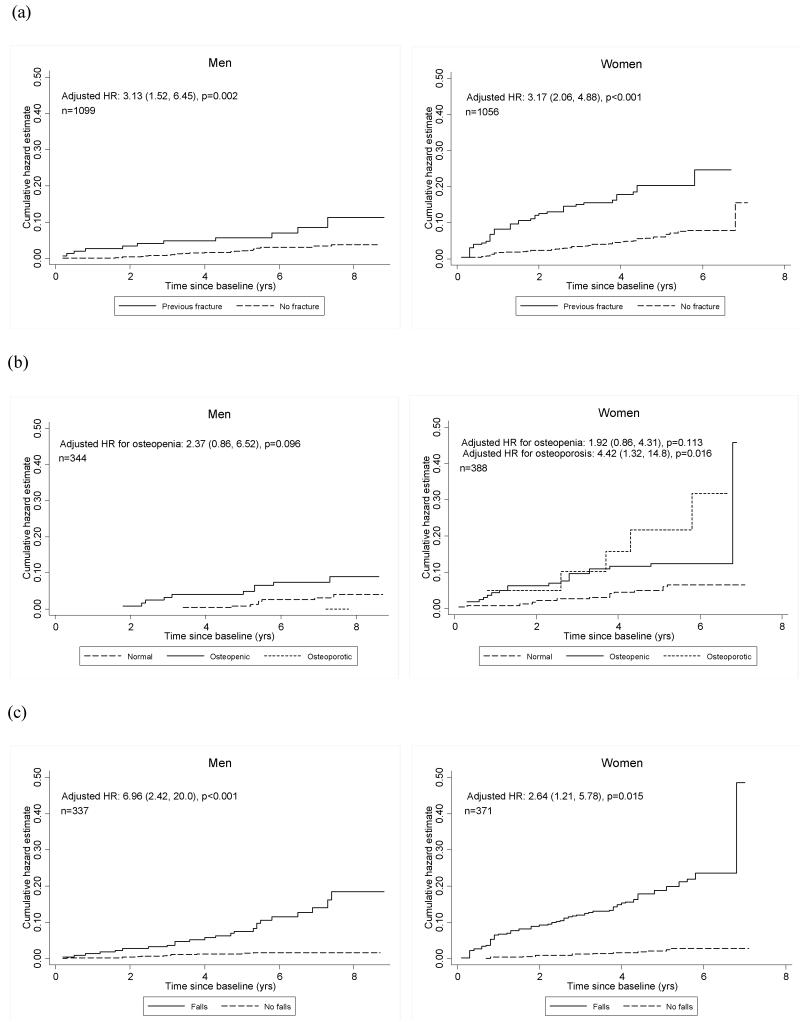
A lower BMD at the femoral neck resulted in an increased risk of fracture in both men (HR 1.73, p=0.032) and women (HR 1.81, p=0.001) (table 4). This association persisted after adjustment for CRFs. A history of falls since the age of 45 years was associated with a marked increase in risk of fracture in both men (HR 7.31, p<0.001) and women (HR 8.56, p<0.001) (table 5). The magnitude of this association was maintained in men after adjustment for CRFs and femoral neck BMD (HR 6.96, p<0.001). In women the level of risk reduced after similar adjustment but remained significantly elevated (HR 2.64, p=0.015). Figure 2a graphically illustrates that the cumulative incidence of fracture is consistently higher in those subjects with a previous fracture (the main CRF), a lower BMD and a history of falls.
Table 4.
Cox’s proportional hazards model with femoral neck BMD negative SD score as an explanatory variable for incident fracture
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Femoral neck BMD negative SD score |
N | HR | 95% CI | p-value | N | HR | 95% CI | p-value |
|
|
|
|||||||
| Unadjusted | 367 | 1.73 | (1.05, 2.87) | 0.032 | 405 | 1.81 | (1.29, 2.54) | 0.001 |
| Adjusted for risk factorsa |
344 | 1.92 | (1.04, 3.54) | 0.036 | 388 | 1.77 | (1.16, 2.71) | 0.008 |
Risk factors: age, weight, height, fracture after aged 45 but before baseline, parent or sibling having a fracture after aged 45, smoker status, diagnosis of rheumatoid arthritis since baseline, number of comorbidities, and alcohol consumption.
Table 5.
Cox’s proportional hazards model with falls history as an explanatory variable for incident fracture
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Fall historya | N | HR | 95% CI | p-value | N | HR | 95% CI | p-value |
|
|
|
|||||||
| Unadjusted | 1121 | 7.31 | (3.78, 14.14) | <0.001 | 1064 | 8.56 | (4.85, 15.13) | <0.001 |
| Adjusted for risk factorsb |
1063 | 6.75 | (3.41, 13.36) | <0.001 | 1008 | 6.33 | (3.50, 11.44) | <0.001 |
| Adjusted for risk factors + femoral neck BMD |
337 | 6.96 | (2.42, 20.01) | <0.001 | 371 | 2.64 | (1.21, 5.78) | 0.015 |
Fallen since the age of 45 years
Risk factors: age, weight, height, fracture after aged 45 but before baseline, parent or sibling having a fracture after aged 45, smoker status, diagnosis of rheumatoid arthritis since baseline, number of comorbidities, and alcohol consumption.
Figure 2.
Cumulative hazard in men and women of developing a fracture in subjects (a) with and without a previous fracture as a clinical risk factor, (b) with different bone mineral densities and (c) with and without a fall since aged 45a.
aHRs are adjusted for risk factors (age, weight, height, family history of fracture, smoker status, number of comorbidities, and alcohol consumption). The bone mineral density HRs (figure 1(b)) are also adjusted for previous fracture; the fallen since aged 45 HRs (Figure 1(c)) are also adjusted for previous fracture and femoral neck BMD.
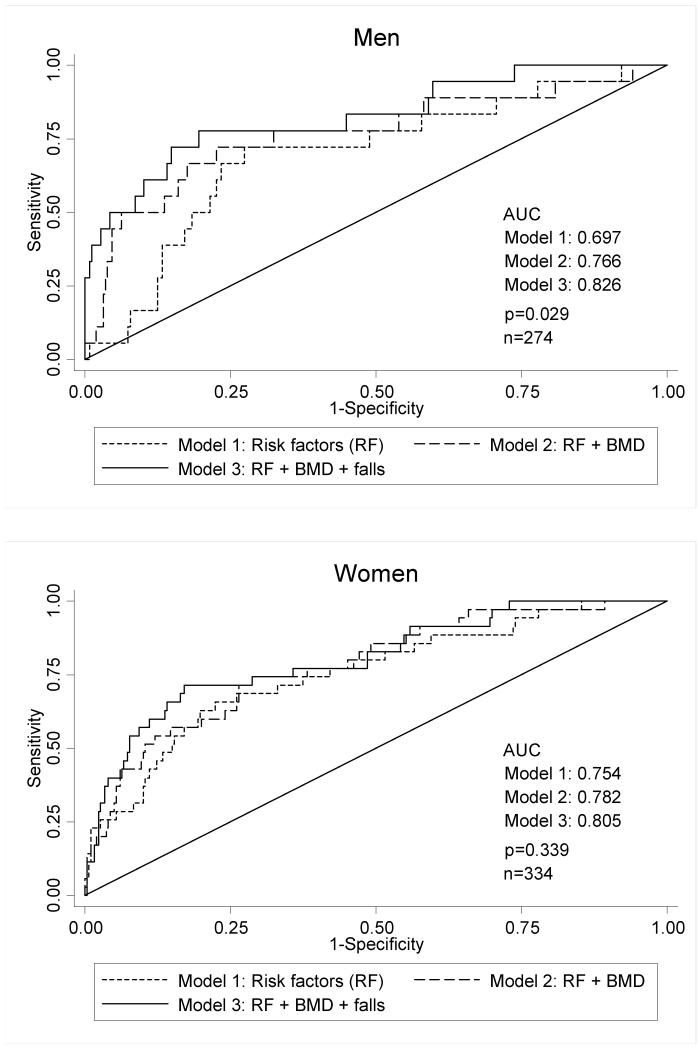
The ROC curve analysis illustrated in figure 3 shows the progressive predictive capacity, area under curve (AUC), as BMD and previous falls are sequentially added to the model in men. The trend was similar but less marked for falls in women. When interpreted as predictive performance, a screening test using CRFs, femoral neck BMD and fall history would potentially have 80% specificity and 77.8% sensitivity in men and 80% specificity and 71.4% sensitivity in women. This would provide positive predictive values of 21.5% and 29.8% in men and women respectively.
Figure 3.
Receiver Operating Characteristic (ROC) curves comparing differing models of fracture prediction.
Akaike Information Criteria confirmed progressively enhanced discriminatory capacity of the model as BMD, and then falls, were added to CRFs (men: 521, 209, 185 for CRF, BMD and falls respectively, and women: 1194, 404, and 357). The NRI with fracture probability as a continuous variable when fall-history was added into the model was assessed. In those that ultimately fractured, the predicted probability of fracture increased in 61.1% and 68.6% of men and women respectively whereas in those who did not fracture the predicted probability reduced in 80.4% and 63.9% of men and women respectively.
In calibration analysis, the observed probability of fracture was 4.2% among men and 10.8% among women; predicted probabilities were as follows: CRFs men 4.3%, women 10.6%; CRFs and BMD men 6.9%, women 11.4%; CRFs, BMD and falls men 6.6%, women 10.5%.
DISCUSSION
This study clearly demonstrates that the addition of fall risk to other CRFs and BMD modestly augments the predictive capacity of the model. Its inclusion in the algorithm appears to be of greatest importance in men to improve classification of fracture.
A number of CRFs have previously been identified as strong predictors of fracture and hence are included in fracture algorithms such as FRAX. For example, the risk of fracture is known to increase with advancing age and this association was evident in female participants in our study. Surprisingly a similar relationship was not seen in men, possibly because of the narrow age range of individuals included. Similarly, a history of previous fracture is known to be one of the most important CRFs [13]. In our study, we found that in those individuals with a previous fracture, the hazards ratio for further fracture was 2.75 and 3.40 in men and women respectively. Figure 2a clearly illustrates the magnitude of the difference between the cumulative fracture risk in those with or without a previous fracture. Associations shown between other CRFs and fracture risk are in keeping with the published literature.
Using our data, a model containing the FRAX CRFs provides a fracture predicting capacity (AUC of ROC analysis) of 0.697 and 0.754 in men and women respectively. In clinical practice the predictive capacity of the FRAX CRF tool is improved by adding in femoral neck BMD. Our study confirms that combining CRFs and BMD within the Hertfordshire cohort also improves the model of fracture prediction, increasing the area under the ROC curve to 0.766 and 0.782 in men and women respectively. The excess risk of all fractures caused by a reduction in BMD of 1 standard deviation (SD) at the femoral neck, after adjustment for CRFs, is 1.92 and 1.77 in men and women respectively. This is in keeping with a meta-analysis investigating BMD and fracture risk, which showed a 1 SD drop in BMD was associated with an approximate doubling of fracture incidence [2]. The graded nature of this association from normal bone density through osteopenia to osteoporosis is highlighted in figure 2b.
Having confirmed that CRFs and BMD predict fracture in our population, we explored whether there was an additional benefit of adding falls into the fracture prediction model. Prior to adjustment the hazards ratios for fracture were 7.31 and 8.56 for men and women respectively. The magnitude of these effects can be seen graphically in figure 2c. Our data shows falls to be a risk factor for fracture independent of other CRFs and femoral neck BMD. When fall history was added into the prediction model, the area under the ROC curve increased to 0.826 and 0.805 for men and women respectively. Thus its inclusion improves the accuracy of prediction in men by around 6%. Additionally, the Akaike Information Criteria take into consideration not only an assessment of the discriminative capacity of the model, through the likelihood function, but also the number of parameters used. It confirms that the progressive improvement occurs even after the small increase in complexity is factored in. The probabilities of fracture predicted by the model that included fall-history were similar in magnitude to those observed within the cohort as a whole, although the values were closer in women (10.47% predicted, 10.77% observed) than in men (6.57% predicted, 4.21% observed).
We then assessed which group of participants was most likely to have their fracture probability correctly modified in the new model. The NRI demonstrates that the greatest effect of adding fall-risk into the model is seen in those men that do not subsequently fracture. In over 80% of cases, the predicted risk reduces in this cohort, and thus more accurately predicts that a fracture will not occur. The increase in the predicted probability of fracture in those that do ultimately fracture is similar in men and women: 61.1% and 68.6% respectively. These findings suggest that the contribution of falls risk to fracture prediction appears greater in men than women. This observation is in accord with previous epidemiological data suggesting a much greater excess mortality among male hip fracture patients compared with females [14] and may be explained by more marked frailty in male hip fracture sufferers compared with their non-fractured male counterparts. Furthermore, the nature of the fall may differ between men and women, with those occurring in men being more likely to cause fracture.
Other studies have also highlighted fall history as a risk factor for fracture independently of other influences [15-17]. A large UK cohort study of 2,424 women aged 65-84 (COSHIBA: Cohort for Skeletal Health in Bristol and Avon) and a French prospective study (OFELY) of 672 postmenopausal women both showed fall history to be an independent risk factor for future fracture OR 1.49 (95% CI 1.13–1.94) [15] and OR 1.76 (95% CI 1.00-3.09) [16] respectively. Furthermore, fall history has already been utilised in some models of fracture prediction. The Garvan fracture risk calculator uses a limited number of risk factors, including the number of falls in the last year (1, 2, 2+), and has been shown to be accurate and reliable [18]. Prior to the publication of this model, Van Staa et al developed a simple clinical score for estimating the risk of fracture in post-menopausal women and this too included an assessment of falls risk [19]. In contrast, the FRAX calculator [9] (the most widely used tool to assess an individual’s risk of fracture) currently does not include history of falls as one of the CRFs. Our results accord with the current literature and support the IOF-ISCD Official Positions on FRAX which state that a 30% increased risk of fracture should be applied for every additional fall in the preceding year [20].
Our study has several caveats when interpreting the results. Firstly, the authors acknowledge that the CRFs investigated are similar but not identical to those used in the FRAX calculator and the discriminatory performance is likely to be maximised as the model has been built and tested on the same data. The period of follow up between identification of CRFs and the assessment of fracture outcomes was relatively short at a mean of 5.5 years, whereas the FRAX tool predicts risk over the subsequent 10 years. The FRAX algorithm considers not only the risk of fracture, but also the competing risk of death in order to calculate its prediction. We were unable to assess for this factor in our analyses and thus how it might have modified the effect of falls if it had been included. Caution must therefore be taken when making direct comparisons between the analysis of this study and implications for the FRAX model. Falls data were collected retrospectively rather than prospectively which can lead to problems with underreporting. However this is often the way in which falls history would be collected in clinical practice for use in fracture risk assessment and so may be considered a valid measure for this indication. Incident fractures were ascertained through self-report. Although this technique has been validated there is still the risk of false positives and false negatives, particularly if the fractures are not of the forearm or hip [12]. Response bias may have occurred due to participant loss between their initial interview and subsequent attendance at clinic. Investigating this we found that there were no major differences in age, social class, alcohol intake, or activity level between interviewed subjects who did or did not attend the clinic. The proportion of current smokers was lower among those who did come to clinic than those who declined, suggesting a “healthy subject” effect. However, as our comparisons were internal, unless the relationships between risk factors and fracture differed between those who did and did not come to clinic, no bias should have been introduced.
CONCLUSION
This study confirms the role of CRFs and BMD in the assessment of fracture risk. Fall history further contributes to this prediction, in particular in men. Its exclusion from FRAX may lead to underestimation of the actual risk in fallers and an overestimation in those that do not fall; our results suggest most strongly the latter effect in men. A history of falls should alert the physician to the opportunity to target falls risk directly as there is strong evidence that falls can be prevented and this is likely to further reduce fracture risk.
Acknowledgements
The Hertfordshire Cohort Study was supported by the Medical Research Council of Great Britain; Arthritis Research UK; and the International Osteoporosis Foundation. The work herein was also supported by the NIHR Nutrition BRU, University of Southampton and the NIHR Musculoskeletal BRU, University of Oxford. We thank all of the men and women who took part in the Hertfordshire Cohort Study; the HCS Research Staff; and Vanessa Cox who managed the data.
REFERENCES
- 1.Walker-Bone K, Dennison E, Cooper C. Osteoporosis. In: Silman AJ, Hochberg MC, editors. Epidemiology of the Rheumatic Diseases. second edition Oxford University Press; 2001. pp. 259–292. [Google Scholar]
- 2.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fracture. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross PD, Davis JW, Vogel JM, Wasnich RD. A critical review of bone mass and the risk of fractures in osteoporosis. Calcif Tissue Int. 1990;46:149–161. doi: 10.1007/BF02555036. [DOI] [PubMed] [Google Scholar]
- 4.Schott AM, Cormier C, Hans D, et al. How hip and whole body bone mineral density predict hip fracture in elderly women: the EPIDOS Prospective Study. Osteoporosis Int. 1998;8:247–254. doi: 10.1007/s001980050061. [DOI] [PubMed] [Google Scholar]
- 5.Black DM, Steinbuch M, Palermo L, et al. An assessment tool for predicting fracture risk in postmenopausal women. Osteoporosis Int. 2001;12:519–528. doi: 10.1007/s001980170072. [DOI] [PubMed] [Google Scholar]
- 6.Lyles KW, Colon Emeric CS, Magaziner SJ, et al. Zolendronic acid in reducing clinical fracture and mortality after hip fracture. N Eng J Med. 2007;357 doi: 10.1056/NEJMoa074941. nihpa40967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allolio B. Risk factors for hip fracture not related to bone mass and their therapeutic implications. Osteoporosis Int. 1999;9(suppl 2):S9–16. doi: 10.1007/pl00004166. [DOI] [PubMed] [Google Scholar]
- 8.Masud T, Morris RO. Epidemiolgoy of Falls. Age Ageing. 2001;30(suppl 4):3–7. doi: 10.1093/ageing/30.suppl_4.3. [DOI] [PubMed] [Google Scholar]
- 9.WHO FRAX fracture risk assessment tool. 2009 Sep 07; [cited 02 November 2009]; Web version 3.0: [Available from]: http://www.shef.ac.uk/FRAX/index.htm. [PubMed]
- 10.Dallosso HM, Morgan K, Bassy EJ, Ebrahim SBJ, Fentem PH, Arie THD. Levels of customary physical activity among the old and very old living at home. J Epidemiol Commun Health. 1988;42:121–127. doi: 10.1136/jech.42.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennison EM, Syddall HE, Sayer AA, Gilbody HJ, Cooper C. Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade: the Hertfordshire cohort study. Pediatr Res. 2005;57(4):582–586. doi: 10.1203/01.PDR.0000155754.67821.CA. [DOI] [PubMed] [Google Scholar]
- 12.Ismail AA, O’Neill TW, Cockerill W, Finn JD, Cannata JB, Hoszowski K, Johnell O, Matthis C, Raspe H, Raspe A, Reeve J, Silman AJ. Validity of self-report of fractures: results from a prospective study in men and women across Europe. EPOS Study Group. European Prospective Osteoporosis Study Group. Osteoporosis Int. 2000;11(3):248–254. doi: 10.1007/s001980050288. [DOI] [PubMed] [Google Scholar]
- 13.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an in creased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Race and sex differences in mortality following fracture of the hip. Am J Public Health. 1992;82(8):1147–1150. doi: 10.2105/ajph.82.8.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark EM, Gould VC, Morrison L, Masud T, Tobias J. Determinants of fracture risk in a UK-population-based cohort of older women: a cross-sectional analysis of the Cohort for Skeletal Health in Bristol and Avon (COSHIBA) Age Ageing. 2012;41:46–52. doi: 10.1093/ageing/afr132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albrand G, Munoz F, Sornay-Rendu E, et al. Independent predictors of all osteoporosis related fractures in healthy postmenopausal women. The OFELY Study. Bone. 2003;32:78–85. doi: 10.1016/s8756-3282(02)00919-5. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook PN, Cameron ID, Chen JS, Cummings RG, Lord SR, March LM, et al. Influence of fall related factors and bone strength on fracture risk in the frail elderly. Osteoporosis Int. 2007;18(5):603–610. doi: 10.1007/s00198-006-0290-z. [DOI] [PubMed] [Google Scholar]
- 18.Bolland MJ, Siu AT, Mason BH, Horne AM, Ames RW, Grey AB, Gamble GD, Reid IR. Evaluation of the FRAX and Garvan fracture risk calculators in older women. J Bone Miner Res. 2011;26:420–427. doi: 10.1002/jbmr.215. [DOI] [PubMed] [Google Scholar]
- 19.van Staa TP, Geusens P, Kanis JA, Leufkens HGM, Gehlbach S, Cooper C. A simple clinical score for estimating the long-term risk of fracture in post-menopausal women. Q J Med. 2006;99:673–682. doi: 10.1093/qjmed/hcl094. [DOI] [PubMed] [Google Scholar]
- 20.Kanis JA, Hans D, Cooper C, Baim S, Bilezikian JP, Binkley N, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22(9):2395–2411. doi: 10.1007/s00198-011-1713-z. [DOI] [PubMed] [Google Scholar]