Abstract
Mitogen-activated protein kinases (MAPKs) are a family of protein kinases that are essential nodes in many cellular regulatory circuits including those that take place on DNA. Most members of the four MAPK subgroups that exist in canonical three kinase cascades - extracellular signal-regulated kinases 1 and 2 (ERK1/2), ERK5, c-Jun N-terminal kinases (JNK1-3), and p38 (α, β, γ, and δ) families - have been shown to perform regulatory functions on chromatin. This review offers a brief update on the variety of processes that involve MAPKs and available mechanisms garnered in the last two years.
Introduction
A wide variety of stimuli are recognized and processed via the sequential phosphorylation and activation of a central module of three kinases terminating in a mitogen-activated protein kinase (MAPK). MAPKs are serine/threonine protein kinases, which respond to activation by phosphorylating a diverse assortment of downstream targets in multiple compartments to execute highly-specific cellular responses. There are four mammalian pathways in which this cascade paradigm holds: extracellular signal-regulated kinases 1 and 2 (ERK1/2), which are often mitogen sensitive, but are also the most widely responsive to a large range of stimuli; ERK5, considered mitogen- and stress-responsive; and the c-Jun N-terminal kinases (JNK1-3) as well as p38 (α, β, γ, and δ) families, categorized as stress-responsive, but which are widely activated, particularly in metabolism and the immune system (1-5). Working outside canonical cascades are several other MAPKs and MAPK-related enzymes, which will not be discussed in this review. The purpose of this review is to provide an update on progress from the perspective of chromatin-tethered kinases.
MAPKs target transcription factors
Activated MAPKs work in the cytoplasm and nucleus to modulate transcriptional responses appropriate to regulatory stimuli and tissue and cellular state. The most extensively studied gene expression drivers are MAPK substrate transcription factors and modifiers, which respond to phosphorylation by altering protein stability, affinity for DNA or protein partners, and nuclear localization (reviewed by Yang, Sharrocks and Whitmarsh, (6)); any of these changes can promote or inhibit transcriptional outputs. Modification of these factors by different MAPKs on one or more phosphoacceptor sites influences both distinct and combinatorial responses to stimulus depending on context.
Our knowledge of the consequences of transcription factor phosphorylation has continued to evolve in the framework of the general transcription machinery and the landscape of chromatin modifications. Twenty years ago, Elk1 was identified as the ternary complex factor, that, once phosphorylated by ERK1/2, partnered with the serum response factor (SRF) to potently enhance transcription of ERK1/2 target genes (7,8). Ten years later, two findings expanded the mechanistic insight. Elk1 phosphorylation was shown to facilitate its interaction with the Mediator complex (9), promoting recruitment and initiation of RNA polymerase II (10). During cardiac differentiation, ERK1/2 activities were found to be suppressed to allow SRF to partner instead with differentiation-promoting factors such as myocardin (11). We return to this system again below.
The advent of genomic mapping has demonstrated that transcription factors can bind vast numbers of sites (12,13), and from our own studies to define MAPK chromatin targets by chromatin immunoprecipitation (ChIP)-sequencing, we find DNA interaction sites in the tens of thousands, consistent with what has been shown for yeast Hog1/p38 and suggested by earlier reviews (14-16). We find a stable pool of chromatin-bound MAPKs under both stimulated and unstimulated conditions. Among possible scenarios, a nuclear pool of ERK1/2 may be refractory to long-term inactivation and/or binding to chromatin may occur in the absence of activation, to keep a subset of genes primed for rapid transcriptional activation, potentially to serve as transcriptional repressors, as some studies have suggested (17,18), or to serve transcription-independent functions.
Next-generation sequencing technologies have revealed a plethora of factor-specific binding sites and have facilitated detailed analyses of cooperative binding among transcription factors and their cognate signaling molecules. A good recent example involves nuclear hormone receptors. ERK2 and JNK1 can each promote the transcription of gene networks activated by estrogen receptor alpha (ERα). The ERK1/2 and JNK pathways are both responsive to stimulation of breast cancer cells by estrogen. ERK1/2 activity is required for the full transcriptional output of ERα-regulated genes (19). In addition to estrogen-dependent targeting of genes crucial for cell cycle progression and proliferation, activated ERK2 also binds to hormone-liganded ERα at genomic targets (20), where it might affect DNA output through phosphorylation of nearby general transcription regulators, including the polymerase machinery (MED1 (21)), chromatin modifiers (p300 (22,23) or ERα itself (24). Similarly, JNK1 and ERα display coordinated regulation in this system. In addition to co-occupying a substantial subset of genes following estrogen stimulation, JNK1 recruitment to estrogen-responsive targets is dependent on the presence of ERα, and the full transcriptional response of these genes requires the presence of both activated proteins (25).
MAPKs target chromatin modification and remodeling factors
Adding another layer of complexity to MAPK-directed transcriptional outcomes, MAPKs can drive histone modifications and direct chromatin remodeling, processes that are frequently complementary to alter gene transcription on a wide scale. Phosphorylation of the mitogen- and stress-activated protein kinases 1 and 2 (MSK1/2) is one such example. MSKs are ERK1/2 and p38 substrates that can directly phosphorylate histone H3 at S10 and S28, modifying nucleosomes in the proximal promoters of immediate early genes (IEGs) during interphase (26). One elegant example shows that ERK1/2 activation leads to phosphorylation of H3S10 in the enhancer of the IEG target FOSL by MSK1/2 (27), which subsequently directs a cascade of events resulting in the recruitment of the Pol II elongation complex Positive Elongation Factor b (P-TEFb) and enhanced transcription of FOSL (22) (reviewed in (28)). MSKs, transcription factors and chromatin remodeling complexes work cooperatively at IEG target promoters, contributing to a permissive transcriptional environment (29,30). Additional functions for MSKs include H3S28 phosphorylation in response to both mitogens and stress, which can oppose the transcriptionally repressive methylation of H3K27 by inhibiting binding of the Polycomb Repressive Complex (31). JNKs have also been implicated in directing H3S10 and S28 phosphorylation in a model of neuronal development, although whether this effect is mediated by MSKs has not been explored (32).
H3K4 methylation coincides with active promoters and enhancers and may be influenced by both p38 and ERK1/2. p38 increases H3K4 methylation in a model of myoblast differentiation (33). Drosophila ERK, along with its scaffold MP1, interact with the chromatin-binding protein Corto; the Corto knockout exhibits a phenotype that suggests involvement either with H3K4 or H3K27 methylating complexes (34). Histone lysine acetylation at multiple sites corresponds with actively transcribed genes, and the ubiquitous p300 protein is a direct ERK target and its phosphorylation promotes not only acetylation of local histones (22), but also transcription factors (35) to drive transcription. In Drosophila, activation of gene targets of the JNK pathway is regulated on many levels, including through recruitment of the transcription factor c-Jun, an event dependent on JNK-driven histone acetylation by the ATAC complex (36). In this case JNK was implicated through mass spectrometry of immunopurified complexes.
The SWI/SNF family and other ATP-dependent chromatin remodeling complexes modulate transcription by changing the accessibility of DNA elements to transcription factors, coactivators, and the transcriptional machinery (37). Recruitment of Hog1/p38 to target promoters in response to osmotic stress directs activity-dependent interaction with the RSC remodeling complex; this complex evicts nucleosomes from promoters for efficient transcription (38). During cardiomyocyte differentiation, as ERK1/2 activity is suppressed, p38 is activated and directs remodeling of genes critical for myogenesis by recruitment of a SWI/SNF family member (39). This phosphorylation-dependent interaction between the basic helix-loop-helix transcription factor MyoD, and the tissue-specific subunit of SWI/SNF, Baf60c, promotes integration of these factors into the Brg1-SWI/SNF complex, driving remodeling of target genes (40). Phosphorylation-dependent subunit incorporation into a SWI/SNF-like complex has additionally been documented in the ERK1/2 response at vitamin D-responsive genes (41), suggesting that MAPKs may serve as a general signaling conduit to effect gene-specific remodeling responses.
MAPKs bind to DNA directly
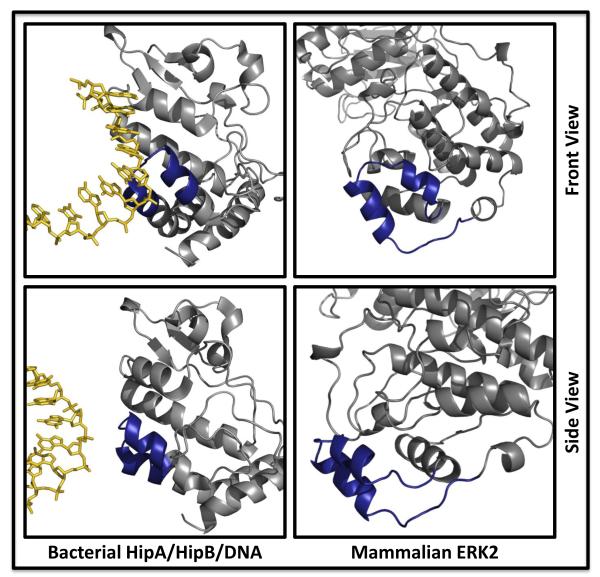
In addition to chromatin localization of MAPKs mediated by interaction with their target transcription factors and other chromatin-bound substrates, ERK2 was demonstrated to bind to DNA directly in vitro at a defined DNA motif, C/GAAAG/C (A-rich motif, (17)). The MAPK insert, a ~40-residue segment containing two antiparallel α-helices which is conserved among MAPKs, CDKs and GSK3, was implicated based on mutagenesis of basic residues in the segment. While most eukaryotic protein kinases do not contain this insert, recent crystallographic studies on a bacterial kinase/transcription factor pair bound to DNA showed that the kinase structure had a eukaryotic-like kinase fold with the component of the molecule structurally analogous to the MAPK insert making direct contacts with DNA ((42); see Figure 1 for a comparison of the structures of ERK2 and HipA/B/DNA). Basic residues in the MAPK insert are in positions analogous to basic residues in HipA that contact DNA. Despite a high level of structural similarity among MAPKs, direct binding to DNA by other family members has not yet been demonstrated, and crystal structures elucidating the mechanism of interaction with DNA are eagerly anticipated.
Figure 1. Structural comparison of a bacterial transcription factor bound to DNA and the mammalian MAPK, ERK2.
Front (top panels) and side (bottom panels) views of the bacterial transcription factor pair HipA/HipB complexed to DNA display structural similarity at a binding interface to the ERK2 MAPK insert (dark blue) which has been implicated in DNA binding. PDB: 3DNV and 1ERK.
MAPKs localize to the coding regions of genes
Binding of protein kinases to the promoters of target genes can be readily explained by their interactions with promoter- and enhancer-associated substrates. However, inducible binding further downstream, within the coding region of the gene and beyond, suggests direct interactions with the Pol II machinery. This was initially reported for Hog1/p38, which is recruited to stress-responsive genes, and binds not only to their promoters, but additionally to coding and 3′ regions through interaction with the elongating polymerase complex (43). Furthermore, chimeras were used to show that the 3′ noncoding region dictates recruitment of Hog1 to the coding region and supports elongating Pol II. This process can be uncoupled from recruitment of the kinase to the promoter, suggesting mechanistically distinct modes of kinase recruitment for different purposes.
The yeast MAPK Mpk1, considered functionally similar to mammalian ERK5, is required for high fidelity transcriptional elongation (44). In addition to phosphorylation of target transcription factors, Mpk1 drives productive transcription by binding to the Paf1 elongation complex. In the absence of Mpk1, target genes are thought to be constitutively primed for transcription but cannot be elongated, resulting in termination by the Sen1 termination complex immediately downstream of the promoter. Binding of Mpk1 to Paf1 allows transcription of the full coding region to proceed in a kinase activity-independent manner. Studies extending to ERK5 suggested that it also controls elongation, but the mechanism remains unclear.
Inhibition of CDK9, an obligate subunit of P-TEFb, prevents processive elongation by Pol II and reduces binding of ERK1/2 and its cascade through the coding region of a target IEG (45), further suggesting intimate interaction between elongating Pol II and MAPKs. It is unclear in this system whether stimulus-dependent Pol II recruitment to target genes is predicated upon interaction of ERK1/2 with the 3′ region of the gene. Interestingly, the ERK1/2 substrate hnRNPK is co-recruited to these sites, and a buildup of signaling factors and Pol II in this region may reflect regulation of the early stages of mRNA processing, that positively feeds back to Pol II (46). ERK1/2 and many of its upstream factors, most dramatically the scaffold Grb2, are enriched downstream of the coding sequence, but testing whether this localization is a requirement for efficient transcription faces substantial technical challenges. These observations suggest that multiple routes of recruitment exist to bring MAPKs to their target genes, and that they have multiple functions once bound.
MAPKs are locally regulated on target genes
Targeted recruitment of MAPKs including ERK1/2, their upstream kinase MEK1/2 and MAPK-regulatory receptor tyrosine kinases has been widely documented to date (47-49). Stimulus-induced localization of signaling cascade components upstream of MEK1/2 was recently demonstrated in cultured cells and mice. Recruitment of ERK1/2 activation machinery to target genes, including B-Raf, SOS and Grb2, largely reflects the binding profiles of ERK1/2, with peaks typically found in the promoter-proximal coding region of target genes. It is unclear from these experiments whether loss of any of these factors could abrogate binding of ERK1/2, but studies on the inducible recruitment of MAPK cascade components in the presence of the MEK1/2 inhibitor U0126 demonstrated that ERK1/2 activity is vital for binding of all cascade components. In Drosophila, the JNK MAPK cascade, including upstream kinases, additionally localizes to target genes, an event that is important both in transcription factor activation, histone acetylation, and negative regulation of upstream kinase activity (36). From these studies, it appears that recruitment of MAPK cascade components is widely conserved and can consequently regulate upstream signal transduction (36). Yet, interactions between MAPKs with binding partners at the promoter and within the coding region can be separable (43). Given the coordinated nature of transcription factor, Pol II and MAPK recruitment, and the complexity of MAPK-driven transcriptional activation, epistatic relationships are difficult to assign. At the least, it is likely that the local presence of upstream MAPK activation machinery is important for orchestrating multiple aspects of recruitment and initiation.
Kinetic studies suggest that kinase recruitment can be transient (47). Kinetics may be affected by rapid dissociation of the activated kinase from the gene, for instance as a consequence of binding to activated Pol II which completes a cycle of transcription and subsequently dissociates or competition by tighter binding factors (12). Recent work has shown that the nuclear MAPK phosphatase-1 (MKP-1) is recruited directly to an ERK1/2 target gene following insulin treatment on the same time-scale as the MAPK (50), suggesting an additional regulatory mechanism - the system may recruit its own off-switch to maintain the reliable termination of signaling. Calcineurin was also shown to be recruited to ERK1/2 binding sites on the insulin gene promoter (48).
Much of this discussion is based on the expectation that active kinases are chromatin-associated and inactive ones are not. Inactive (i.e., unphosphorylated) kinases are also present in quantity on chromatin. What are possible purposes of inactive kinases on chromatin? Inactive MAPKs may interfere with transcription, either by directly blocking binding of site-specific factors or by sequestering factor substrates. Because kinase binding sites identified on chromatin are not restricted to DNA regions associated with transcriptional regulation, kinase repositories may act on enhancer sites distant from proximal promoters, or exist in inactive depots until conscripted to other sites for activation in situ, reflecting the complexity of transcriptional regulation. Inactive kinases may also have activity-independent functions aside from transcription initiation and elongation or DNA repair. Among tantalizing possibilities, MAPKs may be involved in formation of heterochromatin, chromatin looping, or maintenance of chromatin structure by forming bridges to nuclear structural proteins such as nucleoporins.
At this point, we must conclude that not only are protein kinases associated with chromatin through their transcription factor substrates, but are also common components of chromatin regulatory complexes. Regulation of nuclear structural properties are almost certainly among their important functions. We anticipate that the next five years will reveal greater regulatory functions as well as novel mechanisms of action.
Highlights.
MAPKs bind to DNA directly and indirectly via transcriptional regulators
MAPKs target a wide range of chromatin-bound factors
Regulatory proteins that modulate MAPK function can be co-recruited to chromatin
Mechanisms that dictate MAPK function in transcription are widely conserved
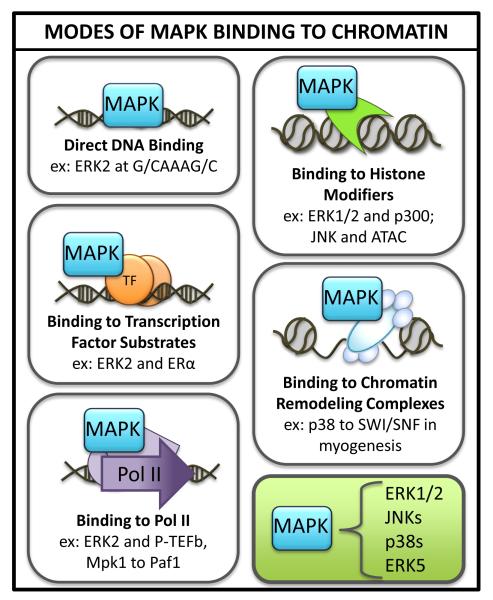
Figure 2. MAPKs interact with chromatin through direct interactions with DNA as well as binding to chromatin-associated substrates.
Examples of each interaction from the MAPK family are provided. Among chromatin-associated substrates, MAPKs bind to transcription factors, RNA polymerase subunits, histone modifying complexes and ATP-dependent chromatin modifiers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Raman M, et al. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 2.Turjanski AG, et al. MAP kinases and the control of nuclear events. Oncogene. 2007;26:3240–3253. doi: 10.1038/sj.onc.1210415. [DOI] [PubMed] [Google Scholar]
- 3.Sabio G, Davis RJ. cJun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends Biochem.Sci. 2010;35:490–496. doi: 10.1016/j.tibs.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Nadal E, Posas F. Multilayered control of gene expression by stress-activated protein kinases. EMBO J. 2010;29:4–13. doi: 10.1038/emboj.2009.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 6.Yang SH, et al. Transcriptional regulation by the MAP kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- 7.Gille H, et al. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992;358:414–416. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- 8.Hill CS, et al. Functional analysis of a growth factor-responsive transcription factor complex. Cell. 1993;73:395–406. doi: 10.1016/0092-8674(93)90238-l. [DOI] [PubMed] [Google Scholar]
- 9.Stevens JL, et al. Transcription control by E1A and MAP kinase pathway via Sur2 mediator subunit. Science. 2002;296:755–758. doi: 10.1126/science.1068943. [DOI] [PubMed] [Google Scholar]
- 10.Wang G, et al. Mediator requirement for both recruitment and postrecruitment steps in transcription initiation. Mol.Cell. 2005;17:683–694. doi: 10.1016/j.molcel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, et al. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428:185–189. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- **12.Li XY, et al. The role of chromatin accessibility in directing the widespread, overlapping patterns of Drosophila transcription factor binding. Genome Biol. 2011;12:R34. doi: 10.1186/gb-2011-12-4-r34. Argues that DNA accessibility not affinity dictates transcription factor binding.
- 13.Biggin MD. Animal transcription networks as highly connected, quantitative continua. Dev.Cell. 2011;21:611–626. doi: 10.1016/j.devcel.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Pokholok DK, et al. Activated signal transduction kinases frequently occupy target genes. Science. 2006;313:533–536. doi: 10.1126/science.1127677. [DOI] [PubMed] [Google Scholar]
- 15.Edmunds JW, Mahadevan LC. MAP kinases as structural adaptors and enzymatic activators in transcription complexes. J.Cell Sci. 2004;117:3715–3723. doi: 10.1242/jcs.01346. [DOI] [PubMed] [Google Scholar]
- 16.Chow CW, Davis RJ. Proteins kinases: chromatin-associated enzymes? Cell. 2006;127:887–890. doi: 10.1016/j.cell.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Hu S, et al. Profiling the human protein-DNA interactome reveals ERK2 as a transcriptional repressor of interferon signaling. Cell. 2009;139:610–622. doi: 10.1016/j.cell.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bardwell L, et al. Repression of yeast Ste12 transcription factor by direct binding of unphosphorylated Kss1 MAPK and its regulation by the Ste7 MEK. Genes Dev. 1998;12:2887–2898. doi: 10.1101/gad.12.18.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madak-Erdogan Z, et al. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol.Endocrinol. 2008;22:2116–2127. doi: 10.1210/me.2008-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *20.Madak-Erdogan Z, et al. Genomic collaboration of estrogen receptor alpha and extracellular signal-regulated kinase 2 in regulating gene and proliferation programs. Mol.Cell Biol. 2011;31:226–236. doi: 10.1128/MCB.00821-10. The authors examine the combinatorial actions of ERK2 and ERα on transcription in breast cancer cells. They implicate ERK2 in transcription, both dependent and independent of physical association with ERα on DNA.
- 21.Belakavadi M, et al. MED1 phosphorylation promotes its association with mediator: implications for nuclear receptor signaling. Mol.Cell Biol. 2008;28:3932–3942. doi: 10.1128/MCB.02191-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YJ, et al. ERK2-mediated C-terminal serine phosphorylation of p300 is vital to the regulation of epidermal growth factor-induced keratin 16 gene expression. J Biol Chem. 2007;282:27215–27228. doi: 10.1074/jbc.M700264200. [DOI] [PubMed] [Google Scholar]
- 23.Dioum EM, et al. A small molecule differentiation inducer increases insulin production by pancreatic beta cells. Proc.Natl.Acad.Sci.U.S.A. 2011;108:20713–20718. doi: 10.1073/pnas.1118526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Likhite VS, et al. Kinase-specific phosphorylation of the estrogen receptor changes receptor interactions with ligand, deoxyribonucleic acid, and coregulators associated with alterations in estrogen and tamoxifen activity. Mol.Endocrinol. 2006;20:3120–3132. doi: 10.1210/me.2006-0068. [DOI] [PubMed] [Google Scholar]
- 25.Sun M, et al. Estrogen regulates JNK1 genomic localization to control gene expression and cell growth in breast cancer cells. Mol.Endocrinol. 2012;26:736–747. doi: 10.1210/me.2011-1158. This study shows that the interaction between JNK1 and ERα is important for full transcriptional output in response to estrogen, and that loss of JNK1 abrogates ERα binding to targets.
- 26.Healy S, et al. Histone H3 phosphorylation, immediate-early gene expression, and the nucleosomal response: a historical perspective. Biochem.Cell Biol. 2012;90:39–54. doi: 10.1139/o11-092. [DOI] [PubMed] [Google Scholar]
- 27.Zippo A, et al. Histone crosstalk between H3S10ph and H4K16ac generates a histone code that mediates transcription elongation. Cell. 2009;138:1122–1136. doi: 10.1016/j.cell.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 28.Galbraith MD, Espinosa JM. Lessons on transcriptional control from the serum response network. Curr.Opin.Genet.Dev. 2011;21:160–166. doi: 10.1016/j.gde.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drobic B, et al. Promoter chromatin remodeling of immediate-early genes is mediated through H3 phosphorylation at either serine 28 or 10 by the MSK1 multi-protein complex. Nucleic Acids Res. 2010;38:3196–3208. doi: 10.1093/nar/gkq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimada M, et al. cAMP-response element-binding protein (CREB) controls MSK1-mediated phosphorylation of histone H3 at the c-fos promoter in vitro. J Biol.Chem. 2010;285:9390–9401. doi: 10.1074/jbc.M109.057745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *31.Gehani SS, et al. Polycomb group protein displacement and gene activation through MSK-dependent H3K27me3S28 phosphorylation. Mol.Cell. 2010;39:886–900. doi: 10.1016/j.molcel.2010.08.020. MSK1/2 phosphorylation of H3S28 physically blocks recruitment of polycomb repressive complex and relieves silencing on genes important for mitogenic and stress signaling.
- 32.Tiwari VK, et al. A chromatin-modifying function of JNK during stem cell differentiation. Nat.Genet. 2012;44:94–100. doi: 10.1038/ng.1036. [DOI] [PubMed] [Google Scholar]
- 33.Rampalli S, et al. p38 MAPK signaling regulates recruitment of Ash2L-containing methyltransferase complexes to specific genes during differentiation. Nat.Struct.Mol.Biol. 2007;14:1150–1156. doi: 10.1038/nsmb1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mouchel-Vielh E, et al. The MAP kinase ERK and its scaffold protein MP1 interact with the chromatin regulator Corto during Drosophila wing tissue development. BMC.Dev.Biol. 2011;11:17. doi: 10.1186/1471-213X-11-17. This study implicates the Drosophila ERK1/2 homolog and its scaffolding partner MP1 in wing vein development through binding and potentially phosphorylating the epigenetic effector protein Corto.
- 35.Liu DX, et al. p300-Dependent ATF5 acetylation is essential for Egr-1 gene activation and cell proliferation and survival. Mol.Cell Biol. 2011;31:3906–3916. doi: 10.1128/MCB.05887-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **36.Suganuma T, et al. The ATAC acetyltransferase complex coordinates MAP kinases to regulate JNK target genes. Cell. 2010;142:726–736. doi: 10.1016/j.cell.2010.07.045. Takes a mass spectrometry approach that identifies JNK pathway components as binding partners of the histone acetyltransferase ATAC; shows that ATAC is a downstream target of the pathway and negatively feeds back to block further JNK activation.
- 37.Wu JI, et al. Understanding the words of chromatin regulation. Cell. 2009;136:200–206. doi: 10.1016/j.cell.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mas G, et al. Recruitment of a chromatin remodelling complex by the Hog1 MAP kinase to stress genes. EMBO J. 2009;28:326–336. doi: 10.1038/emboj.2008.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simone C, et al. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat.Genet. 2004;36:738–743. doi: 10.1038/ng1378. [DOI] [PubMed] [Google Scholar]
- *40.Forcales SV, et al. Signal-dependent incorporation of MyoD-BAF60c into Brg1-based SWI/SNF chromatin-remodelling complex. EMBO J. 2012;31:301–316. doi: 10.1038/emboj.2011.391. The authors show that during myogenesis, p38 helps to promote differentiation by phosphorylating the SWI/SNF subunit Baf60c, promoting association of the remodeling complex with transcription factors to drive appropriate gene expression.
- 41.Oya H, et al. Phosphorylation of Williams syndrome transcription factor by MAPK induces a switching between two distinct chromatin remodeling complexes. J Biol.Chem. 2009;284:32472–32482. doi: 10.1074/jbc.M109.009738. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Schumacher MA, et al. Molecular mechanisms of HipA-mediated multidrug tolerance and its neutralization by HipB. Science. 2009;323:396–401. doi: 10.1126/science.1163806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Proft M, et al. The stress-activated Hog1 kinase is a selective transcriptional elongation factor for genes responding to osmotic stress. Mol.Cell. 2006;23:241–250. doi: 10.1016/j.molcel.2006.05.031. [DOI] [PubMed] [Google Scholar]
- **44.Kim KY, Levin DE. Mpk1 MAPK association with the Paf1 complex blocks Sen1-mediated premature transcription termination. Cell. 2011;144:745–756. doi: 10.1016/j.cell.2011.01.034. This study shows that the yeast MAPK Mpk1 is required for transcriptional elongation of stress responsive genes via its interaction with the Paf1 elongation complex.
- *45.Mikula M, Bomsztyk K. Direct recruitment of ERK cascade components to inducible genes is regulated by heterogeneous nuclear ribonucleoprotein (hnRNP) K. J Biol.Chem. 2011;286:9763–9775. doi: 10.1074/jbc.M110.213330. This is a detailed analysis of ERK1/2 binding dynamics along an entire target gene following activation, as well as recruitment of upstream signaling molecules, including Raf, SOS and Grb2.
- 46.Moore MJ, Proudfoot NJ. Pre-mRNA processing reaches back to transcription and ahead to translation. Cell. 2009;136:688–700. doi: 10.1016/j.cell.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Lawrence MC, et al. Chromatin-bound mitogen-activated protein kinases transmit dynamic signals in transcription complexes in {beta}-cells. Proc.Natl.Acad.Sci.U.S.A. 2008;105:13315–13320. doi: 10.1073/pnas.0806465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawrence MC, et al. Multiple chromatin-bound protein kinases assemble factors that regulate insulin gene transcription. Proc.Natl.Acad.Sci.USA. 2009;106:22181–22186. doi: 10.1073/pnas.0912596106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang YN, et al. Nuclear trafficking of the epidermal growth factor receptor family membrane proteins. Oncogene. 2010;29:3997–4006. doi: 10.1038/onc.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **50.Nelson JD, et al. Direct recruitment of insulin receptor and ERK signaling cascade to insulin-inducible gene loci. Diabetes. 2011;60:127–137. doi: 10.2337/db09-1806. This paper shows that MAPK pathway components bind to insulin-responsive genes in vivo, and that recruitment profiles are perturbed in ob/ob mice, reflecting issues in efficient insulin-responsive gene transcription.